Two Co(II)-Based MOFs Constructed from Resorcin[4]Arene Ligand: Syntheses, Structures, and Heterogeneous Catalyst for Conversion of CO2
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [(CH3)2NH2]2[Co5L(H2O)8]·4H2O (1)
2.3. Synthesis of [Co6L(DMF)2(H2O)8]·2H2O (2)
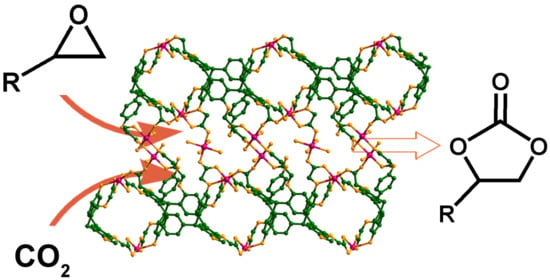
2.4. Coupling of CO2 with Epoxides
2.5. X-ray Crystallography
3. Results and Discussion
3.1. Structure of [(CH3)2NH2]2[Co5L(H2O)8]·4H2O (1)
3.2. Structure of [Co6L(DMF)2(H2O)8]·2H2O (2)
3.3. Characterization of the Crystal Structure of 1 and 2
3.4. Coupling of CO2 with Epoxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitne, W. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Janaky, C. Recent Advances in Solar-Driven Carbon Dioxide Conversion: Expectations versus Reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Francke, R.; Schille, B.; Roemelt, M. Homogeneously Catalyzed Electroreduction of Carbon Dioxide Methods, Mechanisms, and Catalysts. Chem. Rev. 2018, 118, 4631–4701. [Google Scholar] [CrossRef]
- Yan, X.; Chen, C.; Wu, Y.; Liu, S.; Chen, Y.; Feng, R.; Zhang, J.; Han, B. Efficient electroreduction of CO2 to C2+ products on CeO2 modified CuO. Chem. Sci. 2021, 12, 6638–6645. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, H.; An, K.; Lee, J.S. Recycling Carbon Dioxide through Catalytic Hydrogenation: Recent Key Developments and Perspectives. ACS Catal. 2020, 10, 11318–11345. [Google Scholar] [CrossRef]
- Dey, S.; Bhunia, A.; Breitzke, H.; Groszewicz, P.B.; Buntkowsky, G.; Janiak, C. Two linkers are better than one: Enhancing CO2 capture and separation with porous covalent triazine-based frameworks from mixed nitrile linkers. J. Mater. Chem. A 2017, 5, 3609–3620. [Google Scholar] [CrossRef]
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal-organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Fang, Z.-B.; Liu, T.-T.; Liu, J.; Jin, S.; Wu, X.-P.; Gong, X.-Q.; Wang, K.; Yin, Q.; Liu, T.-F.; Cao, R.; et al. Boosting Interfacial Charge-Transfer Kinetics for Efficient Overall CO2 Photoreduction via Rational Design of Coordination Spheres on Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12515–12523. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Huang, H.-H.; Liu, M.; Yao, S.; Guo, S.; Wang, J.-W.; Zhang, Z.-M.; Lu, T.-B. Encapsulation of Single Iron Sites in a Metal-Porphyrin Framework for High-Performance Photocatalytic CO2 Reduction. Inorg. Chem. 2020, 59, 6301–6307. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhou, Y.; Wang, W.; Zhang, C.; Zeng, G.; Huang, D.; Wang, L.; Wang, H.; Yang, Y.; Lei, L.; et al. Recent advances in two-dimensional nanomaterials for photocatalytic reduction of CO2: Insights into performance, theories and perspective. J. Mater. Chem. A 2020, 8, 19156–19195. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, D.; Liu, H.; Sun, X.; Chen, C.; Bi, J.; Liu, J.; Wu, H.; Han, B. Hollow metal organic framework-mediated in-situ architecture of copper dendrites for enhanced CO2 electroreduction. Angew. Chem. Int. Ed. 2020, 59, 8896–8901. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.-X.; Lang, Z.-L.; Liu, Y.; Liu, J.; Yuan, L.; Lu, W.-Y.; Xia, Y.-S.; Dong, L.-Z.; Yuan, D.-Q.; et al. Enhanced Cuprophilic Interactions in Crystalline Catalysts Facilitate the Highly Selective Electroreduction of CO2 to CH4. J. Am. Chem. Soc. 2021, 143, 3808–3816. [Google Scholar] [CrossRef]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic Strategies for the Cycloaddition of Pure, Diluted, and Waste CO2 to Epoxides under Ambient Conditions. ACS Catal. 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Chen, H.; Jie, K.; Yang, Z.; Li, T.; Dai, S. Entropy-Driven Mechanochemical Synthesis of Polymetallic ZeoliticImidazolate Frameworks for CO2 Fixation. Angew. Chem. Int. Ed. 2019, 58, 5018–5022. [Google Scholar] [CrossRef]
- Ji, H.; Naveen, K.; Lee, W.; Kim, T.S.; Kim, D.; Cho, D.-H. Pyridinium-Functionalized Ionic Metal-Organic Frameworks Designed as Bifunctional Catalysts for CO2 Fixation into Cyclic Carbonates. ACS Appl. Mater. Interfaces 2020, 12, 24868–24876. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-B.; Darensbourg, D.J. Cobalt Catalysts for The Coupling of CO2 and Epoxides to Provide Polycarbonates and Cyclic Carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Liu, J.; Lim, J.S.; Zou, R.; Zhao, Y.L. A triazole-containing metal-organic framework as a highly effective and substrate size-dependent catalyst for CO2 conversion. J. Am. Chem. Soc. 2016, 138, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Jiang, H.-L. Incorporation of Imidazolium-Based Poly(ionic liquid)s into a Metal-Organic Framework for CO2 Capture and Conversion. ACS Catal. 2018, 8, 3194–3201. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Sun, W.; Yang, N.-N.; Li, P.; Gong, T.; Sun, W.-J.; Sui, Q.; Gao, E.-Q. A Facile and Versatile “Click” Approach Toward Multifunctional Ionic Metal-organic Frameworks for Efficient Conversion of CO2. ChemSusChem 2019, 12, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Kuruppathparambil, R.R.; Babu, R.; Jeong, H.M.; Hwang, G.-Y.; Jeong, G.S.; Kim, M.-I.; Kim, D.-W.; Park, D.-W. A Solid Solution Zeolitic Imidazolate Framework as A Room Temperature Efficient Catalyst for the Chemical Fixation of CO2. Green Chem. 2016, 18, 6349–6356. [Google Scholar] [CrossRef]
- Cuesta-Aluja, L.; Masdeu-Bultó, A.M. Iron(III) Versatile Catalysts for Cycloaddition of CO2 to Epoxides and Epoxidation of Alkenes. ChemistrySelect 2016, 1, 2065–2070. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Sun, X.; Li, G.; Liu, Y.; Verma, G.; Ma, S. Indium-Organic Frameworks Based on Dual Secondary Building Units Featuring Halogen-Decorated Channels for Highly Effective CO2 Fixation. Chem. Mater. 2019, 31, 1084–1091. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Yue, C.; Fan, Y.; Qi, C.; Jiang, H. Highly Stable Chiral Zirconium-Metallosalen Frameworks for CO2 Conversion and Asymmetric C-H Azidation. ACS Appl. Mater. Interfaces 2018, 10, 36047–36057. [Google Scholar] [CrossRef]
- Lu, B.-B.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. A Polyoxovanadate-Resorcin[4]arene-Based Porous Metal-Organic Framework as an Efficient Multifunctional Catalyst for the Cycloaddition of CO2 with Epoxides and the Selective Oxidation of Sulfides. Inorg. Chem. 2017, 56, 11710–11720. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-P.; Xue, Y.-S.; Li, N.-F.; Gong, J.-J.; Kang, R.-K.; Xu, Y. Lewis Acid Dominant Windmill-Shaped V8 Clusters: A Bifunctional Heterogeneous Catalyst for CO2 Cycloaddition and Oxidation of Sulfides. J. Am. Chem. Soc. 2019, 141, 19487–19497. [Google Scholar] [CrossRef]
- Zhao, Y.-Q.; Liu, Y.-Y.; Ma, J.-F. Polyoxometalate-Based Organic−Inorganic Hybrids as Heterogeneous Catalysts for Cycloaddition of CO2 with Epoxides and Oxidative Desulfurization Reactions. Cryst. Growth Des. 2021, 21, 1019–1027. [Google Scholar] [CrossRef]
- Drake, T.; Ji, P.; Lin, W. Site Isolation in Metal-Organic Frameworks Enables Novel Transition Metal Catalysis. ACC. Chem. Res. 2018, 51, 2129–2138. [Google Scholar] [CrossRef]
- Pei, W.-Y.; Xu, G.; Yang, J.; Wu, H.; Chen, B.; Zhou, W.; Ma, J.-F. Versatile Assembly of Metal-Coordinated Calix[4]resorcinarene Cavitands and Cages through Ancillary Linker Tuning. J. Am. Chem. Soc. 2017, 139, 7648–7656. [Google Scholar] [CrossRef]
- Wei, Y.-S.; Zhang, M.; Zou, R.; Xu, Q. Metal-Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-Organic Frameworks for CO2 Chemical Transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Lu, W.; Wei, Z.; Gu, Z.-Y.; Liu, T.-F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Thomas, G., III; et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, J.; Liu, Y.-Y.; Song, S.-Y.; Ma, J.-F. A porphyrin-based porous rtl metal-organic framework as an efficient catalyst for the cycloaddition of CO2 to epoxides. Chem. Eur. J. 2016, 22, 16991–16997. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, J.; Su, Z.-M.; Batten, S.R.; Ma, J.-F. An exceptional 54-fold interpenetrated coordination polymer with 103-srs network topology. J. Am. Chem. Soc. 2011, 133, 11406–11409. [Google Scholar] [CrossRef]
- Kalaj, M.; Cohen, S.M. Postsynthetic Modification: An Enabling Technology for the Advancement of Metal-Organic Frameworks. ACS Cent. Sci. 2020, 6, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-B.; Chen, X.-Y.; Feng, C.-J.; Chang, J.; Ye, F. Palladium Nanoparticles Immobilized on a Resorcin[4]arene-Based Metal-Organic Framework for Hydrogenation of Nitroarenes. ACS Appl. Nano Mater. 2021, 4, 2278–2284. [Google Scholar] [CrossRef]
- Han, X.; Xu, Y.-X.; Yang, J.; Xu, X.; Li, C.-P.; Ma, J.-F. Metal-Assembled, Resorcin[4]arene-Based Molecular Trimer for Efficient Removal of Toxic Dichromate Pollutants and Knoevenagel Condensation Reaction. ACS Appl. Mater. Interfaces 2019, 11, 15591–15597. [Google Scholar] [CrossRef]
- Liu, J.-H.; Shen, Q.-T.; Yang, J.; Yu, M.-Y.; Ma, J.-F. Polyoxometalate-Templated Cobalt-Resorcin[4]arene Frameworks: Tunable Structure and Lithium-Ion Battery Performance. Inorg. Chem. 2021, 60, 3729–3740. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, W.; Du, S.; Ji, C.; Zhou, M.; Yuan, D. Reticular Chemistry in the Construction of Porous Organic Cages. J. Am. Chem. Soc. 2020, 142, 18060–18072. [Google Scholar] [CrossRef]
- Lu, B.-B.; Yang, J.; Che, G.-B.; Pei, W.-Y.; Ma, J.-F. Highly Stable Copper(I)-Based Metal-Organic Framework Assembled with Resorcin[4]arene and Polyoxometalate for Efficient Heterogeneous Catalysis of Azide-Alkyne “Click” Reaction. ACS Appl. Mater. Interfaces 2018, 10, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, J.; Liu, Y.-Y.; Song, S.; Ma, J.-F. A Family of Metal-Organic Frameworks with A New Chair Conformation Resorcin[4]arene-Based Ligand: Selective Luminescent Sensing of Amine and Aldehyde Vapors, and Solvent-Mediated Structural Transformations. Cryst. Growth Des. 2016, 16, 3244–3255. [Google Scholar] [CrossRef]
- Lv, L.-L.; Yang, J.; Zhang, H.-M.; Liu, Y.-Y.; Ma, J.-F. Metal-Ion Exchange, Small-Molecule Sensing, Selective Dye Adsorption, and Reversible Iodine Uptake of Three Coordination Polymers Constructed by A New Resorcin[4]arene-Based Tetracarboxylate. Inorg. Chem. 2015, 54, 1744–1755. [Google Scholar] [CrossRef]
- Lu, B.-B.; Jiang, W.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Resorcin[4]arene-Based Microporous Metal-Organic Framework as an Efficient Catalyst for CO2 Cycloaddition with Epoxides and Highly Selective Luminescent Sensing of Cr2O72−. ACS Appl. Mater. Interfaces 2017, 9, 39441–39449. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXS-2014, Program for the Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014, Program for the Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Farrugia, L.J. WINGX: A Windows Program for Crystal Structure Analysis; University of Glasgow: Glasgow, UK, 1988. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Hawxwell, S.M.; Brammer, L. Solvent hydrolysis leads to an unusual Cu(II) metal-organic framework. CrystEngComm 2006, 8, 473–476. [Google Scholar] [CrossRef]
- Murphy, C.A.; Cameron, T.S.; Cooke, M.W.; Aquino, M.A.S. Synthesis and structure of trans-dimethylammonium bis(oxalato)diaquaruthenate(III) tetrahydrate. Inorg. Chim. Acta 2000, 305, 225–229. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Liu, H.; Zheng, T.-T.; Liu, P.; Song, J.-X.; Wang, Y.-Y. The effect of coordinated solvent molecules on metal coordination environments in singlecrystal-to-single-crystal transformations. CrystEngComm 2020, 22, 6750–6775. [Google Scholar] [CrossRef]






| Parameters | 1 | 2 |
|---|---|---|
| Formula | C80 H92 O48 N2 Co5 | C82 H86 O48 N2 Co6 |
| Mr | 2144.20 | 2221.10 |
| Cryst syst | Triclinic | Triclinic |
| Space group | P-1 | P-1 |
| a (Å) | 10.5320(6) | 11.2564(6) |
| b (Å) | 13.2619(7) | 15.9785(9) |
| c (Å) | 18.1118(10) | 16.5583(10) |
| α (°) | 70.883(5) | 62.347(6) |
| β (°) | 74.056(5) | 73.527(5) |
| γ (°) | 85.860(4) | 70.219(5) |
| V (Å3) | 2297.9(2) | 2453.6(3) |
| Z | 1 | 1 |
| Dcalc (g cm−3) | 1.550 | 1.503 |
| F(000) | 1105 | 1138 |
| Rint | 0.0498 | 0.0446 |
| GOF on F2 | 1.211 | 1.175 |
| R1 a [I > 2σ(I)] | 0.0847 | 0.0663 |
| wR2 b (all data) | 0.1883 | 0.1559 |
| Entry | 1 (mg) | Temperature (°C) | Time (h) | Conversion (%) b |
|---|---|---|---|---|
| 1 | 10 | 80 | 1 | 24 |
| 2 | 20 | 80 | 1 | 26 |
| 3 | 30 | 80 | 1 | 48 |
| 4 | 0 | 80 | 1 | 23 |
| 5 | 30 | 25 | 1 | 0 |
| 6 | 30 | 50 | 1 | 12 |
| 7 | 30 | 80 | 2 | 51 |
| 8 | 30 | 80 | 4 | 80 |
| 9 | 30 | 80 | 6 | 83 |
| 10 | 30 | 80 | 8 | 98 |
| Entry | Epoxides | Products | Conversion (%) b |
|---|---|---|---|
| 1 |  |  | 99 |
| 2 |  |  | 99 |
| 3 |  |  | 99 |
| 4 |  |  | 95 |
| 5 |  |  | 75 |
| 6 |  |  | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.-B.; Han, X.; Feng, C.-J.; Wang, D.; Ye, F. Two Co(II)-Based MOFs Constructed from Resorcin[4]Arene Ligand: Syntheses, Structures, and Heterogeneous Catalyst for Conversion of CO2. Crystals 2021, 11, 574. https://doi.org/10.3390/cryst11060574
Lu B-B, Han X, Feng C-J, Wang D, Ye F. Two Co(II)-Based MOFs Constructed from Resorcin[4]Arene Ligand: Syntheses, Structures, and Heterogeneous Catalyst for Conversion of CO2. Crystals. 2021; 11(6):574. https://doi.org/10.3390/cryst11060574
Chicago/Turabian StyleLu, Bing-Bing, Xue Han, Cheng-Jie Feng, Duo Wang, and Fei Ye. 2021. "Two Co(II)-Based MOFs Constructed from Resorcin[4]Arene Ligand: Syntheses, Structures, and Heterogeneous Catalyst for Conversion of CO2" Crystals 11, no. 6: 574. https://doi.org/10.3390/cryst11060574