Functional Equations for Calculating the Properties of Low-GWP R1234ze(E) Refrigerant
Abstract
:1. Introduction
2. Modelling the Thermodynamic and Thermokinetic Properties of Refrigerants
3. Correlations for Calculating the Thermodynamic and Thermokinetic Properties of the R1234ze(E) Refrigerant
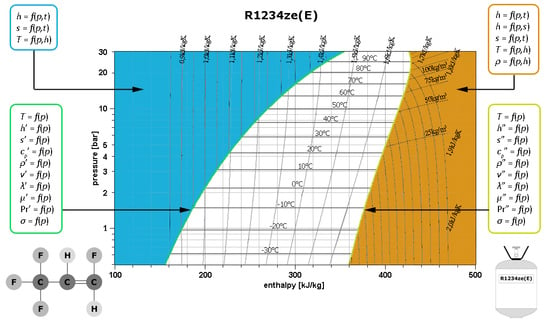
3.1. Saturated Liquid Region
3.2. Saturated Vapour Region
3.3. Superheated Vapour Region
- h = f(p,t)—specific enthalpy as a function of the pressure and temperature (Equation (19));
- h = f(p,s)—specific enthalpy as a function of the pressure and specific entropy (Equation (20));
- s = f(p,t)—specific entropy as a function of the pressure and temperature (Equation (21));
- T = f(p,h)—temperature as a function of the pressure and specific enthalpy (Equation (22));
- ρ = f(p,h)—density as a function of the pressure and specific enthalpy (Equation (23)).
3.4. Subcooled Liquid Region
- h = f(p,t)—specific enthalpy as a function of the pressure and temperature (Equation (24));
- s = f(p,t)—specific entropy as a function of the pressure and temperature (Equation (25));
- T = f(p,h)—temperature as a function of the pressure and specific enthalpy (Equation (26)).
4. Statistical Verification of the Equations used to Calculate the Thermodynamic and Thermokinetic Properties of the R1234ze(E) Refrigerant
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006. OJ L 150, 20.5.2014; pp. 195–230. Available online: http://data.europa.eu/eli/reg/2014/517/oj (accessed on 2 December 2019).
- European Environment Agency. Fluorinated Greenhouse Gases 2018. Data Reported by Companies on the Production, Import, Export and Destruction of Fluorinated Greenhouse Gases in the European Union, 2007–2017; EEA Report No 21/2018; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. REFPROP, NIST Standard Reference Database 23, v.9.1; National Institute of Standards: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F.D., Qin, G.-K., Plattner, M., Tignor, S.K., Allen, J., Boschung, A., Nauels, Y., Xia, V.B., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Sethi, A.; Becerra, E.V.; Motta, S.Y. Low GWP R134a replacements for small refrigeration (plug-in) applications. Int. J. Refrig. 2016, 66, 64–72. [Google Scholar] [CrossRef]
- Moles, F.; Navarro-Esbri, J.; Peris, B.; Mota-Babiloni, A.; Mateu-Royo, C. R1234yf and R1234ze as alternatives to R134a in Organic Rankine Cycles for low temperature heat sources. Energy Procedia 2017, 142, 1192–1198. [Google Scholar] [CrossRef]
- Devecioglu, A.G.; Oruc, V. Improvement on the energy performance of a refrigeration system adapting a plate-type heat exchanger and low-GWP refrigerants as alternatives to R134a. Energy 2018, 155, 105–116. [Google Scholar] [CrossRef]
- Devecioglu, A.G.; Oruc, V. A comparative energetic analysis for some low-GWP refrigerants as R134a replacements in various vapor compression refrigeration systems. Isı Bilimi Ve Tek. Tek. I Derg. J. Therm. Sci. Technol. 2018, 38, 51–61. [Google Scholar]
- Ben Jemaa, R.; Mansouri, R.; Boukholda, I.; Bellagi, A. Energy and exergy investigation of R1234ze as R134a replacement in vapor compression chillers. Int. J. Hydrog. Energy 2017, 42, 12877–12887. [Google Scholar] [CrossRef]
- Jankovic, Z.; Sieres Atienza, J.; Martinez Suarez, J.A. Thermodynamic and heat transfer analyses for R1234yf and R1234ze(E) as drop-in replacements for R134a in a small power refrigerating system. Appl. Therm. Eng. 2015, 80, 42–54. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Navarro-Esbri, J.; Barragan-Cervera, A.; Moles, F.; Peris, B. Drop-in analysis of an internal heat exchanger in a vapour compression system using R1234ze(E) and R450A as alternatives for R134a. Energy 2015, 90, 1636–1644. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, D.; Cabello, R.; Llopis, R.; Arauzo, I.; Catalan-Gil, J.; Torrella, E. Energy performance evaluation of R1234yf, R1234ze(E), R600a, R290 and R152a as low-GWP R134a alternatives. Int. J. Refrig. 2017, 74, 269–282. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, H.; Qiu, J.; Lei, M. Theoretical analysis of R1234ze(E), R152a, and R1234ze(E)/R152a mixtures as replacements of R134a in vapor compression system. Adv. Mech. Eng. 2016, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bansal, P.; Shen, B. Analysis of environmentally friendly refrigerant options for window air conditioners. Sci. Technol. Built Environ. 2015, 21, 483–490. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Navarro-Esbri, J.; Moles, F.; Barragan-Cervera, A.; Peris, B.; Verdu, G. A review of refrigerant R1234ze(E) recent investigations. Appl. Therm. Eng. 2016, 95, 211–222. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0; Standard Reference Data Program; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and Pseudo-pure Fluid Thermophysical Property Evaluation and the Open-Source Thermophysical Property Library CoolProp. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butrymowicz, D.; Baj, P.; Śmierciew, K. Technika Chłodnicza; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2014. [Google Scholar]
- Bobbo, S.; Di Nicola, G.; Zilio, C.; Brown, J.S.; Fedele, L. Low GWP halocarbon refrigerants: A review of thermophysical properties. Int. J. Refrig. 2018, 90, 181–201. [Google Scholar] [CrossRef]
- De Monte, F. Calculation of thermodynamic properties of R407C and R410A by the Martin-Hou equation of state—Part I: Theoretical development. Int. J. Refrig. 2002, 25, 306–313. [Google Scholar] [CrossRef]
- De Monte, F. Calculation of thermodynamic properties of R407C and R410A by the Martin-Hou equation of state—Part II: Technical interpretation. Int. J. Refrig. 2002, 25, 314–329. [Google Scholar] [CrossRef]
- Feroiu, V.; Geana, D. Volumetric and thermodynamic properties for pure refrigerants and refrigerant mixtures from cubic equations of state. Fluid Phase Equilibria 2003, 207, 283–300. [Google Scholar] [CrossRef]
- Feroiu, V.; Geana, D. Properties of pure refrigerants and refrigerant mixtures from cubic equations of state. Rev. Roum. De Chmie 2011, 56, 517–525. [Google Scholar]
- Ding, G.; Wu, Z.; Liu, J.; Inagaki, T.; Wang, K.; Fukaya, M. An implicit curve-fitting method for fast calculation of thermal properties of pure and mixed refrigerant. Int. J. Refrig. 2005, 28, 921–932. [Google Scholar] [CrossRef]
- Sozen, A.; Arcaklioglu, E.; Menlik, T.; Ozalp, M. Determination of thermodynamic properties of an alternative refrigerant (R407C) using artificial neural network. Expert Syst. Appl. 2009, 36, 4346–4356. [Google Scholar] [CrossRef]
- Sozen, A.; Arcaklioglu, E.; Menlik, T. Derivation of empirical equations for thermodynamic properties of a ozone safe refrigerant (R404a) using artificial neural network. Expert Syst. Appl. 2010, 37, 1158–1168. [Google Scholar] [CrossRef]
- Sencan, A.; Kose, I.I.; Selbas, R. Comparative analysis of neural network and neuro-fuzzy system for thermodynamic properties of refrigerants. Appl. Artif. Intell. 2012, 26, 662–672. [Google Scholar] [CrossRef]
- Yilmaz, H.; Sencan, A.; Selbas, R. An estimation of thermodynamic properties of hydrocarbon refrigerants. Int. J. Green Energy 2014, 11, 500–526. [Google Scholar] [CrossRef]
- Mora, R.J.E.; Perez, T.C.; Gonzalez, N.F.F.; Ocampo, D.J.D.D. Thermodynamic properties of refrigerants using artificial neural networks. Int. J. Refrig. 2014, 46, 9–16. [Google Scholar] [CrossRef]
- Kucuksille, E.U.; Selbas, R.; Sencan, A. Data mining techniques for thermophysical properties of refrigerants. Energy Convers. Manag. 2009, 50, 399–412. [Google Scholar] [CrossRef]
- Kucuksille, E.U.; Selbas, R.; Sencan, A. Prediction of thermodynamic properties of refrigerants using data mining. Energy Convers. Manag. 2011, 52, 836–848. [Google Scholar] [CrossRef]
- Sieres, J.; Varas, F.; Martinez-Suarez, J.A. A hybrid formulation for fast explicit calculation of thermodynamic properties of refrigerants. Int. J. Refrig. 2012, 35, 1021–1034. [Google Scholar] [CrossRef]
- StatSoft. STATISTICA (Data Analysis Software System), version 12. 2014. Available online: www.statsoft.pl (accessed on 1 October 2019).
- Życzkowski, P. Korelacje obliczeniowe parametrów termodynamicznych i termokinetycznych czynnika chłodniczego R1234yf. Cz. 1, Ciecz nasycona i para nasycona sucha. Chłodnictwo 2016, 3, 18–22. [Google Scholar] [CrossRef]
- Życzkowski, P. Korelacje obliczeniowe parametrów termodynamicznych i termokinetycznych czynnika chłodniczego R1234yf. Cz. 2, Obszar pary przegrzanej i cieczy przechłodzonej. Chłodnictwo 2016, 4, 22–25. [Google Scholar] [CrossRef]
- Nowak, B.; Życzkowski;Łuczak, R. Functional dependence of thermodynamic and thermokinetic parameters of refrigerants used in mine air refrigerators. Part 1, Refrigerant R407C. Arch. Min. Sci. 2017, 62, 55–72. [Google Scholar] [CrossRef] [Green Version]
- Nowak, B.; Życzkowski;Łuczak, R. Functional dependencies of thermodynamic and thermokinetic parameters of refrigerants used in mine air refrigerators. Pt. 2, Refrigerant R404A. Arch. Min. Sci. 2018, 63, 27–41. [Google Scholar] [CrossRef]
- Pires, R.; Mili, L.; Chagas, G. Robust complex-valued Levenberg-Marquardt algorithm as applied to power flow analysis. Electr. Power Energy Syst. 2019, 113, 383–392. [Google Scholar] [CrossRef]
- Cui, M.; Yang, K.; Xu, X.L.; Wang, S.D.; Gao, X.W. A modified Levenberg–Marquardt algorithm for simultaneous estimation of multi-parameters of boundary heat flux by solving transient nonlinear inverse heat conduction problems. Int. J. Heat Mass Transf. 2016, 97, 908–916. [Google Scholar] [CrossRef]
- Yang, K.; Jiang, G.H.; Peng, H.F.; Gao, X.W. A new modified Levenberg-Marquardt algorithm for identifying the temperature-dependent conductivity of solids based on the radial integration boundary element method. Int. J. Heat Mass Transf. 2019, 144, 118615. [Google Scholar] [CrossRef]
- Tossa, A.K.; Soro, Y.M.; Azoumah, Y.; Yamegueu, D. A new approach to estimate the performance and energy productivity of photovoltaic modules in real operating conditions. Sol. Energy 2014, 110, 543–560. [Google Scholar] [CrossRef]
- Blaifi, S.; Moulahoum, S.; Taghezouit, B.; Saim, A. An enhanced dynamic modeling of PV module using Levenberg-Marquardt algorithm. Renew. Energy 2019, 135, 745–760. [Google Scholar] [CrossRef]
- Koppel, T.; Vassiljev, A. Calibration of a model of an operational water distribution system containing pipes of different age. Adv. Eng. Softw. 2009, 40, 659–664. [Google Scholar] [CrossRef]






| Property | Value |
|---|---|
| Group | HFO |
| Chemical formula | trans-CF3CH = CHF |
| Molar mass, kg/kmol | 114 |
| Critical temperature, °C | 109.36 |
| Critical pressure, bar | 36.4 |
| Critical density, kg/m3 | 489.2 |
| Normal boiling point, °C | −19.3 |
| Triple point, °C | −104.53 |
| ODP (ozone depletion potential) | 0 |
| GWP20 (global warming potential) 20 years | 4 |
| GWP100 (global warming potential) 100 years | 1 |
| Atmospheric lifetime of individual components, days | 16.4 |
| State | Scope | Amount of Data |
|---|---|---|
| Saturated liquid | p: 0.5–30.0 bar | 591 |
| Saturated vapour | p: 0.5–30.0 bar | 591 |
| Superheated vapour | p: 0.5–30.0 bar; t: t″ − 120 °C | 18,186 1 |
| Subcooled liquid | p: 0.5–30.0 bar; t: −80 °C−t′ | 41,901 |
| Coef | Equation | |||
|---|---|---|---|---|
| T = f(p) (1) | h′ = f(p) (2) | s′ = f(p) (3) | cp′ = f(p) (4) | |
| a0 | 2.53879921713140 × 102 | 1.74968285360988 × 102 | 9.05279244602078 × 10−1 | 1.24083270657197 |
| a1 | 2.28770347319237 × 101 | 2.93484680604720 × 101 | 1.15356542959209 × 10−1 | 4.51902938229883 × 10−2 |
| a2 | 2.45670755735841 | 3.00047504671966 | 7.24618471191152 × 10−3 | −5.63723496516354 × 10−3 |
| a3 | 3.14117394078267 × 10−1 | 1.19253718641735 | 2.73012551482764 × 10−3 | 6.42959196900272 × 10−4 |
| a4 | −6.76941445013617 × 10−2 | 6.99952705225739 × 10−1 | 1.71764538194706 × 10−3 | −3.54112766926212 × 10−5 |
| a5 | 4.07277016474858 × 10−2 | −1.51604024263818 | −3.86378154015459 × 10−3 | 5.37559165828602 × 10−7 |
| a6 | −5.95404205600932 × 10−3 | 9.67067796211943 × 10−1 | 2.45968598111626 × 10−3 | 3.84386880084408 × 10−8 |
| a7 | - | −2.62092375289440 × 10−1 | −6.67334247116959 × 10−4 | −1.88423766967265 × 10−9 |
| a8 | - | 2.66563442687942 × 10−2 | 6.78181882644024 × 10−5 | 2.54741811878324 × 10−11 |
| Coef | Equation | ||
|---|---|---|---|
| ρ′ = f(p) (5) | ν′ = f(p) (6) | λ′ = f(p) (7) | |
| a0 | 1.29396343579499 × 103 | 7.29710663744474 × 10−4 | 9.03143423982640 × 10−2 |
| a1 | −6.20669713392757 × 101 | 5.01608980784443 × 10−5 | −8.80066599536261 × 10−3 |
| a2 | −2.53752547194416 | −9.30886654653688 × 10−6 | −9.99542811098308 × 10−4 |
| a3 | −9.31562506661149 | 1.37045258345160 × 10−6 | 2.92095921761060 × 10−4 |
| a4 | −7.20333951838005 | −1.23148305617849 × 10−7 | 3.50549588319595 × 10−4 |
| a5 | 1.64294878085487 × 101 | 6.76679411090488 × 10−9 | −7.30082138730070 × 10−4 |
| a6 | −1.04093686983023 × 101 | −2.20602504790423 × 10−10 | 4.40360054711903 × 10−4 |
| a7 | 2.81591566729637 | 3.91341788788786 × 10−12 | −1.14689158182047 × 10−4 |
| a8 | −2.86461830242391 × 10−1 | −2.90062006624196 × 10−14 | 1.10956525980123 × 10−5 |
| Coef | Equation | ||
|---|---|---|---|
| µ′ = f(p) (8) | Pr′ = f(p) (9) | σ = f(p) (10) | |
| a0 | 3.30126989957222 × 10−4 | 4.67836170077957 | 1.59343062468816 × 10−2 |
| a1 | −1.03228385472871 × 10−4 | −8.07941153808255 × 10−1 | −4.27582523407763 × 10−3 |
| a2 | 1.09405636740428 × 10−5 | 1.78574619681131 × 10−1 | 1.08824350295615 × 10−5 |
| a3 | −1.54688847220722 × 10−6 | −4.54256602128744 × 10−1 | −2.88012392508621 × 10−5 |
| a4 | −2.44961988256085 × 10−7 | 1.75299143854024 × 10−1 | 1.02007105181399 × 10−5 |
| a5 | 9.19517023370312 × 10−7 | 5.54715409594885 × 10−1 | −3.46263550356762 × 10−5 |
| a6 | −6.03952237218337 × 10−7 | −6.97630428910946 × 10−1 | 2.31347786949313 × 10−5 |
| a7 | 1.66816987767627 × 10−7 | 3.38689978995674 × 10−1 | −6.30855999198157 × 10−6 |
| a8 | −1.71375788331681 × 10−8 | −7.62417081213265 × 10−2 | 6.68652044821767 × 10−7 |
| a9 | - | 6.62560394142489 × 10−3 | - |
| Coef | Equation | |||
|---|---|---|---|---|
| h″ = f(p) (11) | s″ = f(p) (12) | cp″ = f(p) (13) | ρ″ = f(p) (14) | |
| a0 | 3.70657366815286 × 102 | 1.67622654205859 | 7.63398360227738 × 10−1 | 2.27768588078390 × 10−1 |
| a1 | 1.62926056610703 × 101 | −5.81493952118715 × 10−3 | 7.32627000545461 × 10−2 | 5.54068367754339 |
| a2 | 2.46905883267318 | 6.61688503765656 × 10−3 | −1.06783110270413 × 10−2 | −1.70610755621257 × 10−1 |
| a3 | −1.13621954845305 | −2.94796323909036 × 10−3 | 1.22686997407115 × 10−3 | 3.58297189467255 × 10−2 |
| a4 | −1.08070437166134 | −2.75644193419488 × 10−3 | −7.15472829720056 × 10−5 | −3.97722125575951 × 10−3 |
| a5 | 2.49195790709675 | 6.33081705594435 × 10−3 | 1.61584178260620 × 10−6 | 2.97586911550852 × 10−4 |
| a6 | −1.57431720780436 | −3.99095180784525 × 10−3 | 3.78799626428319 × 10−8 | −1.42888359942333 × 10−5 |
| a7 | 4.25121219497403 × 10−1 | 1.07579556459832 × 10−3 | −2.62846106511664 × 10−9 | 4.26062135708768 × 10−7 |
| a8 | −4.32428413533420 × 10−2 | −1.09130621835064 × 10−4 | 3.84463040954545 × 10−11 | −7.16416594446346 × 10−9 |
| a9 | - | - | - | 5.23362423501008 × 10−11 |
| Coef | Equation | |||
|---|---|---|---|---|
| ν″ = f(p) (15) | λ″ = f(p) (16) | µ″ = f(p) (17) | Pr″ = f(p) (18) | |
| a0 | 1.77433953172014 × 10−1 | 1.00685420897056 × 10−2 | 1.05062529128478 × 10−5 | 8.74440749215016 × 10−1 |
| a1 | −1.66936428639188 × 10−1 | 2.01521099378549 × 10−3 | 9.52821100098587 × 10−7 | −2.39936282800067 × 10−2 |
| a2 | 7.75579038246079 × 10−2 | 4.91117080105326 × 10−4 | −3.00951147568936 × 10−7 | 1.25205016486864 × 10−2 |
| a3 | −2.41489018240415 × 10−2 | −1.75824023764422 × 10−3 | 5.32152051905147 × 10−7 | −2.27389415072141 × 10−3 |
| a4 | 5.61391538794325 × 10−3 | 7.60350748872995 × 10−4 | 4.70502421614829 × 10−7 | 2.36745600186706 × 10−4 |
| a5 | −9.65998396886106 × 10−4 | 2.19935489007404 × 10−3 | −1.09002347169414 × 10−6 | −1.45822262459754 × 10−5 |
| a6 | 9.87363959178734 × 10−5 | −2.83720578521593 × 10−3 | 6.84901655972941 × 10−7 | 5.30654276070867 × 10−7 |
| a7 | −7.48240927743627 × 10−7 | 1.40136740639282 × 10−3 | −1.84631268281049 × 10−7 | −1.05453244596624 × 10−8 |
| a8 | −7.07631682979349 × 10−7 | −3.20515361896471 × 10−4 | 1.86385265681204 × 10−8 | 8.89411840080677 × 10−11 |
| a9 | - | 2.83753380724076 × 10−5 | - | - |
| Coef | Equation | ||
|---|---|---|---|
| h = f(p,t) (19) | h = f(p,s) (20) | s = f(p,t) (21) | |
| a1 | −1.22538219116946 × 101 | 9.70737789929653 | 1.02201325065485 × 103 |
| a2 | −1.19684176263820 × 10−1 | 2.61874436156932 × 10−1 | 2.11930425863317 |
| a3 | 2.56418987553419 × 10−2 | 7.03198731354432 × 10−2 | −2.25093762714879 × 10−1 |
| a4 | 1.12370072751876 × 10−1 | 2.04713651916276 × 10−1 | −4.13156661967710 × 10−1 |
| a5 | −7.84589730210466 × 10−2 | - | −2.66667859197166 × 10−1 |
| b1 | 1.07613121977651 × 101 | 2.14480351606632 × 102 | −7.38442317160207 |
| b2 | 1.27909162098184 × 10−1 | 8.78348541751969 | −1.54691107365295 × 10−2 |
| b3 | −2.17557771059542 × 10−2 | 2.33196204712552 | 1.61160247230101 × 10−3 |
| b4 | −1.33475632720526 × 10−2 | 6.31343053191083 | 4.11509116217012 × 10−3 |
| b5 | 1.30152145421200 × 10−2 | - | 2.68867618687700 × 10−3 |
| c1 | 9.20939997738017 × 102 | 1.30915602277249 × 102 | 1.00415786839940 × 104 |
| c2 | −1.70804798986579 × 101 | 6.23649470729840 | −1.12883262503797 × 102 |
| c3 | −9.36024775369566 | 1.07200578512649 | −2.83482394816380 × 101 |
| c4 | 6.34415745181292 × 10−1 | −2.26086399298466 | −9.09256164033981 × 10−1 |
| c5 | −1.25745577415437 | - | −1.13294272169830 |
| Coef | Equation | |
|---|---|---|
| T = f(p,h) (22) | ρ = f(p,h) (23) | |
| a1 | 1.80123820111647 × 101 | 2.81203869542640 × 101 |
| a2 | 8.24003323530265 × 10−2 | 1.15184541343782 × 10−1 |
| a3 | 2.51686613531001 × 10−2 | 2.45938820598331 |
| a4 | - | 8.43110685237400 × 10−1 |
| a5 | - | −2.05663545341847 × 10−3 |
| a6 | - | 1.15342507562934 |
| b1 | −9.57478728374795 | −2.27670387576552 × 101 |
| b2 | −4.92373151059871 × 10−2 | 3.83041059824037 |
| b3 | 1.88344691904411 × 10−2 | −4.86420752353526 |
| b4 | - | −1.09064973382657 × 101 |
| b5 | - | −1.20058186844573 × 10−1 |
| b6 | - | −4.74405743126640 |
| c1 | −6.16759701982095 × 103 | 2.65873399640243 × 102 |
| c2 | −8.19380014241848 × 101 | −2.22556557307299 × 101 |
| c3 | −1.11076735383728 × 101 | 2.57855441990927 × 101 |
| c4 | - | 6.47202322127270 × 101 |
| c5 | - | −1.99964641247140 |
| c6 | - | 2.66402045824145 × 101 |
| Coef | Equation | ||
|---|---|---|---|
| h = f(p,t) (24) | s = f(p,t) (25) | T = f(p,h) (26) | |
| a1 | −1.24632495027227 × 102 | 1.53492509731654 | −2.54401494933800 |
| a2 | −8.93767310439441 × 10−2 | 1.14188030931082 × 10−2 | 1.41981737305051 × 10−2 |
| a3 | −5.13970645120413 × 10−3 | −2.27977637362198 × 10−3 | 4.17004268014005 × 10−3 |
| a4 | −7.81419045658094 × 10−4 | −9.09151613042750 × 10−4 | −4.57959473157251 × 10−3 |
| a5 | 4.22545925349730 × 10−4 | −4.02681309084223 × 10−4 | - |
| a6 | 3.59229939083742 × 10−4 | - | - |
| b1 | −8.14460833381591 × 103 | −8.64303643796513 | 8.02627024648105 |
| b2 | −6.05289428492502 | −6.37870935254633 × 10−2 | −4.09757320861048 × 10−2 |
| b3 | −3.94885910243123 × 10−1 | 1.30109052596914 × 10−2 | −1.18576883918572 × 10−2 |
| b4 | −7.25230146369704 × 10−2 | 5.37778256355485 × 10−3 | 1.08815116545496 × 10−3 |
| b5 | 2.69851262120827 × 10−2 | 4.62322186390055 × 10−3 | - |
| b6 | 1.40090835277157 × 10−2 | - | - |
| c1 | 5.51597947695862 × 105 | 1.05287623121195 × 103 | −7.10087317464252 × 103 |
| c2 | −3.62158965210490 × 102 | −2.75983526288628 × 101 | 8.50746165042646 × 101 |
| c3 | −5.21112983842346 × 101 | −1.25845749595145 × 101 | −2.84134460440402 |
| c4 | −1.64400591142357 × 101 | −3.66028024779350 | −6.47159328560425 × 10−1 |
| c5 | −1.44044662909068 × 101 | −3.23703650445058 × 10−1 | - |
| c6 | −4.26763585954523 | - | - |
| Equation | Region | Correlation Coefficient R | Determination Coefficient R2 | |
|---|---|---|---|---|
| T = f(p) | (1) | Saturated liquid, saturated vapour | 0,9999999955 | 0,9999999910 |
| h′ = f(p) | (2) | Saturated liquid | 0.9999999077 | 0.9999998155 |
| s′ = f(p) | (3) | Saturated liquid | 0.9999999361 | 0.9999998722 |
| cp′ = f(p) | (4) | Saturated liquid | 0.9999992806 | 0.9999985612 |
| ρ′ = f(p) | (5) | Saturated liquid | 0.9999986910 | 0.9999973820 |
| ν′ = f(p) | (6) | Saturated liquid | 0.9999976596 | 0.9999953191 |
| λ′ = f(p) | (7) | Saturated liquid | 0.9999991886 | 0.9999983771 |
| µ′ = f(p) | (8) | Saturated liquid | 0.9999999800 | 0.9999999600 |
| Pr′ = f(p) | (9) | Saturated liquid | 0.9998688664 | 0.9997377499 |
| σ = f(p) | (10) | Saturated liquid, saturated vapour | 0.9999999943 | 0.9999999886 |
| h″ = f(p) | (11) | Saturated vapour | 0.9999976439 | 0.9999952879 |
| s″ = f(p) | (12) | Saturated vapour | 0.9998810558 | 0.9997621258 |
| cp″ = f(p) | (13) | Saturated vapour | 0.9999988802 | 0.9999977605 |
| ρ″ = f(p) | (14) | Saturated vapour | 0.9999999996 | 0.9999999992 |
| ν″ = f(p) | (15) | Saturated vapour | 0.9999999996 | 0.9999999991 |
| λ″ = f(p) | (16) | Saturated vapour | 0.9999895746 | 0.9999791493 |
| µ″ = f(p) | (17) | Saturated vapour | 0.9999806274 | 0.9999612552 |
| Pr″ = f(p) | (18) | Saturated vapour | 0.9999988314 | 0.9999976628 |
| h = f(p,t) | (19) | Superheated vapour | 0.9999843304 | 0.9999686611 |
| h = f(p,s) | (20) | Superheated vapour | 0.9995097223 | 0.9990196850 |
| s = f(p,t) | (21) | Superheated vapour | 0.9999351748 | 0.9998703538 |
| T = f(p,h) | (22) | Superheated vapour | 0.9999471203 | 0.9998942434 |
| ρ = f(p,h) | (23) | Superheated vapour | 0.9999989879 | 0.9999979758 |
| h = f(p,t) | (24) | Subcooled liquid | 0.9999996924 | 0.9999993847 |
| s = f(p,t) | (25) | Subcooled liquid | 0.9999996140 | 0.9999992279 |
| T = f(p,h) | (26) | Subcooled liquid | 0.9999981133 | 0.9999962267 |
| Equation | Region | Absolute Deviation | ||
|---|---|---|---|---|
| T = f(p) | (1) | Saturated liquid, saturated vapour | average, K | 0.002499 |
| maximum, K | 0.015994 | |||
| h′ = f(p) | (2) | Saturated liquid | average, kJ/kg | 0.016781 |
| maximum, kJ/kg | 0.091438 | |||
| s′ = f(p) | (3) | Saturated liquid | average, kJ/(kg·K) | 0.000043 |
| maximum, kJ/(kg·K) | 0.000234 | |||
| cp′ = f(p) | (4) | Saturated liquid | average, kJ/(kg·K) | 0.000344 |
| maximum, kJ/(kg·K) | 0.002936 | |||
| ρ′ = f(p) | (5) | Saturated liquid | average, kg/m3 | 0.180720 |
| maximum, kg/m3 | 0.977343 | |||
| ν′ = f(p) | (6) | Saturated liquid | average, m3/kg | 1.99 × 10−7 |
| maximum, m3/kg | 3.00 × 10−6 | |||
| λ′ = f(p) | (7) | Saturated liquid | average, W/(m·K) | 1.01 × 10−5 |
| maximum, W/(m·K) | 6.89 × 10−5 | |||
| µ′ = f(p) | (8) | Saturated liquid | average, kg/(m·s) | 1.03 × 10−8 |
| maximum, kg/(m·s) | 5.70 × 10−8 | |||
| Pr′ = f(p) | (9) | Saturated liquid | average, - | 0.005346 |
| maximum, - | 0.035289 | |||
| σ = f(p) | (10) | Saturated liquid, saturated vapour | average, N/m | 3.49 × 10−7 |
| maximum, N/m | 1.70 × 10−6 | |||
| h″ = f(p) | (11) | Saturated vapour | average, kJ/kg | 0.027309 |
| maximum, kJ/kg | 0.144573 | |||
| s″ = f(p) | (12) | Saturated vapour | average, kJ/(kg·K) | 0.000069 |
| maximum, kJ/(kg·K) | 0.000368 | |||
| cp″ = f(p) | (13) | Saturated vapour | average, kJ/(kg·K) | 0.000643 |
| maximum, kJ/(kg·K) | 0.006157 | |||
| ρ″ = f(p) | (14) | Saturated vapour | average, kg/m3 | 0.001151 |
| maximum, kg/m3 | 0.017800 | |||
| ν″ = f(p) | (15) | Saturated vapour | average, m3/kg | 9.46 × 10−7 |
| maximum, m3/kg | 4.97 × 10−6 | |||
| λ″ = f(p) | (16) | Saturated vapour | average, W/(m·K) | 1.99 × 10−5 |
| maximum, W/(m·K) | 1.18 × 10−4 | |||
| µ″ = f(p) | (17) | Saturated vapour | average, kg/(m·s) | 1.23 × 10−8 |
| maximum, kg/(m·s) | 6.80 × 10−8 | |||
| Pr″ = f(p) | (18) | Saturated vapour | average, - | 0.000263 |
| maximum, - | 0.005100 | |||
| h = f(p,t) | (19) | Superheated vapour | average, kJ/kg | 0.094327 |
| maximum, kJ/kg | 1.561120 | |||
| h = f(p,s) | (20) | Superheated vapour | average, kJ/kg | 0.683322 |
| maximum, kJ/kg | 3.484118 | |||
| s = f(p,t) | (21) | Superheated vapour | average, kJ/(kg·K) | 0.000729 |
| maximum, kJ/(kg·K) | 0.013665 | |||
| T = f(p,h) | (22) | Superheated vapour | average, K | 0.237796 |
| maximum, K | 2.891388 | |||
| ρ = f(p,h) | (23) | Superheated vapour | average, kg/m3 | 0.047104 |
| maximum, kg/m3 | 0.437241 | |||
| h = f(p,t) | (24) | Subcooled liquid | average, kJ/kg | 0.028842 |
| maximum, kJ/kg | 1.109729 | |||
| s = f(p,t) | (25) | Subcooled liquid | average, kJ/(kg·K) | 0.000121 |
| maximum, kJ/(kg·K) | 0.004597 | |||
| T = f(p,h) | (26) | Subcooled liquid | average, K | 0.058954 |
| maximum, K | 1.540900 | |||
| Equation | Region | Absolute Deviation, % | ||
|---|---|---|---|---|
| T = f(p) | (1) | Saturated liquid, saturated vapour | average | 0.000762 |
| maximum | 0.006690 | |||
| h′ = f(p) | (2) | Saturated liquid | average | 0.005986 |
| maximum | 0.048023 | |||
| s′ = f(p) | (3) | Saturated liquid | average | 0.003384 |
| maximum | 0.023007 | |||
| cp′ = f(p) | (4) | Saturated liquid | average | 0.020681 |
| maximum | 0.233195 | |||
| ρ′ = f(p) | (5) | Saturated liquid | average | 0.018672 |
| maximum | 0.124958 | |||
| ν′ = f(p) | (6) | Saturated liquid | average | 0.021500 |
| maximum | 0.400597 | |||
| λ′ = f(p) | (7) | Saturated liquid | average | 0.016861 |
| maximum | 0.133656 | |||
| µ′ = f(p) | (8) | Saturated liquid | average | 0.009928 |
| maximum | 0.085166 | |||
| Pr′ = f(p) | (9) | Saturated liquid | average | 0.164049 |
| maximum | 0.973922 | |||
| σ = f(p) | (10) | Saturated liquid, saturated vapour | average | 0.018004 |
| maximum | 0.297914 | |||
| h″ = f(p) | (11) | Saturated vapour | average | 0.006575 |
| maximum | 0.034191 | |||
| s″ = f(p) | (12) | Saturated vapour | average | 0.004143 |
| maximum | 0.022158 | |||
| cp″ = f(p) | (13) | Saturated vapour | average | 0.050334 |
| maximum | 0.777996 | |||
| ρ″ = f(p) | (14) | Saturated vapour | average | 0.006875 |
| maximum | 0.605068 | |||
| ν″ = f(p) | (15) | Saturated vapour | average | 0.009808 |
| maximum | 0.109992 | |||
| λ″ = f(p) | (16) | Saturated vapour | average | 0.108580 |
| maximum | 0.897582 | |||
| µ″ = f(p) | (17) | Saturated vapour | average | 0.082363 |
| maximum | 0.548505 | |||
| Pr″ = f(p) | (18) | Saturated vapour | average | 0.026480 |
| maximum | 0.585967 | |||
| h = f(p,t) | (19) | Superheated vapour | average | 0.021344 |
| maximum | 0.417555 | |||
| h = f(p,s) | (20) | Superheated vapour | average | 0.155244 |
| maximum | 0.817447 | |||
| s = f(p,t) | (21) | Superheated vapour | average | 0.040509 |
| maximum | 0.822705 | |||
| T = f(p,h) | (22) | Superheated vapour | average | 0.065669 |
| maximum | 0.775753 | |||
| ρ = f(p,h) | (23) | Superheated vapour | average | 0.078893 |
| maximum | 0.867133 | |||
| h = f(p,t) | (24) | Subcooled liquid | average | 0.014884 |
| maximum | 0.312942 | |||
| s = f(p,t) | (25) | Subcooled liquid | average | 0.012436 |
| maximum | 0.312932 | |||
| T = f(p,h) | (26) | Subcooled liquid | average | 0.021539 |
| maximum | 0.413421 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Życzkowski, P.; Borowski, M.; Łuczak, R.; Kuczera, Z.; Ptaszyński, B. Functional Equations for Calculating the Properties of Low-GWP R1234ze(E) Refrigerant. Energies 2020, 13, 3052. https://doi.org/10.3390/en13123052
Życzkowski P, Borowski M, Łuczak R, Kuczera Z, Ptaszyński B. Functional Equations for Calculating the Properties of Low-GWP R1234ze(E) Refrigerant. Energies. 2020; 13(12):3052. https://doi.org/10.3390/en13123052
Chicago/Turabian StyleŻyczkowski, Piotr, Marek Borowski, Rafał Łuczak, Zbigniew Kuczera, and Bogusław Ptaszyński. 2020. "Functional Equations for Calculating the Properties of Low-GWP R1234ze(E) Refrigerant" Energies 13, no. 12: 3052. https://doi.org/10.3390/en13123052