Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries
Abstract
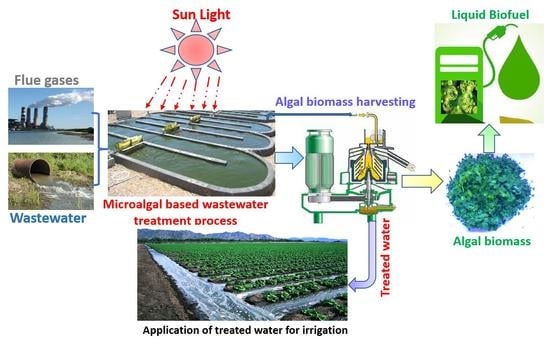
:1. Introduction
2. Wastewater Treatment Processes
3. Microalgae Cultivation Systems
3.1. Open Systems
3.1.1. Raceways Ponds
3.1.2. Circular Ponds
3.1.3. Unstirred Open Ponds
3.2. Closed Systems
3.2.1. Flat Plates
3.2.2. Tubular Reactors
3.3. Advance Cultivation Systems
3.3.1. Algal Turf Scrubber (ATS) Cultivation Systems
3.3.2. Hybrid Cultivation Systems (HCS)
4. Mode of Microalgae Cultivation
5. Microalgal Biorefinery for Biofuels Production
5.1. Biodiesel
5.2. Biogas
5.3. Biohydrogen
5.4. Bioelectricity
5.5. Biochar
6. Challenges of Microalgal Biorefineries and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arun, J.; Gopinath, K.P.; Sivaramakrishnan, R.; SundarRajan, P.; Malolan, R.; Pugazhendhi, A. Technical insights into the production of green fuel from CO2 sequestered algal biomass: A conceptual review on green energy. Sci. Total Environ. 2021, 755, 142636. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Mehariya, S.; Obulisamy, P.K.; Verma, P. Advanced microalgae-based renewable biohydrogen production systems: A review. Bioresour. Technol. 2021, 320, 124301. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Mehariya, S.; Molino, A.; Musmarra, D.; Verma, P. Microalgae-Based Biorefinery for Utilization of Carbon Dioxide for Production of Valuable Bioproducts. In Chemo-Biological Systems for CO2 Utilization; CRC Press: Boca Raton, FL, USA, 2020; pp. 203–228. [Google Scholar]
- Meier, L.; Barros, P.; Torres, A.; Vilchez, C.; Jeison, D. Photosynthetic biogas upgrading using microalgae: Effect of light/dark photoperiod. Renew. Energy 2017, 106, 17–23. [Google Scholar] [CrossRef]
- Libutti, A.; Gatta, G.; Gagliardi, A.; Vergine, P.; Pollice, A.; Beneduce, L.; Disciglio, G.; Tarantino, E. Agro-industrial wastewater reuse for irrigation of a vegetable crop succession under Mediterranean conditions. Agric. Water Manag. 2018, 196, 1–14. [Google Scholar] [CrossRef]
- Awaleh, M.O.; Soubaneh, Y.D. Waste Water Treatment in Chemical Industries: The Concept and Current Technologies. J. Waste Water Treat. Anal. 2014, 5, 1–13. [Google Scholar]
- Guimarães, N.R.; Filho, S.S.F.; Hespanhol, B.P.; Piveli, R.P. Evaluation of chemical sludge production in wastewater treatment processes. Desalin. Water Treat. 2016, 57, 16346–16352. [Google Scholar] [CrossRef]
- Sparn, B.; Hunsberger, R. Opportunities and Challenges for Water and Wastewater Industries to Provide Exchangeable Services; National Renewable Energy Lab (NREL): Golden, CO, USA, 2015.
- Castellaños-Estupiñana, M.A.; Sánchez-Galvisa, E.M.; García-Martínezb, J.B.; Barajas-Ferreirab, C.; Zuorroc, A.; Barajas-Solano, A.F. Design of an Electroflotation System for the Concentration and Harvesting of Freshwater Microalgae. Chem. Eng. Trans. 2018, 64, 1–6. [Google Scholar]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.-Y.; Zhu, J.-N.; Kong, F.; Xing, D.; Zhao, L.; Ma, J.; Ren, N.-Q.; Liu, B.-F. Ultrasonic enhanced simultaneous algal lipid production and nutrients removal from non-sterile domestic wastewater. Energy Convers. Manag. 2019, 180, 680–688. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Yang, Y.-H. An overview of microdiesel—A sustainable future source of renewable energy. Renew. Sustain. Energy Rev. 2017, 79, 1078–1090. [Google Scholar] [CrossRef]
- García-Martíneza, J.B.; Urbina-Suarezb, N.A.; Zuorroc, A.; Barajas-Solanob, A.F.; Kafarova, V. Fisheries wastewater as a sustainable media for the production of algae-based products. Chem. Eng. Trans. 2019, 76, 1339–1344. [Google Scholar]
- Vargas, S.R.; dos Santos, P.V.; Giraldi, L.A.; Zaiat, M.; Calijuri, M. do C. Anaerobic phototrophic processes of hydrogen production by different strains of microalgae Chlamydomonas sp. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [Green Version]
- Mehariya, S.; Marino, T.; Casella, P.; Iovine, A.; Leone, G.P.; Musmarra, D.; Molino, A. Biorefinery for Agro-Industrial Waste into Value-Added Biopolymers: Production and Applications. In Biorefineries: A Step Towards Renewable and Clean Energy; Verma, P., Ed.; Springer: Singapore, 2020; pp. 1–19. ISBN 978-981-15-9593-6. [Google Scholar]
- Mehariya, S.; Iovine, A.; Casella, P.; Musmarra, D.; Chianese, S.; Marino, T.; Figoli, A.; Sharma, N.; Molino, A. Bio-based and agriculture resources for production of bioproducts. In Current Trends and Future Developments on (Bio-) Membranes; Figoli, A., Li, Y., Basile, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–282. ISBN 978-0-12-816778-6. [Google Scholar]
- Mehariya, S.; Sharma, N.; Iovine, A.; Casella, P.; Marino, T.; Larocca, V.; Molino, A.; Musmarra, D. An Integrated Strategy for Nutraceuticals from Haematoccus pluvialis: From Cultivation to Extraction. Antioxidants 2020, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Rizza, L.; Coronel, C.D.; Sanz Smachetti, M.E.; Do Nascimento, M.; Curatti, L. A semi-closed loop microalgal biomass production-platform for ethanol from renewable sources of nitrogen and phosphorous. J. Clean. Prod. 2019, 219, 217–224. [Google Scholar] [CrossRef]
- Wirth, R.; Lakatos, G.; Böjti, T.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Anaerobic gaseous biofuel production using microalgal biomass—A review. Anaerobe 2018, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solé-Bundó, M.; Garfí, M.; Ferrer, I. Pretreatment and co-digestion of microalgae, sludge and fat oil and grease (FOG) from microalgae-based wastewater treatment plants. Bioresour. Technol. 2020, 298, 122563. [Google Scholar] [CrossRef]
- Ling, J.; Xu, Y.; Lu, C.; Lai, W.; Xie, G.; Zheng, L.; Talawar, M.P.; Du, Q.; Li, G. Enhancing Stability of Microalgae Biocathode by a Partially Submerged Carbon Cloth Electrode for Bioenergy Production from Wastewater. Energies 2019, 12, 3229. [Google Scholar] [CrossRef] [Green Version]
- Logroño, W.; Pérez, M.; Urquizo, G.; Kadier, A.; Echeverría, M.; Recalde, C.; Rákhely, G. Single chamber microbial fuel cell (SCMFC) with a cathodic microalgal biofilm: A preliminary assessment of the generation of bioelectricity and biodegradation of real dye textile wastewater. Chemosphere 2017, 176, 378–388. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-scale cultivation of microalgae Scenedesmus almeriensis for CO2 capture and lutein production. Energies 2019, 12, 2806. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Mehariya, S.; Iovine, A.; Casella, P.; Marino, T.; Karatza, D.; Chianese, S.; Musmarra, D. Enhancing Biomass and Lutein Production from Scenedesmus almeriensis: Effect of Carbon Dioxide Concentration and Culture Medium Reuse. Front. Plant Sci. 2020, 11, 415. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process. Eng. 2020, 101747. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Xu, X.-Q.; Wang, X.-X.; Hu, H.-Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.H.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263, 322–333. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kurade, M.B.; Abou-Shanab, R.A.I.; El-Dalatony, M.M.; Yang, I.-S.; Min, B.; Jeon, B.-H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Mohd Udaiyappan, A.F.; Abu Hasan, H.; Takriff, M.S.; Sheikh Abdullah, S.R. A review of the potentials, challenges and current status of microalgae biomass applications in industrial wastewater treatment. J. Water Process. Eng. 2017, 20, 8–21. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S. Palm oil mill effluent treatment and CO2 sequestration by using microalgae—Sustainable strategies for environmental protection. Environ. Sci. Pollut. Res. 2017, 24, 20209–20240. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control. 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic modeling of azo dye adsorption on non-living cells of nannochloropsis oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Zuorro, A.; Malavasi, V.; Cao, G.; Lavecchia, R. Use of cell wall degrading enzymes to improve the recovery of lipids from chlorella sorokiniana. Chem. Eng. J. 2019, 377. [Google Scholar] [CrossRef]
- Gill, S.S.; Mehmood, M.A.; Ahmad, N.; Ibrahim, M.; Rashid, U.; Ali, S.; Nehdi, I.A. Strain selection, growth productivity and biomass characterization of novel microalgae isolated from fresh and wastewaters of upper Punjab, Pakistan. Front. Life Sci. 2016, 9, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Ribeiro, B.; Marques, P.A.S.S.; Ferreira, A.F.; Dias, A.P.; Pinheiro, H.M.; Reis, A.; Gouveia, L. Scenedesmus obliquus mediated brewery wastewater remediation and CO2 biofixation for green energy purposes. J. Clean. Prod. 2017, 165, 1316–1327. [Google Scholar] [CrossRef]
- Calicioglu, O.; Demirer, G.N. Carbon-to-nitrogen and substrate-to-inoculum ratio adjustments can improve co-digestion performance of microalgal biomass obtained from domestic wastewater treatment. Environ. Technol. 2019, 40, 614–624. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Lay, C.-H.; Chen, C.-C.; Wu, S.-Y. Lipid accumulating microalgae cultivation in textile wastewater: Environmental parameters optimization. J. Taiwan Inst. Chem. Eng 2017, 79, 1–6. [Google Scholar] [CrossRef]
- Xie, P.; Ho, S.-H.; Peng, J.; Xu, X.-J.; Chen, C.; Zhang, Z.-F.; Lee, D.-J.; Ren, N.-Q. Dual purpose microalgae-based biorefinery for treating pharmaceuticals and personal care products (PPCPs) residues and biodiesel production. Sci. Total Environ. 2019, 688, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Basheer, F.; Sengar, A.; Irfanullah; Khan, S.U.; Farooqi, I.H. Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef]
- Khan, S.; Shamshad, I.; Waqas, M.; Nawab, J.; Ming, L. Remediating industrial wastewater containing potentially toxic elements with four freshwater algae. Ecol. Eng. 2017, 102, 536–541. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Nguyen, M.-L.T.; Lay, C.-H. Starch-containing textile wastewater treatment for biogas and microalgae biomass production. J. Clean. Prod. 2017, 168, 331–337. [Google Scholar] [CrossRef]
- Jayakumar, S.; Yusoff, M.M.; Rahim, M.H.A.; Maniam, G.P.; Govindan, N. The prospect of microalgal biodiesel using agro-industrial and industrial wastes in Malaysia. Renew. Sustain. Energy Rev. 2017, 72, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Guo, R.; Cai, X.; Liu, H.; Yang, Z.; Meng, Y.; Chen, F.; Li, Y.; Wang, B. In Situ Growth of Metal–Organic Frameworks in Three-Dimensional Aligned Lumen Arrays of Wood for Rapid and Highly Efficient Organic Pollutant Removal. Environ. Sci. Technol. 2019, 53, 2705–2712. [Google Scholar] [CrossRef]
- Ejraei, A.; Aroon, M.A.; Ziarati Saravani, A. Wastewater treatment using a hybrid system combining adsorption, photocatalytic degradation and membrane filtration processes. J. Water Process. Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Affam, A.C.; Chaudhuri, M.M.; Kutty, S.R. Comparison of Five Advanced Oxidation Processes for Degradation of Pesticide in Aqueous Solution. Bull. Chem. React. Eng. Catal. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.; Chen, J.; Wang, X. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Peng, C.; Khan, Z.M.; Naz, I.; Sultan, M.; Ali, M.; Abbasi, I.A.; Islam, T.; Ye, T. Overview of microbes based fabricated biogenic nanoparticles for water and wastewater treatment. J. Environ. Manag. 2019, 230, 128–150. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.K.; Singh, A.; Misra, K.; Sahoo, A.K.; Samanta, S.K. Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. J. Environ. Manag. 2019, 231, 734–748. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Ur Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hernández, D.; Cruz García-González, M. Microalgae and Wastewater Treatment: Advantages and Disadvantages. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 505–533. ISBN 978-981-13-2264-8. [Google Scholar]
- Mohd-Salleh, S.N.A.; Mohd-Zin, N.S.; Othman, N. A review of wastewater treatment using natural material and its potential as aid and composite coagulant. Sains Malays. 2019, 48, 155–164. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef]
- Wang, X.; Gao, S.; Zhang, Y.; Zhao, Y.; Cao, W. Performance of different microalgae-based technologies in biogas slurry nutrient removal and biogas upgrading in response to various initial CO2 concentration and mixed light-emitting diode light wavelength treatments. J. Clean. Prod. 2017, 166, 408–416. [Google Scholar] [CrossRef]
- Eyvaz, M.; Arslan, S.; Gürbulak, E.; Yüksel, E. Textile Materials in Liquid Filtration Practices. Current Status and Perspectives in Water and Wastewater Treatment. Text Adv. Appl. InTech. 2017, 11, 293. [Google Scholar]
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Wichern, M.; Lübken, M. Olive mill wastewater pretreatment by combination of filtration on olive stone filters and coagulation–flocculation. Environ. Technol. 2019, 40, 2135–2146. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Dahiya, S.; Amulya, K.; Katakojwala, R.; Vanitha, T.K. Can circular bioeconomy be fueled by waste biorefineries—A closer look. Bioresour. Technol. Rep. 2019, 7, 100277. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of Microalgae Cultivation in Photobioreactor, Open Raceway Pond, and a Two-Stage Hybrid System. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Yen, H.-W.; Hu, I.-C.; Chen, C.-Y.; Nagarajan, D.; Chang, J.-S. Chapter 10—Design of photobioreactors for algal cultivation. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 225–256. ISBN 978-0-444-64192-2. [Google Scholar]
- Ting, H.; Haifeng, L.; Shanshan, M.; Zhang, Y.; Zhidan, L.; Na, D. Progress in microalgae cultivation photobioreactors and applications in wastewater treatment: A review. Int. J. Agric. Biol. Eng. 2017, 10, 1–29. [Google Scholar]
- El-Baz, F.K.; Abd El Baky, H.H. Pilot Scale of Microalgal Production Using Photobioreactor. In Photosynthesis from its Evolution to Future Improvements in Photosynthetic Efficiency Using Nanomaterials; Intech Open: London, UK, 2018; p. 53. [Google Scholar]
- Saha, S.K.; Murray, P. Exploitation of Microalgae Species for Nutraceutical Purposes: Cultivation Aspects. Fermentation 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- İhsan, E. Types of microalgae cultivation photobioreactors and production process of microalgal biodiesel as alternative fuel. Acta Biol. Turc. 2020, 33, 114–131. [Google Scholar]
- Show, P.L.; Tang, M.S.Y.; Nagarajan, D.; Ling, T.C.; Ooi, C.-W.; Chang, J.-S. A Holistic Approach to Managing Microalgae for Biofuel Applications. Int. J. Mol. Sci. 2017, 18, 215. [Google Scholar] [CrossRef] [Green Version]
- Elrayies, G.M. Microalgae: Prospects for greener future buildings. Renew. Sustain. Energy Rev. 2018, 81, 1175–1191. [Google Scholar] [CrossRef]
- Chang, J.-S.; Show, P.-L.; Ling, T.-C.; Chen, C.-Y.; Ho, S.-H.; Tan, C.-H.; Nagarajan, D.; Phong, W.-N. 11-Photobioreactors. In Current Developments in Biotechnology and Bioengineering; Larroche, C., Sanromán, M.Á., Du, G., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 313–352. ISBN 978-0-444-63663-8. [Google Scholar]
- Duan, Y.; Shi, F. Chapter 2—Bioreactor design for algal growth as a sustainable energy source. In Reactor and Process Design in Sustainable Energy Technology; Shi, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 27–60. ISBN 978-0-444-59566-9. [Google Scholar]
- Płaczek, M.; Patyna, A.; Witczak, S. Technical evaluation of photobioreactors for microalgae cultivation. E3S Web Conf. 2017, 19, 2032. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Adey, W.H. Algal Turf Scrubber. Patent US4333263A, 8 June 1982. [Google Scholar]
- D’Aiuto, P.E.; Patt, J.M.; Albano, J.P.; Shatters, R.G.; Evens, T.J. Algal turf scrubbers: Periphyton production and nutrient recovery on a South Florida citrus farm. Ecol. Eng. 2015, 75, 404–412. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.-M.; Ling, T.C.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Hoffman, J.; Pate, R.C.; Drennen, T.; Quinn, J.C. Techno-economic assessment of open microalgae production systems. Algal Res. 2017, 23, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An innovative low-cost equipment for electro-concentration of microalgal biomass. Appl. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Hewes, C.D. Timing is everything: Optimizing crop yield for Thalassiosira pseudonana (Bacillariophyceae) with semi-continuous culture. J. Appl. Phycol. 2016, 28, 3213–3223. [Google Scholar] [CrossRef]
- Siville, B.; Boeing, W.J. Optimization of algal turf scrubber (ATS) technology through targeted harvest rate. Bioresour. Technol. Rep. 2020, 9, 100360. [Google Scholar] [CrossRef]
- Pal, P.; Chew, K.W.; Yen, H.-W.; Lim, J.W.; Lam, M.K.; Show, P.L. Cultivation of Oily Microalgae for the Production of Third-Generation Biofuels. Sustainability 2019, 11, 5424. [Google Scholar] [CrossRef] [Green Version]
- Adesanya, V.O.; Cadena, E.; Scott, S.A.; Smith, A.G. Life cycle assessment on microalgal biodiesel production using a hybrid cultivation system. Bioresour. Technol. 2014, 163, 343–355. [Google Scholar] [CrossRef]
- Yun, J.-H.; Cho, D.-H.; Lee, S.; Heo, J.; Tran, Q.-G.; Chang, Y.K.; Kim, H.-S. Hybrid operation of photobioreactor and wastewater-fed open raceway ponds enhances the dominance of target algal species and algal biomass production. Algal Res. 2018, 29, 319–329. [Google Scholar] [CrossRef]
- Swain, P.; Tiwari, A.; Pandey, A. Enhanced lipid production in Tetraselmis sp. by two stage process optimization using simulated dairy wastewater as feedstock. Biomass Bioenergy 2020, 139, 105643. [Google Scholar] [CrossRef]
- Javed, F.; Aslam, M.; Rashid, N.; Shamair, Z.; Khan, A.L.; Yasin, M.; Fazal, T.; Hafeez, A.; Rehman, F.; Rehman, M.S.U.; et al. Microalgae-based biofuels, resource recovery and wastewater treatment: A pathway towards sustainable biorefinery. Fuel 2019, 255, 115826. [Google Scholar] [CrossRef]
- Ryu, K.H.; Kim, B.; Lee, J.H. A model-based optimization of microalgal cultivation strategies for lipid production under photoautotrophic condition. Comput. Chem. Eng. 2019, 121, 57–66. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A realistic scenario on microalgae based biodiesel production: Third generation biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Sanz Smachetti, M.E.; Coronel, C.D.; Salerno, G.L.; Curatti, L. Sucrose-to-ethanol microalgae-based platform using seawater. Algal Res. 2020, 45, 101733. [Google Scholar] [CrossRef]
- Garoma, T.; Nguyen, D. Anaerobic Co-Digestion of Microalgae Scenedesmus sp. and TWAS for Biomethane Production. Water Environ. Res. 2016, 88, 13–20. [Google Scholar] [CrossRef]
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A look back at the US Department of Energy’s aquatic species program: Biodiesel from algae. Natl. Renew. Energy Lab. 1998, 328, 1–294. [Google Scholar]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef]
- Behera, B.; Acharya, A.; Gargey, I.A.; Aly, N.P.B. Bioprocess engineering principles of microalgal cultivation for sustainable biofuel production. Bioresour. Technol. Rep. 2019, 5, 297–316. [Google Scholar] [CrossRef]
- Rangel-Basto, Y.; Garcia-Ochoa, I.; Suarez-Gelvez, J.H.; Zuorro, A.; Barajas-Solano, A.F.; Urbina-Suarez, N.A. The effect of temperature and enzyme concentration in the transesterification process of synthetic microalgae oil. Chem. Eng. Trans. 2018, 64, 331–336. [Google Scholar]
- Zuorro, A.; Lavecchiaa, R.; Maffeia, G.; Marraa, F.; Migliettab, S.; Petrangelia, A.; Familiarib, G.; Valentea, T. Enhanced lipid extraction from unbroken microalgal cells using enzymes. Chem. Eng. Trans. 2015, 43, 211–216. [Google Scholar]
- Jacob, A.; Ashok, B.; Alagumalai, A.; Chyuan, O.H.; Le, P.T.K. Critical review on third generation micro algae biodiesel production and its feasibility as future bioenergy for IC engine applications. Energy Convers. Manag. 2021, 228, 113655. [Google Scholar] [CrossRef]
- Bose, A.; O’Shea, R.; Lin, R.; Murphy, J.D. A perspective on novel cascading algal biomethane biorefinery systems. Bioresour. Technol. 2020, 304, 123027. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabro, V. Biofuel Production and Phosphorus Recovery through an Integrated Treatment of Agro-Industrial Waste. Sustainability 2018, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Solé-Bundó, M.; Passos, F.; Romero-Güiza, M.S.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Garfí, M.; Matamoros, V.; Ferrer, I. Co-digestion of microalgae and primary sludge: Effect on biogas production and microcontaminants removal. Sci. Total Environ. 2019, 660, 974–981. [Google Scholar] [CrossRef]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, O.P.; Mehariya, S.; Chung Wong, J.W. Bio-refining of food waste for fuel and value products. Energy Proc. 2017, 136, 14–21. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Kaur, G.; Mehariya, S.; Karthikeyan, O.P.; Chen, G. Food waste treatment by anaerobic co-digestion with saline sludge and its implications for energy recovery in Hong Kong. Bioresour. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nagendranatha Reddy, C.; Mehariya, S.; Kavitha, S.; Yukesh Kannah, R.; Jayaprakash, K.; Yadavalli, R.; Rajesh Banu, J.; Obulisamy, P.K. Electro-Fermentation of Biomass for High-Value Organic Acids. In Biorefineries: A Step Towards Renewable and Clean Energy; Verma, P., Ed.; Springer: Singapore, 2020; pp. 417–436. ISBN 978-981-15-9593-6. [Google Scholar]
- Beltrán, C.; Jeison, D.; Fermoso, F.G.; Borja, R. Batch anaerobic co-digestion of waste activated sludge and microalgae (Chlorella sorokiniana) at mesophilic temperature. J. Environ. Sci. Health Part A 2016, 51, 847–850. [Google Scholar] [CrossRef] [Green Version]
- Wágner, D.S.; Radovici, M.; Smets, B.F.; Angelidaki, I.; Valverde-Pérez, B.; Plósz, B.G. Harvesting microalgae using activated sludge can decrease polymer dosing and enhance methane production via co-digestion in a bacterial-microalgal process. Algal Res. 2016, 20, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Algaculture integration in conventional wastewater treatment plants: Anaerobic digestion comparison of primary and secondary sludge with microalgae biomass. Bioresour. Technol. 2015, 184, 236–244. [Google Scholar] [CrossRef]
- Meneses-Reyes, J.C.; Hernández-Eugenio, G.; Huber, D.H.; Balagurusamy, N.; Espinosa-Solares, T. Biochemical methane potential of oil-extracted microalgae and glycerol in co-digestion with chicken litter. Bioresour. Technol. 2017, 224, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Sahu, A.K.; Rusten, B.; Park, C. Anaerobic co-digestion of microalgae Chlorella sp. and waste activated sludge. Bioresour. Technol. 2013, 142, 585–590. [Google Scholar] [CrossRef]
- Wang, M.; Park, C. Investigation of anaerobic digestion of Chlorella sp. and Micractinium sp. grown in high-nitrogen wastewater and their co-digestion with waste activated sludge. Biomass Bioenergy 2015, 80, 30–37. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, X.J. Biogas production from anaerobic codigestion of microalgae and septic sludge. J. Environ. Eng. 2016, 142, 4016049. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, M.J.; Rincón, B.; Fermoso, F.G.; Jiménez, A.M.; Borja, R. Assessment of two-phase olive mill solid waste and microalgae co-digestion to improve methane production and process kinetics. Bioresour. Technol. 2014, 157, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, P.; Torres, A.; Fermoso, F.G.; Borja, R.; Jeison, D. Anaerobic co-digestion of lipid-spent microalgae with waste activated sludge and glycerol in batch mode. Int. Biodeterior. Biodegrad. 2015, 100, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Schwede, S.; Kowalczyk, A.; Gerber, M.; Span, R. Anaerobic co-digestion of the marine microalga Nannochloropsis salina with energy crops. Bioresour. Technol. 2013, 148, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Suárez, J.L.; Carreras, N. Use of microalgae residues for biogas production. Chem. Eng. J. 2014, 242, 86–95. [Google Scholar] [CrossRef]
- Ramos-Suárez, J.L.; Martínez, A.; Carreras, N. Optimization of the digestion process of Scenedesmus sp. and Opuntia maxima for biogas production. Energy Convers. Manag. 2014, 88, 1263–1270. [Google Scholar] [CrossRef]
- Astals, S.; Musenze, R.S.; Bai, X.; Tannock, S.; Tait, S.; Pratt, S.; Jensen, P.D. Anaerobic co-digestion of pig manure and algae: Impact of intracellular algal products recovery on co-digestion performance. Bioresour. Technol. 2015, 181, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, J.; Forkman, T.; Gentili, F.G.; Zambrano, J.; Schwede, S.; Thorin, E.; Nehrenheim, E. Anaerobic co-digestion of sludge and microalgae grown in municipal wastewater—A feasibility study. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2018, 77, 682–694. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Trobajo, R.; Caiola, N.; Ibáñez, C.; Fabregat, A.; Bengoa, C. Biogas production from sewage sludge and microalgae co-digestion under mesophilic and thermophilic conditions. Renew. Energy 2015, 75, 374–380. [Google Scholar] [CrossRef]
- Du, X.; Tao, Y.; Liu, Y.; Li, H. Stimulating methane production from microalgae by alkaline pretreatment and co-digestion with sludge. Environ. Technol. 2020, 41, 1546–1553. [Google Scholar] [CrossRef]
- El-Mashad, H.M. Biomethane and ethanol production potential of Spirulina platensis algae and enzymatically saccharified switchgrass. BioChem. Eng. J. 2015, 93, 119–127. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 2012, 114, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Marín, D.; Posadas, E.; Cano, P.; Pérez, V.; Blanco, S.; Lebrero, R.; Muñoz, R. Seasonal variation of biogas upgrading coupled with digestate treatment in an outdoors pilot scale algal-bacterial photobioreactor. Bioresour. Technol. 2018, 263, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Cervantes, A.; Serejo, M.L.; Blanco, S.; Pérez, R.; Lebrero, R.; Muñoz, R. Photosynthetic biogas upgrading to bio-methane: Boosting nutrient recovery via biomass productivity control. Algal Res. 2016, 17, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Franco-Morgado, M.; Alcántara, C.; Noyola, A.; Muñoz, R.; González-Sánchez, A. A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: Influence of the illumination regime. Sci. Total Environ. 2017, 592, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Prandini, J.M.; da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Michelon, W.; Soares, H.M. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour. Technol. 2016, 202, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Posadas, E.; Marín, D.; Blanco, S.; Lebrero, R.; Muñoz, R. Simultaneous biogas upgrading and centrate treatment in an outdoors pilot scale high rate algal pond. Bioresour. Technol. 2017, 232, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Sun, S.; Hu, C.; Zhang, H.; Xu, J.; Ping, L. Performance of three microalgal strains in biogas slurry purification and biogas upgrade in response to various mixed light-emitting diode light wavelengths. Bioresour. Technol. 2015, 187, 338–345. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Madrid-Chirinos, C.; Cantera, S.; Lebrero, R.; Muñoz, R. Influence of the gas-liquid flow configuration in the absorption column on photosynthetic biogas upgrading in algal-bacterial photobioreactors. Bioresour. Technol. 2017, 225, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posadas, E.; Serejo, M.L.; Blanco, S.; Pérez, R.; García-Encina, P.A.; Muñoz, R. Minimization of biomethane oxygen concentration during biogas upgrading in algal–bacterial photobioreactors. Algal Res. 2015, 12, 221–229. [Google Scholar] [CrossRef]
- Kumar, P.; Pant, D.C.; Mehariya, S.; Sharma, R.; Kansal, A.; Kalia, V.C. Ecobiotechnological Strategy to Enhance Efficiency of Bioconversion of Wastes into Hydrogen and Methane. Indian J. Microbiol. 2014, 54, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.K.S.; Kumar, P.; Mehariya, S.; Purohit, H.J.; Lee, J.-K.; Kalia, V.C. Enhancement in hydrogen production by co-cultures of Bacillus and Enterobacter. Int. J. Hydrog. Energy 2014. [Google Scholar] [CrossRef]
- Kumar, P.; Mehariya, S.; Ray, S.; Mishra, A.; Kalia, V.C. Biodiesel Industry Waste: A Potential Source of Bioenergy and Biopolymers. Indian J. Microbiol. 2015, 55, 1–7. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, R.; Ray, S.; Mehariya, S.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Dark fermentative bioconversion of glycerol to hydrogen by Bacillus thuringiensis. Bioresour. Technol. 2015, 182, 383–388. [Google Scholar] [CrossRef]
- Kumar, P.; Mehariya, S.; Ray, S.; Mishra, A.; Kalia, V.C. Biotechnology in Aid of Biodiesel Industry Effluent (Glycerol): Biofuels and Bioplastics. In Microbial Factories: Biofuels, Waste Treatment: Volume 1; Kalia, V.C., Ed.; Springer: New Delhi, India, 2015; pp. 105–119. ISBN 978-81-322-2598-0. [Google Scholar]
- Mehariya, S.; Iovine, A.; Casella, P.; Musmarra, D.; Figoli, A.; Marino, T.; Sharma, N.; Molino, A. Fischer–Tropsch Synthesis of Syngas to Liquid Hydrocarbons. In Lignocellulosic Biomass to Liquid Biofuels; Yousuf, A., Pirozzi, D., Sannino, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–248. ISBN 978-0-12-815936-1. [Google Scholar]
- Sharma, A.; Arya, S.K. Hydrogen from algal biomass: A review of production process. Biotechnol. Rep. 2017, 15, 63–69. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chang, J.S.; Lee, D.J. Pretreatment of microalgal biomass for efficient biohydrogen production—Recent insights and future perspectives. Bioresour. Technol. 2020, 302, 122871. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Kossalbayev, B.D.; Zayadan, B.K.; Tomo, T.; Veziroglu, T.N.; Allakhverdiev, S.I. Hydrogen production from phototrophic microorganisms: Reality and perspectives. Int. J. Hydrog. Energy 2019, 44, 5799–5811. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, C.Y.; Liao, Q.; Zhu, X.; Liao, C.F.; Chang, J.S. Biohydrogen production by a novel integration of dark fermentation and mixotrophic microalgae cultivation. Int. J. Hydrog. Energy 2013, 38, 15807–15814. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, H.Y.; Chang, J.S. Producing carbohydrate-rich microalgal biomass grown under mixotrophic conditions as feedstock for biohydrogen production. Int. J. Hydrog. Energy 2016, 41, 4413–4420. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, Z.; Zhang, H.; Lee, D.J.; Zhang, Q.; Lu, C.; He, C. Evaluation of hydrogen yield potential from Chlorella by photo-fermentation under diverse substrate concentration and enzyme loading. Bioresour. Technol. 2020, 303, 122956. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Sharma, N.; Rautela, I.; Nanda, M.; Arora, N.; Singh, A.; Chauhan, P.K. Microalgae fuel cell for wastewater treatment: Recent advances and challenges. J. Water Process. Eng. 2020, 38, 101549. [Google Scholar] [CrossRef]
- Rashid, N.; Cui, Y.-F.; Rehman, M.S.U.; Han, J.-I. Enhanced electricity generation by using algae biomass and activated sludge in microbial fuel cell. Sci. Total Environ. 2013, 456, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Chang, J.S.; Lai, J.Y. Microalgae-microbial fuel cell: A mini review. Bioresour. Technol. 2015, 198, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Choi, Y.-K.; Kim, H.J.; Song, H.-S.; Lee, S.M.; Lee Park, S.; Lee, H.S.; Koh, J.; et al. Application of macroalgal biomass derived biochar and bioelectrochemical system with Shewanella for the adsorptive removal and biodegradation of toxic azo dye. Chemosphere 2021, 264, 128539. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Curtis, T.P.; Logan, B.E. Energy from algae using microbial fuel cells. Biotechnol. Bioeng. 2009, 103, 1068–1076. [Google Scholar] [CrossRef]
- Liu, T.; Rao, L.; Yuan, Y.; Zhuang, L. Bioelectricity Generation in a Microbial Fuel Cell with a Self-Sustainable Photocathode. Sci. World J. 2015, 2015, 864568. [Google Scholar] [CrossRef] [Green Version]
- González del Campo, A.; Cañizares, P.; Rodrigo, M.A.; Fernández, F.J.; Lobato, J. Microbial fuel cell with an algae-assisted cathode: A preliminary assessment. J. Power Sour. 2013, 242, 638–645. [Google Scholar] [CrossRef]
- He, H.; Zhou, M.; Yang, J.; Hu, Y.; Zhao, Y. Simultaneous wastewater treatment, electricity generation and biomass production by an immobilized photosynthetic algal microbial fuel cell. Bioprocess Biosyst. Eng. 2014, 37, 873–880. [Google Scholar] [CrossRef]
- Wu, X.; Song, T.; Zhu, X.; Wei, P.; Zhou, C.C. Construction and Operation of Microbial Fuel Cell with Chlorella vulgaris Biocathode for Electricity Generation. Appl. Biochem. Biotechnol. 2013, 171, 2082–2092. [Google Scholar] [CrossRef]
- Zhou, M.; He, H.; Jin, T.; Wang, H. Power generation enhancement in novel microbial carbon capture cells with immobilized Chlorella vulgaris. J Power Sources 2012, 214, 216–219. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Liu, J.; Lee, H.; Li, C.; Li, N.; Ren, N. Sequestration of CO2 discharged from anode by algal cathode in microbial carbon capture cells (MCCs). Biosens. Bioelectron. 2010, 25, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Evitts, R.W.; Hill, G.A.; Bolster, J.C. A Microbial Fuel Cell with a Photosynthetic Microalgae Cathodic Half Cell Coupled to a Yeast Anodic Half Cell. Energy Sour. Part A Recover. Util. Environ. Eff. 2011, 33, 440–448. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhou, M. A photosynthetic algal microbial fuel cell for treating swine wastewater. Environ. Sci. Pollut. Res. 2019, 26, 6182–6190. [Google Scholar] [CrossRef]
- Juang, D.F.; Lee, C.H.; Hsueh, S.C. Comparison of electrogenic capabilities of microbial fuel cell with different light power on algae grown cathode. Bioresour. Technol. 2012, 123, 23–29. [Google Scholar] [CrossRef]
- Lobato, J.; González del Campo, A.; Fernández, F.J.; Cañizares, P.; Rodrigo, M.A. Lagooning microbial fuel cells: A first approach by coupling electricity-producing microorganisms and algae. Appl. Energy 2013, 110, 220–226. [Google Scholar] [CrossRef]
- Gadhamshetty, V.; Belanger, D.; Gardiner, C.-J.; Cummings, A.; Hynes, A. Evaluation of Laminaria-based microbial fuel cells (LbMs) for electricity production. Bioresour. Technol. 2013, 127, 378–385. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Evaluation of microbial fuel cell operation using algae as an oxygen supplier: Carbon paper cathode vs. carbon brush cathode. Bioprocess Biosyst. Eng. 2014, 37, 2453–2461. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Choi, K.S.; Kakarla, R.; Min, B. Microalgae Scenedesmus obliquus as renewable biomass feedstock for electricity generation in microbial fuel cells (MFCs). Front. Environ. Sci. Eng. 2014, 8, 784–791. [Google Scholar] [CrossRef]
- Walter, X.A.; Greenman, J.; Taylor, B.; Ieropoulos, I.A. Microbial fuel cells continuously fuelled by untreated fresh algal biomass. Algal Res. 2015, 11, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chi-Wei Lan, J.; Chen, W.H.; Chang, J.S. Microalgae from wastewater treatment to biochar—Feedstock preparation and conversion technologies. Energy Convers. Manag. 2017, 150, 1–13. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Ong, H.C.; Show, P.L.; Ling, T.C.; Chen, W.H.; Yu, K.L.; Abdullah, R. Torrefaction of microalgal biochar as potential coal fuel and application as bio-adsorbent. Energy Convers. Manag. 2018, 165, 152–162. [Google Scholar] [CrossRef]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A review on the pyrolysis of algal biomass for biochar and bio-oil—Bottlenecks and scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Abnisa, F.; Wan Daud, W.M.A.; Abu Bakar, N.; Loh, S.K. A review of torrefaction of oil palm solid wastes for biofuel production. Energy Convers. Manag. 2017, 149, 101–120. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A.; Kalemba, I. Characterisation of renewable fuels’ torrefaction process with different instrumental techniques. Energy 2015, 87, 259–269. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass Bioenergy 2011, 35, 3748–3762. [Google Scholar] [CrossRef]
- Chen, W.-H.; Huang, M.-Y.; Chang, J.-S.; Chen, C.-Y.; Lee, W.-J. An energy analysis of torrefaction for upgrading microalga residue as a solid fuel. Bioresour. Technol. 2015, 185, 285–293. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, W.-H.; Lin, B.-J.; Chang, J.-S.; Ong, H.C. Impact of torrefaction on the composition, structure and reactivity of a microalga residue. Appl. Energy 2016, 181, 110–119. [Google Scholar] [CrossRef]
- Chen, W.-H.; Huang, M.-Y.; Chang, J.-S.; Chen, C.-Y. Torrefaction operation and optimization of microalga residue for energy densification and utilization. Appl. Energy 2015, 154, 622–630. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Chen, W.-H.; Lin, S.-C.; Sheen, H.-K.; Chang, J.-S. Wet torrefaction of microalga Chlorella vulgaris ESP-31 with microwave-assisted heating. Energy Convers. Manag. 2017, 141, 163–170. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wu, Z.-Y.; Chang, J.-S. Isothermal and non-isothermal torrefaction characteristics and kinetics of microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2014, 155, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shimanouchi, T.; Iwamura, M.; Takahashi, Y.; Mano, R.; Takashima, K.; Tanifuji, T.; Kimura, Y. Elevating the fuel properties of Humulus lupulus, Plumeria alba and Calophyllum inophyllum L. through wet torrefaction. Fuel 2015, 146, 88–94. [Google Scholar] [CrossRef]
- Yue, Y.; Singh, H.; Singh, B.; Mani, S. Torrefaction of sorghum biomass to improve fuel properties. Bioresour. Technol. 2017, 232, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.-C.; Chang, C.-C.; Yuan, M.-H.; Chang, C.-Y.; Chen, Y.-H.; Lin, C.-F.; Ji, D.-R.; Shie, J.-L.; Manh, D.; Wu, C.-H.; et al. Upgrading of Jatropha-seed residue after mechanical extraction of oil via torrefaction. Energy 2018, 142, 773–781. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chang, C.-C.; Chang, C.-Y.; Yuan, M.-H.; Ji, D.-R.; Shie, J.-L.; Lee, C.-H.; Chen, Y.-H.; Chang, W.-R.; Yang, T.-Y.; et al. Production of a solid bio-fuel from waste bamboo chopsticks by torrefaction for cofiring with coal. J. Anal. Appl. Pyrolysis 2017, 126, 315–322. [Google Scholar] [CrossRef]
- Pahla, G.; Ntuli, F.; Muzenda, E. Torrefaction of landfill food waste for possible application in biomass co-firing. Waste Manag. 2018, 71, 512–520. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Cheng, P.-H.; Chiueh, P.-T.; Lo, S.-L. Leucaena biochar produced by microwave torrefaction: Fuel properties and energy efficiency. Appl. Energy 2017, 204, 1018–1025. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Sung, H.-T.; Chiueh, P.-T.; Lo, S.-L. Microwave torrefaction of sewage sludge and leucaena. J. Taiwan Inst. Chem. Eng. 2017, 70, 236–243. [Google Scholar] [CrossRef]
- Bilgic, E.; Yaman, S.; Haykiri-Acma, H.; Kucukbayrak, S. Limits of variations on the structure and the fuel characteristics of sunflower seed shell through torrefaction. Fuel Process. Technol. 2016, 144, 197–202. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; González-Delgado, Á.D.; García-Martinez, J.B.; L’Abbate, P. Optimization of enzyme-assisted extraction of flavonoids from corn husks. Processes 2019, 7, 804. [Google Scholar] [CrossRef] [Green Version]
- Zuorro, A.; Lavecchia, R.; Monaco, M.M.; Iervolino, G.; Vaiano, V. Photocatalytic degradation of azo dye reactive violet 5 on Fe-doped titania catalysts under visible light irradiation. Catalysts 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]






| Treatment Process | Principle | Source of Pollutants | Removal Efficiency | Advantage | Disadvantage | Reference |
|---|---|---|---|---|---|---|
| Adsorption | Adsorption of specific contaminate on the surface of absorbent | Agricultural, industrial, and municipal wastewater with organic pollutants | 96% | Chemical less, eco-friendly, economical, better metal strap capability | Low selectivity, difficult maintenance, formation of waste products | [45,46] |
| Adsorption, membrane-filtration, and photo-catalytic degradation (Hybrid) | Pollutant removal by serial treatment | Industrial wastewater with organic pollutants | 88–92% of COD; 85–91% of detergents & 91–98% of TS | More efficient, proved highly treated water | Difficult up-scaling | [47] |
| Advance oxidation | Removal of contaminate through oxidation of reactive species | Industrial wastewater rich with organic and pesticidal contaminates | 53–96% of COD & 21–85% of TOC | Broad application, removal of odor molecules, efficient for removal of organic contaminants | High cost, incomplete removal of pollutants | [48] |
| Biochar | Absorption contaminates for removal or degradation | Wastewater rich with dyes, heavy metals, organic inorganic pollutants, and phenolic compounds | 65–99% of dyes & >90% of phenols | Economical, large surface area, more pores, highly efficient | Low removal efficiency of raw biochar, less sustainable | [49] |
| Biogenic Nanoparticles | Reduction or oxidation of metals by natural chemicals-based nanoparticles | Radio-active contamination, inorganic and organic pollutant | 75–99% of dyes & 66–85% of heavy metals | Sustainable, less toxic, inexpensive, less energy requirement | Instability, tricky recovery of intracellularly synthesized nanoparticles | [50,51] |
| Biological method | Assimilation and dissimilation of pollutants | Nitrates, phosphates, dairy waste | >90% of COD & 38–90% of N2 | Economical, high biodegradability, efficient elimination of pollutants | Slow, requires constant maintenance, lower applicability, performance limited by operational conditions | [52,53] |
| Microalgae | Uptake of pollutants as nutrients source for cell proliferation | Municipal and industrial wastewater rich with heavy metals, dyes, organic and inorganic pollutants | 20–98% of TP | Higher removal efficiency, non-toxic, less energy requirement, self-sustainable, produced biomass can be used for biofuel production | Performance limited due to operational conditions as well as type of wastewater, challenging biomass recovery, demands larger land | [54] |
| Coagulation | Dissociation and hydrolysis | Heavy metals, textile, petroleum, cosmetics wastewater | >70% of COD & 90–100% of heavy metals | Ecofriendly, lower operational cost, efficient pollutant removal, energy efficient | Higher maintenance cost, difficult up-scaling, expensive | [55,56] |
| Filtration | Separation via porous membrane | Industrial, municipal, and textile wastewater | 77% of COD; 99% of dyes & TC 74% of TN | Easy operation, cost-effective, and capable to remove suspended solid, alkalinity, inorganic and organic pollutants | Clogging of filter, limited removal of micro pollutant, higher cost of raw material | [31,57,58] |
| Filtration and coagulant-flocculation (Hybrid) | Integration of filtration, coagulating and flocculant | Phenolic compounds, organic pollutants, suspended solids | 36% of COD; 81% fatty matter & 92% of TS | Highly efficient for removal of pollutants, lesser energy requirement | High maintenance cost | [59] |
| Microbial electro-chemical Technology | Oxidation or reduction of pollutants by respiring microbes | Recalcitrant matter, industrial, domestic and food-processing wastewater | >25–63% of COD | Wide applicability, production of electricity, and other valuable commodities | Challenging up-scaling, high cost | [60] |
| Nanomaterials | Play an action as absorbent for the photolytic degradation of contaminate | Inorganic and organic emerging pollutants, petrochemicals as well as heavy metals | 90–100% of heavy metals | Highly efficient with higher adsorption efficiency, friendly with other techniques | Less ecofriendly, expensive, toxic | [51,61] |
| Cultivation System | Advantage | Disadvantage |
|---|---|---|
| Open system | Easy construction and operation Lower cost due to less energy consumption Large open outdoor is widely used to cultivate microalgae for biofuel production using wastewater | Poor light utilization Evaporative losses of water Require Larger areas Chance of contamination Climatical dependency Biomass harvesting challenging and expensive due to lower cell density |
| Closed system | Optimal growth parameters can be controlled Minimize the CO2 and water losses Higher biomass productivity Lesser contamination | Capital expenditures (CAPEX) and operating expense (OPEX) are high Over-heating and biofouling Up-scaling very expensive |
| Algal turf scrubber (ATS) | Very low and fuel production cost Higher biomass productivities and easy biomass harvesting over open cultivation system Low maintenance and monitoring Carbon sequestration from environment | Need sufficient space but lesser as compare to open cultivation system Lower wastewater handling capacity Need considerable infrastructure |
| Hybrid cultivation system (HCS) | Higher biomass productivity Lesser contamination risk Low maintenance and monitoring Carbon sequestration from environment | Higher CAPEX and OPEX of process Require large infrastructure Need high maintenance and continuous monitoring |
| Type of Cultivation Mode | Energy Supply | Type of Carbon Source | Advantages | Disadvantages |
|---|---|---|---|---|
| Autotrophic | Light | Inorganic carbon source (CO2) | Lower CAPEX and OPEX Higher productivity of pigments Ability to remove nutrients from wastewater | Lower biomass production rate |
| Heterotrophic | Organic carbon source | Organic carbon source (glucose etc.) | Higher biomass production rate | Higher cost of carbon sources Chance of microbial contamination Higher CAPEX and OPEX |
| Mixotrophic | Light and organic carbon source | Inorganic and organic carbon source (CO2 & glucose) | Higher growth rate Ability to remove nutrients from wastewater | Higher cost of carbon sources Chance of microbial contamination Higher CAPEX and OPEX |
| Algae Species | CN | CP (°C) | CV (MJ/kg) | FP (°C) | PP (°C) | SG | Viscosity (Centipoise) at 40 °C |
|---|---|---|---|---|---|---|---|
| ASTM) D6751 standards | 40 | - | - | 52 | - | 0.86–0.90 | 1.9–6.0 |
| Botryococcus braunii | – | – | 50.00 | 138 | – | 0.85 | 5.34 |
| Chlorella protothecoides | 52 | −27 | 40.04 | 124 | −11 | 0. 87 | 2.80 |
| Chlorella vulgaris | 60 | 0 | 17.44 | 140 | −11 | 0.87 | 4.80 |
| Cladophora | – | – | 33.60 | 110 | −12 | 0.89 | 3.80 |
| Crypthecodinium cohnii | 46.5 | 16.1 | 39.86 | 95.0 | 0.91 | 5.06 | |
| Dunaliella salina | 50.5 | 0 | 34.00 | 129 | −6 | 0.85 | 2.40 |
| Entromorpha | 50 | −1 | 39.94 | 194 | −6 | 0.86 | 3.12 |
| Nannochloropsis oculata | 46 | 3.39–12.14 | 16.80 | 180 | −4 | 0.85 | 5.76 |
| Neochloris oleoabundans | 55 | −10 | 39.76 | 126 | −12 | 0.89 | 5.54 |
| Spirogyra | – | 3 | 13.62 | 78 | −7 | 0.88 | 4.40 |
| Spirulina | 70 | −3 | 34.50 | 130 | −9 | 0.99 | 0.27 at 300 °C |
| Stoechospermum marginatum | 63 | – | 42.052 | 128 | – | 0.89 | 4.84 |
| Microalgae | Co-Substrate | Mixture Ratio | T (°C) | MYC (mLCH4/gVS) | YI (%) | Reference |
|---|---|---|---|---|---|---|
| Chlorella sorokiniana | WAS | 25:75 (VS) | 37 | 442 | 26 | [108] |
| Chlorella sorokiniana & Scenedesmus sp. | WAS | 9:91 (VS) | 37 | 400 | 11 | [109] |
| Chlorella sorokiniana & Scenedesmus sp. | WAS | 9:91 (VS) | 37 | 560 | 28 | [109] |
| Chlorella vulgaris | WAS | 75:25 (COD) | 35 | 107 | 6 | [110] |
| Chlorella vulgaris | PS | 50:50 (COD) | 35 | 283 | 16 | [110] |
| Chlorella vulgaris | PS | 75:25 (COD) | 35 | 293 | 13 | [110] |
| Chlorella vulgaris | Chicken litter & glycerol | 30:67:3 (TS) | 37 | 131 | 16 | [111] |
| Chlorella sp. | WAS | 41:59 (TS) | 37 | 468 | 23 | [112] |
| Chlorella sp. | WAS | 21:79 (VS) | 37 | 253 | 5 | [113] |
| Chlorella sp. | Septic sludge | 50:50 (VS) | 35 | 547 | 84 | [114] |
| Dunaliella salina | Olive mill solid waste | 50:50 (VS) | 35 | 285 | 48 | [115] |
| Lipid-spent Botryococcus braunii | WAS | 75:25 (VS) | 35 | 393 | 7 | [116] |
| Micractinium sp. | WAS | 21:79 (VS) | 37 | 236 | 0 | [113] |
| Nannochloropsis salina | Corn cob | 25:75 (v/v) | 610 | 18 | [117] | |
| Nannochloropsis salina | Corn silage | 14:86 (v/v) | 37 | 660 | 15 | [117] |
| Scenedesmus quadricauda | WAS | 49:51 (VS) | 35 | 222 | 12 | [92] |
| Scenedesmus sp. | Opuntia maxima | 25:75 (VS) | 37 | 234 | 65 | [118] |
| Scenedesmus sp. | Opuntia maxima | 10:90 (VS) | 37 | 166 | 7 | [118] |
| Scenedesmus sp. | Paper sludge | 74:26 (VS) | 37 | 173 | 35 | [119] |
| Scenedesmus sp. | Pig manure | 15:85 (VS) | 37 | 319 | 5 | [120] |
| Scenedesmus sp. and Chlorella sp. | SS | 40:60 (VS) | 35 | 237 | 2 | [121] |
| Selenastrum capricornutum | SS | 50:50 (VS) | 33 | 392 | 9 | [122] |
| Spirulina platensis | SS | 67:37 (VS) | 35 | 343 | 15 | [123] |
| Spirulina platensis | Pretreated switchgrass | 50:50 (TS) | 50 | 354 | 10 | [124] |
| Taihu blue algae | Corn straw | 20:80 (VS) | 35 | 325 | 114 | [125] |
| Microalgae Species | Cultivation System | H2S Removal | CO2 Removal % | O2% | CH4 Recovery % | Reference | |
|---|---|---|---|---|---|---|---|
| In-/Outdoor | System | ||||||
| Chlorella sp. | Outdoor | HRAP | Yes | 95 | 0.1–2.0 | 94 | [131] |
| Chlorella vulgaris | Outdoor | EPB | 55.39 | 80.4 | [132] | ||
| Scenedesmus obliquus | 62.31 | 0.62 | 82.64 | ||||
| Neochloris oleoabundans | 54.39 | 80.06 | |||||
| Geitlerinema sp. (61.5%), Staurosira sp. (1.5%) & Stigeoclonium tenue (37%) | Indoor | HRAP | Yes | 98.8 | 0.03 | 97.2 | [128] |
| Mychonastes homosphaera | Indoor | HRAP | Yes | 98.8 | 0.7 | 96.2 | [133] |
| Planktolynga brevicellularis (81%), Stigeoclonium tenue (14%) and Limnothrix planktonica (5%) | Indoor | HRAP | Yes | 72–79 | 1.2–0.7 | 81 | [134] |
| Scenedesmus sp. | Indoor | EPB | Yes | 66.7 | 17.8 | 64.7 | [129] |
| Microalgae | Cathode | Anode | Power Density | Reference |
|---|---|---|---|---|
| Chlorella vulfaris; Ulva lactuca | Graphite fiber brush | Air cathode with platinum (Pt) catalyst | 980.0 mW/m2 | [151] |
| Chlorella vulgaris | Carbon fiber brushes | Carbon felt containing Pt catalyst | 187.0 mW/m2 | [152] |
| Toray carbon cloth | Toray carbon cloth | 13.5 mW/m2 | [153] | |
| Graphite felt | Carbon fiber cloth | 2572.8 mW/m2 | [154] | |
| Carbon felt | Carbon felt | 24.4 mW/m2 | [155] | |
| Carbon felt | Carbon fiber cloth | 2485.3 mW/m3 | [156] | |
| Carbon fiber brushes | Carbon cloth | 5600.0 mW/m3 | [157] | |
| Graphite rod | Graphite rod | 0.95 mW/m2 | [158] | |
| Carbon felt | Carbon fiber cloth | 3720.0 mW/m3 | [159] | |
| Chlorella sp. | Graphite carbon | Graphite carbon | 3.35 mW/m2 | [160] |
| Lagoon (algae culture) | Plain carbon cloths | Plain carbon cloths | 11.5 mW/m2 | [161] |
| Laminaria saccharina | Graphite felt | Graphite felt | 250.0 Mw/m2 | [162] |
| Mixed algae culture | Carbon fiber brush | Carbon cloth coated with platinum | 30.0 mW/m2 | [163] |
| Scenedesmus obliquus | Toraycarbon paper | Toray carbon paper | 102.0 mW/m2 | [164] |
| Synechococcus leopoliensis | Black acrylic | Carbon fiber veil | 42.5 mW/m3 | [165] |
| Type of Biomass | t & T | Ultimate Analysis (wt%) | HHV (MJ/kg) | EY (%) | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | ||||||
| Microalgae | Chalamydomonas sp. JSC4 residue | 15–60 min; 200–300 °C | 51.6–72.6 | 7.2–4.4 | 4.0–6.4 | 37.2–16.5 | 17.6–24.8 | 74.3–99.8 | [172] |
| Chlamydomonas sp. JSC4 | 30 min; 300 °C | 63.6 | 5.01 | 6.0 | 25.4 | – | – | [173] | |
| Chlorella vulgaris ESP.31 | 15–60 min; 200–300 °C | 49.1–65.3 | 7.9–5.1 | 5.0–6.7 | 38.0–22.9 | 17.9–25.2 | – | [174] | |
| Chlorella vulgaris ESP.31 by wet torrefaction | 30 min; 170 °C | 59.0 | 7.8 | 8.6 | 24.5 | 26.02 | 62.95 | [175] | |
| Scenedesmus obliquus CNW-N | 60 min; 200–300 °C | 36.9–39.3 | 5.5–3.6 | 6.5–7.3 | 28.2–23.2 | – | – | [176] | |
| Other biomass | Calophyllum inophyllum L | 10 min; 260 °C | 59.1 | 4.9 | 0.3 | 35.7 | 23.6 | 65.2 | [177] |
| Energy sorghum | 30 min; 275 °C | 55.2 | 4.9 | 1.7 | 38.1 | 23.80 | 73 | [178] | |
| Humulud lupulud | 10 min; 260 °C | 60.5 | 6.0 | 2.7 | 30.8 | 25.3 | 38.5 | [177] | |
| Jatropha-seed residue | 30 min; 300 °C | 61.1 | 5.2 | 4.2 | 20.7 | 27.01 | – | [179] | |
| Waste bamboo chopsticks | 40 min; 290 °C | 55.5 | 5.4 | 0.2 | 38.3 | 23.04 | – | [180] | |
| Landfill food waste | 40 min; 275 °C | 61.2 | 5.8 | 3.4 | 29.6 | 26.15 | 77.2 | [181] | |
| Leucaena by microwave torrefaction | 250 W | 76.3 | 2.6 | 1.0 | 15.1 | 28.25 | 34.04 | [182] | |
| Leucaena by microwave torrefaction | 250 W | 80.3 | 2.8 | 1.1 | 15.9 | 29.72 | 36 | [183] | |
| Plumeria alba | 10 min; 260 °C | 60.7 | 6.8 | 0.6 | 31.9 | 25.7 | 45.7 | [177] | |
| Sewage sludge | 400 W | 66.8 | 2.3 | 2.7 | 28.3 | 13.21 | 19 | [183] | |
| Sunflower seed shell | 60 min; 300 °C | 69.5 | 5.3 | 0.5 | 24.6 | 27.6 | – | [184] | |
| Sweet sorghum bagasse | 30 min; 300 °C | 59.3 | 4.6 | 0.9 | 35.2 | 26.88 | 70 | [178] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehariya, S.; Goswami, R.K.; Verma, P.; Lavecchia, R.; Zuorro, A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies 2021, 14, 2282. https://doi.org/10.3390/en14082282
Mehariya S, Goswami RK, Verma P, Lavecchia R, Zuorro A. Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries. Energies. 2021; 14(8):2282. https://doi.org/10.3390/en14082282
Chicago/Turabian StyleMehariya, Sanjeet, Rahul Kumar Goswami, Pradeep Verma, Roberto Lavecchia, and Antonio Zuorro. 2021. "Integrated Approach for Wastewater Treatment and Biofuel Production in Microalgae Biorefineries" Energies 14, no. 8: 2282. https://doi.org/10.3390/en14082282