Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases
Abstract
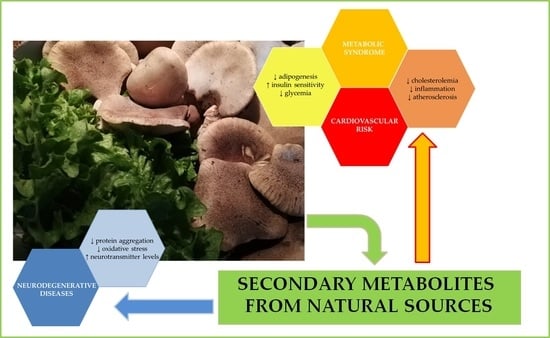
:1. Introduction
2. Natural Compounds and Neurodegenerative Diseases
2.1. Alzheimer’s Disease
2.2. Parkinson’s Disease
3. Metabolic Syndrome and Cardiovascular Risk
3.1. Diabetes
3.2. Obesity
3.3. Hypertension and Hyperinflammation-Managing Cardiovascular Risk in Metabolic Syndrome
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salem, M.A.; De Souza, L.P.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saloustros, E.; Mavroudis, D.; Georgoulias, V. Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin. Pharmacother. 2008, 9, 2603–2616. [Google Scholar] [CrossRef]
- Kumar, A. Vincristine and vinblastine: A review. Int. J. Med. Pharm. Sci. 2016, 6, 23–30. [Google Scholar]
- Vacca, R.A.; Valenti, D.; Caccamese, S.; Daglia, M.; Braidy, N.; Nabavi, S.M. Plant polyphenols as natural drugs for the management of Down syndrome and related disorders. Neurosci. Biobehav. Rev. 2016, 71, 865–877. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-T.; Li, X.; Xie, M.-L.; Huang, Z.; Huang, Y.-X.; Wu, G.-X.; Peng, Z.-R.; Sun, Y.-N.; Ming, Q.-L.; Liu, Y.-X.; et al. Resveratrol: Review on its discovery, anti-leukemia effects and pharmacokinetics. Chem. Biol. Interactions 2019, 306, 29–38. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Misiek, M.; Hoffmeister, D. Fungal genetics, genomics, and secondary metabolites in pharmaceutical sciences. Planta Med. 2007, 73, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahlan, K.; Jensen, S.E. Origins of the β-lactam rings in natural products. J. Antibiot. 2013, 66, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, V.C. The use of ciclosporin in psoriasis: A clinical review. Br. J. Dermatol. Suppl. 2004, 150, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leuci, R.; Brunetti, L.; Laghezza, A.; Tortorella, P.; Loiodice, F.; Piemontese, L. A Review of Recent Patents (2016-2019) on Plant Food Supplements with Potential Application in the Treatment of Neurodegenerative and Metabolic Disorders. Recent Pat. Food. Nutr. Agric. 2020, 11, 145–153. [Google Scholar] [CrossRef]
- Journoud, M.; Jones, P.J.H. Red yeast rice: A new hypolipidemic drug. Life Sci. 2004, 74, 2675–2683. [Google Scholar] [CrossRef]
- Brunetti, L.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Combining fatty acid amide hydrolase (FAAH) inhibition with peroxisome proliferator-activated receptor (PPAR) activation: A new potential multi-target therapeutic strategy for the treatment of Alzheimer’s disease. Neural Regen. Res. 2020, 15, 67–68. [Google Scholar] [CrossRef]
- Fancellu, G.; Chand, K.; Tomás, D.; Orlandini, E.; Piemontese, L.; Silva, D.F.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Novel tacrine–benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer’s Disease. J. Enzym. Inhib. Med. Chem. 2020, 35, 211–226. [Google Scholar] [CrossRef] [Green Version]
- Piemontese, L.; Loiodice, F.; Chaves, S.; Santos, M.A. The Therapy of Alzheimer’s Disease: Towards a New Generation of Drugs. Front. Clin. Drug Res. Alzheimer Disord. 2019, 8, 33–80. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Dumitrascu, D.; Capitanescu, B.; Petcu, E.; Surugiu, R.; Fang, W.-H.; Dumbrava, D.-A. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen. Res. 2020, 15, 394–400. [Google Scholar] [CrossRef]
- Fernández-Moriano, C.; Divakar, P.K.; Crespo, A.; Gómez-Serranillos, M.P. In vitro neuroprotective potential of lichen metabolite fumarprotocetraric acid via intracellular redox modulation. Toxicol. Appl. Pharmacol. 2017, 316, 83–94. [Google Scholar] [CrossRef]
- Leirós, M.; Sánchez, J.A.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Spongionella secondary metabolites protect mitochondrial function in cortical neurons against oxidative stress. Mar. Drugs 2014, 12, 700–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugnani, K.S.; Vu, N.; Rondón-Ortiz, A.N.; Böhlke, M.; Maher, T.J.; Pino-Figueroa, A.J. Neuroprotective activity of macamides on manganese-induced mitochondrial disruption in U-87 MG glioblastoma cells. Toxicol. Appl. Pharmacol. 2018, 340, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Ou-Yang, H.; Yan, X.; Tang, B.W.; Fang, M.J.; Wu, Z.; Chen, J.W.; Qiu, Y.K. Open-ring butenolides from a marine-derived anti-neuroinflammatory fungus aspergillus terreus Y10. Mar. Drugs 2018, 16, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.G.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom hericium erinaceus and the rare species, hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakoyun, Ç.; Bozkurt, B.; Çoban, G.; Masi, M.; Cimmino, A.; Evidente, A.; Somer, N.U. A comprehensive study on narcissus tazetta subsp. tazetta L.: Chemo-profiling, isolation, anticholinesterase activity and molecular docking of amaryllidaceae alkaloids. S. Afr. J. Bot. 2020, 130, 148–154. [Google Scholar] [CrossRef]
- Nuthakki, V.K.; Sharma, A.; Kumar, A.; Bharate, S.B. Identification of embelin, a 3-undecyl-1,4-benzoquinone from Embelia ribes as a multitargeted anti-Alzheimer agent. Drug Dev. Res. 2019, 80, ddr.21544. [Google Scholar] [CrossRef]
- Augustin, N.; Nuthakki, V.K.; Abdullaha, M.; Hassan, Q.P.; Gandhi, S.G.; Bharate, S.B. Discovery of Helminthosporin, an Anthraquinone Isolated from Rumex abyssinicus Jacq as a Dual Cholinesterase Inhibitor. ACS Omega 2020, 5, 1616–1624. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Irfan, A.; Ibrahim, M.; Assiri, M.A.; Khalid, N.; Ullah, S.; Al-Sehemi, A.G. Carbonic anhydrase and cholinesterase inhibitory activities of isolated flavonoids from Oxalis corniculata L. and their first-principles investigations. Ind. Crops Prod. 2020, 148, 112285. [Google Scholar] [CrossRef]
- Reddy, R.G.; Veeraval, L.; Maitra, S.; Chollet-Krugler, M.; Tomasi, S.; Dévéhat, F.L.L.; Boustie, J.; Chakravarty, S. Lichen-derived compounds show potential for central nervous system therapeutics. Phytomedicine 2016, 23, 1527–1534. [Google Scholar] [CrossRef]
- Piemontese, L.; Vitucci, G.; Catto, M.; Laghezza, A.; Perna, F.M.; Rullo, M.; Loiodice, F.; Capriati, V.; Solfrizzo, M. Natural scaffolds with multi-target activity for the potential treatment of Alzheimer’s disease. Molecules 2018, 23, 2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Ryu, H.W.; Kang, M.G.; Park, D.; Oh, S.R.; Kim, H. Potent selective monoamine oxidase B inhibition by maackiain, a pterocarpan from the roots of Sophora flavescens. Bioorganic Med. Chem. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, N.D.; León, F.; Ding, Y.; Gómez-Betancur, I.; Benjumea, D.; Walker, L.A.; Cutler, S.J.; Tekwani, B.L. Interactions of Desmethoxyyangonin, a Secondary Metabolite from Renealmia alpinia, with Human Monoamine Oxidase-A and Oxidase-B. Evid. Based Complement. Altern. Med. 2017, 2017, 4018724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumaran, S.; Nakamura, Y.; Henley, J.M.; Pountney, D.L. Ginkgolic acid promotes autophagy-dependent clearance of intracellular alpha-synuclein aggregates. Mol. Cell. Neurosci. 2019, 101, 103416. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Sang, J.; Cui, W.; Zhao, W.; Wei, W.; Chen, B.; Lu, F.; Liu, F. Dihydromyricetin Inhibits α-Synuclein Aggregation, Disrupts Preformed Fibrils, and Protects Neuronal Cells in Culture against Amyloid-Induced Cytotoxicity. J. Agric. Food Chem. 2019, 67, 3946–3955. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, B.S.; Shivakumar, M.; Joshi, V.; Subbanna, S. Endocannabinoid system in neurodegenerative disorders. J. Neurochem. 2017, 142, 624–648. [Google Scholar] [CrossRef]
- González, J.F.; Alcántara, A.R.; Doadrio, A.L.; Sánchez-Montero, J.M. Developments with multi-target drugs for Alzheimer’s disease: An overview of the current discovery approaches. Expert Opin. Drug Discov. 2019, 14, 879–891. [Google Scholar] [CrossRef]
- Mendez, M.F. Early-onset Alzheimer disease and its variants. Continuum 2019, 25, 34–51. [Google Scholar] [CrossRef]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, M.H.; Ahmad, K.; Rabbani, G.; Choi, I. Use of Peptides for the Management of Alzheimer’s Disease: Diagnosis and Inhibition. Front. Aging Neurosci. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Esposito, Z.; Koch, G. Beyond the Cholinergic Hypothesis: Do Current Drugs Work in Alzheimer’s Disease? CNS Neurosci. Ther. 2010, 16, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. The pharmacology of galanthamine and its analogues. Pharmacol. Ther. 1995, 68, 113–128. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Leem, Y.H.; Park, J.E.; Kim, D.Y.; Kim, H.S. Neuroprotective effect of β-lapachone in MPTP-induced parkinson’s disease mouse model: Involvement of astroglial p-AMPK/Nrf2/HO-1 signaling pathways. Biomol. Ther. 2019, 27, 178–184. [Google Scholar] [CrossRef]
- Gazewood, J.D.; Richards, D.R.; Clebak, K. Parkinson Disease: An Update. Am. Fam. Physician 2013, 87, 267–273. [Google Scholar]
- Kumar, B.; Sheetal, S.; Mantha, A.K.; Kumar, V. Recent developments on the structure-activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv. 2016, 6, 42660–42683. [Google Scholar] [CrossRef]
- Fernandez, H.H.; Chen, J.J. Monoamine oxidase-B inhibition in the treatment of Parkinson’s disease. Pharmacotherapy 2007, 27, 174S–185S. [Google Scholar] [CrossRef]
- Finberg, J.P.M. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: Focus on modulation of CNS monoamine neurotransmitter release. Pharmacol. Ther. 2014, 143, 133–152. [Google Scholar] [CrossRef]
- Carradori, S.; Gidaro, M.C.; Petzer, A.; Costa, G.; Guglielmi, P.; Chimenti, P.; Alcaro, S.; Petzer, J.P. Inhibition of Human Monoamine Oxidase: Biological and Molecular Modeling Studies on Selected Natural Flavonoids. J. Agric. Food Chem. 2016, 64, 9004–9011. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recchia, A.; Debetto, P.; Negro, A.; Guidolin, D.; Skaper, S.D.; Giusti, P. α-Synuclein and Parkinson’s disease. FASEB J. 2004, 18, 617–626. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ye, M.; Guo, H. An Updated Review of Randomized Clinical Trials Testing the Improvement of Cognitive Function of Ginkgo biloba Extract in Healthy People and Alzheimer’s Patients. Front Pharmacol. 2019, 10, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Connell, J.M.C. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M.Á. Vasodilator compounds derived from plants and their mechanisms of action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.Q.; Li, P.H.; Chao, Y.X.; Chen, H.; Du, N.; He, Q.X.; Liu, K.C. Alkaloids with cardiovascular effects from the marine-derived fungus Penicillium expansum Y32. Mar. Drugs 2015, 13, 6489–6504. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Carrieri, A.; Giudici, M.; Parente, M.; De Rosas, M.; Piemontese, L.; Fracchiolla, G.; Laghezza, A.; Tortorella, P.; Carbonara, G.; Lavecchia, A.; et al. Molecular determinants for nuclear receptors selectivity: Chemometric analysis, dockings and site-directed mutagenesis of dual peroxisome proliferator-activated receptors α/γ agonists. Eur. J. Med. Chem. 2013, 63, 321–332. [Google Scholar] [CrossRef]
- Laghezza, A.; Piemontese, L.; Cerchia, C.; Montanari, R.; Capelli, D.; Giudici, M.; Crestani, M.; Tortorella, P.; Peiretti, F.; Pochetti, G.; et al. Identification of the First PPARα/γ Dual Agonist Able to Bind to Canonical and Alternative Sites of PPARγ and to Inhibit Its Cdk5-Mediated Phosphorylation. J. Med. Chem. 2018, 61, 8282–8298. [Google Scholar] [CrossRef]
- Schoonjans, K.; Staels, B.; Auwerx, J. Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J. Lipid Res. 1996, 37, 907–925. [Google Scholar] [PubMed]
- Michalik, L.; Wahli, W. Peroxisome proliferator-activated receptors: Three isotypes for a multitude of functions. Curr. Opin. Biotechnol. 1999, 10, 564–570. [Google Scholar] [CrossRef]
- Piemontese, L.; Fracchiolla, G.; Carrieri, A.; Parente, M.; Laghezza, A.; Carbonara, G.; Sblano, S.; Tauro, M.; Gilardi, F.; Tortorella, P.; et al. Design, synthesis and biological evaluation of a class of bioisosteric oximes of the novel dual peroxisome proliferator-activated receptor α/γ ligand LT175. Eur. J. Med. Chem. 2015, 90, 583–594. [Google Scholar] [CrossRef]
- Lamichane, S.; Dahal Lamichane, B.; Kwon, S.-M. Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int. J. Mol. Sci. 2018, 19, 949. [Google Scholar] [CrossRef] [Green Version]
- Penumetcha, M.; Santanam, N. Nutraceuticals as ligands of PPARγ. PPAR Res. 2012, 2012, 858352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracchiolla, G.; Lavecchia, A.; Laghezza, A.; Piemontese, L.; Trisolini, R.; Carbonara, G.; Tortorella, P.; Novellino, E.; Loiodice, F. Synthesis, biological evaluation, and molecular modeling investigation of chiral 2-(4-chloro-phenoxy)-3-phenyl-propanoic acid derivatives with PPARα and PPARγ agonist activity. Bioorganic Med. Chem. 2008, 16, 9498–9510. [Google Scholar] [CrossRef]
- Fracchiolla, G.; Laghezza, A.; Piemontese, L.; Parente, M.; Lavecchia, A.; Pochetti, G.; Montanari, R.; Di Giovanni, C.; Carbonara, G.; Tortorella, P.; et al. Synthesis, biological evaluation and molecular investigation of fluorinated peroxisome proliferator-activated receptors α/γ dual agonists. Bioorganic Med. Chem. 2012, 20, 2141–2151. [Google Scholar] [CrossRef]
- Laghezza, A.; Montanari, R.; Lavecchia, A.; Piemontese, L.; Pochetti, G.; Iacobazzi, V.; Infantino, V.; Capelli, D.; DeBellis, M.; Liantonio, A.; et al. On the Metabolically Active Form of Metaglidasen: Improved Synthesis and Investigation of Its Peculiar Activity on Peroxisome Proliferator-Activated Receptors and Skeletal Muscles. ChemMedChem 2015, 10, 555–565. [Google Scholar] [CrossRef]
- Fawzy, G.A.; Al-Taweel, A.M.; Perveen, S.; Khan, S.I.; Al-Omary, F.A. Bioactivity and chemical characterization of Acalypha fruticosa Forssk. growing in Saudi Arabia. Saudi Pharm. J. 2017, 25, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Vásquez, Y.; Zhao, J.; Khana, S.I.; Gupta, M.P.; Khana, I.A. Constituents of talisia nervosa with potential utility against metabolic syndrome. Nat. Prod. Commun. 2019, 14, 51–54. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Su, M.; Song, S.J.; Hong, J.; Kim, S.; Im, D.S.; Jung, J.H. A bile acid derivative with PPARγ-mediated anti-inflammatory activity. Steroids 2018, 137, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.; Silva, N.G.; Sousa, M.L.; Ribeiro, T.; Rosa, F.; Leão, P.N.; Vasconcelos, V.; Reis, M.A.; Urbatzka, R. Chlorophyll derivatives from marine cyanobacteria with lipid-reducing activities. Mar. Drugs 2019, 17, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schinkovitz, A.; Le Pogam, P.; Derbré, S.; Roy-Vessieres, E.; Blanchard, P.; Thirumaran, S.L.; Breard, D.; Aumond, M.C.; Zehl, M.; Urban, E.; et al. Secondary metabolites from lichen as potent inhibitors of advanced glycation end products and vasodilative agents. Fitoterapia 2018, 131, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Liang, S.; Che, J.Y.; Li, H.; Sun, H.X.; Chen, J.G.; Du, X.X.; Wang, C.M. Antidiabetic activity of acidic polysaccharide from schisandra chinensis in STZ-induced diabetic mice. Nat. Prod. Commun. 2019, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Thissera, B.; Visvanathan, R.; Khanfar, M.A.; Qader, M.M.; Hassan, M.H.A.; Hassan, H.M.; Bawazeer, M.; Behery, F.A.; Yaseen, M.; Liyanage, R.; et al. Sesbania grandiflora L. Poir leaves: A dietary supplement to alleviate type 2 diabetes through metabolic enzymes inhibition. S. Afr. J. Bot. 2020, 130, 282–299. [Google Scholar] [CrossRef]
- Silva, T.D.C.; Justino, A.B.; Prado, D.G.; Koch, G.A.; Martins, M.M.; Santos, P.D.S.; De Morais, S.A.L.; Goulart, L.R.; Cunha, L.C.S.; Sousa, R.M.F.; et al. Chemical composition, antioxidant activity and inhibitory capacity of α-amylase, α-glucosidase, lipase and non-enzymatic glycation, in vitro, of the leaves of Cassia bakeriana Craib. Ind. Crops Prod. 2019, 140, 111641. [Google Scholar] [CrossRef]
- Ruiz-Vargas, J.A.; Morales-Ferra, D.L.; Ramírez-Ávila, G.; Zamilpa, A.; Negrete-León, E.; Acevedo-Fernández, J.J.; Peña-Rodríguez, L.M. α-Glucosidase inhibitory activity and in vivo antihyperglycemic effect of secondary metabolites from the leaf infusion of Ocimum campechianum mill. J. Ethnopharmacol. 2019, 243, 112081. [Google Scholar] [CrossRef]
- Dewi, R.T.; Tachibana, S.; Darmawan, A. Effect on α-glucosidase inhibition and antioxidant activities of butyrolactone derivatives from Aspergillus terreus MC751. Med. Chem. Res. 2014, 23, 454–460. [Google Scholar] [CrossRef]
- Budipramana, K.; Junaidin, J.; Wirasutisna, K.R.; Pramana, Y.B.; Sukrasno, S. An integrated in silico and in vitro assays of dipeptidyl peptidase-4 and α-glucosidase inhibition by stellasterol from Ganoderma australe. Sci. Pharm. 2019, 87, 21. [Google Scholar] [CrossRef] [Green Version]
- Wiese, J.; Aldemir, H.; Schmaljohann, R.; Gulder, T.A.M.; Imhoff, J.F.; Kerr, R. Asperentin B, a new inhibitor of the protein tyrosine phosphatase 1B. Mar. Drugs 2017, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yuan, C.; Wang, Y. Bioactivity-guided identification of anti-adipogenic isothiocyanates in the moringa (Moringa oleifera) seed and investigation of the structure-activity relationship. Molecules 2020, 25, 2504. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Nam, K.H.; Yi, S.A.; Jo, M.S.; Lee, K.H.; Lee, Y.H.; Lee, J.; Kim, K.H. Anti-adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum). Foods 2019, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, Y.; Yoshimura, N.; Matsumura, S.; Wada, H.; Hoshino, M.; Makino, S.; Morimoto, M. α-Glucosidase and pancreatic lipase inhibitory activities of diterpenes from indian mango ginger (curcuma amada roxb.) and its derivatives. Molecules 2019, 24, 4071. [Google Scholar] [CrossRef] [Green Version]
- Chi, Z.; Le, T.P.H.; Lee, S.K.; Guo, E.; Kim, D.; Lee, S.; Seo, S.Y.; Lee, S.Y.; Kim, J.H.; Lee, S.Y. Honokiol ameliorates angiotensin II-induced hypertension and endothelial dysfunction by inhibiting HDAC6-mediated cystathionine γ-lyase degradation. J. Cell Mol. Med. 2020, 24, 10663–10676. [Google Scholar] [CrossRef]
- Lu, S.; Luo, Y.; Sun, G.B.; Sun, X.B. Ginsenoside Compound K Attenuates Ox-LDL-Mediated Macrophage Inflammation and Foam Cell Formation via Autophagy Induction and Modulating NF-κB, p38, and JNK MAPK Signaling. Front. Pharmacol. 2020, 11, 567238. [Google Scholar] [CrossRef]
- Saha, S.; Profumo, E.; Togna, A.R.; Riganò, R.; Saso, L.; Buttari, B. Lupeol Counteracts the Proinflammatory Signalling Triggered in Macrophages by 7-Keto-Cholesterol: New Perspectives in the Therapy of Atherosclerosis. Oxid. Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Lee, J.; Hernandez, M.A.S.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [Green Version]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 diabetes mellitus: A review of current trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Hays, N.P.; Galassetti, P.R.; Coker, R.H. Prevention and treatment of type 2 diabetes: Current role of lifestyle, natural product, and pharmacological interventions. Pharmacol. Ther. 2008, 118, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Salehi, P.; Asghari, B.; Esmaeili, M.A.; Dehghan, H.; Ghazi, I. -Glucosidase and -amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J. Med. Plants Res. 2013, 7, 257–266. [Google Scholar] [CrossRef]
- Ahrén, B.; Schmitz, O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Horm. Metab. Res. 2004, 36, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Montalibet, J.; Kennedy, B.P. Therapeutic strategies for targeting PTP1B in diabetes. Drug Discov. Today Ther. Strateg. 2005, 2, 129–135. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Yanovski, S.Z.; Carroll, M.D.; Flegal, K.M. The Epidemiology of Obesity. Gastroenterology 2007, 132, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Bloom, S.R. The pharmacological treatment and management of obesity. Postgrad. Med. 2011, 123, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Prasad, A.; Haque, F.; Ray, S.; De, B.; Ray, S.S. In silico investigation of black tea components on α-amylase, α-glucosidase and lipase. J. Appl. Pharm. Sci. 2015, 5, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Paracchini, V.; Pedotti, P.; Taioli, E. Genetics of leptin and obesity: A HuGE review. Am. J. Epidemiol. 2005, 162, 101–114. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A review. Nutr. Neurosci. 2015, 18, 49–65. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, 56. [Google Scholar] [CrossRef]
- Bhansali, S.; Khatri, S.; Dhawan, V. Terminalia Arjuna bark extract impedes foam cell formation and promotes apoptosis in ox-LDL-stimulated macrophages by enhancing UPR-CHOP pathway. Lipids Health Dis. 2019, 18, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piemontese, L. Plant Food Supplements with Antioxidant Properties for the Treatment of Chronic and Neurodegenerative Diseases: Benefits or Risks? J. Diet Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) No 212/2014 of 6 March 2014. Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of the Contaminant Citrinin in Food Supplements based on Rice Fermented with Red Yeast Monascus purpureus (Text with EEA relevance); European Union: Brussels, Belgium, 2014. [Google Scholar]
- Piemontese, L.; Perna, F.M.; Logrieco, A.; Capriati, V.; Solfrizzo, M. Deep eutectic solvents as novel and effective extraction media for quantitative determination of Ochratoxin A in wheat and derived products. Molecules 2017, 22, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncalvo, A.; Marinoni, L.; Dordoni, R.; Garrido, G.D.; Lavelli, V.; Spigno, G. Waste grape skins: Evaluation of safety aspects for the production of functional powders and extracts for the food sector. Food Addit. Contam. Part A Chem. Anal. Control. Exp. Risk Assess. 2016, 33, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, V.; Chaves, S.; Brunetti, L.; Loiodice, F.; Carrieri, A.; Laghezza, A.; Tortorella, P.; Magalhaes, J.D.; Cardoso, S.M.; Santos, M.A.; et al. Derivatives of Tenuazonic Acid as Potential New Multi-Target Anti-Alzheimer’s Disease Agents. Biomolecules 2021, 11, 111. [Google Scholar] [CrossRef]
| Source | Bioactive Compounds | Effects | Main Activities | Ref. |
|---|---|---|---|---|
| Cetraria islandica L. Ach | Furmarprotocetraric acid | Neuroprotective and antioxidant activities | Oxygen radical absorbance capacity (ORAC) 5.07 ± 0.43 μmol TE/mg | [20] |
| Spongionella sp. | Gracilin A, H, K, J, L, and tetrahydroaplysulphurin-1 | Neuroprotective activity | (Caspase 3 inh.) 3.88–4.04 × 103 RFU | [21] |
| Lepidium meyenii | N-(3-methoxybenzyl)oleamide, (N-(3-methoxybenzyl)linolenamide, N-(3-methoxybenzyl)linolenamide | Neuroprotective activity, peroxisome proliferator-activated receptor (PPAR) γ interaction, inhibition of fatty acid amide hydrolase (FAAH) | (PPARγ act., EC50) 20.4–22.6 μM | [22] |
| Aspergillus terreus Y10 | Asperteretal F, G1, G2, H and others | Inhibition of Tumor Necrosis Factor α (TNFα) | (TNFα inh., IC50) 7.6 9.9 μM | [23] |
| Sarcophyton glaucum | Sarcophytolide | Antimicrobic and cytoprotective activities | (MIC) 0.13–0.22 μg/mL | [24] |
| Hericius erinaceus and Hericius flagellum | Erinacine A, B, C, E, F, and others | Neurotrophic activity | (increased NGF expression) 0.8–12 μg/mL | [25] |
| Narcissus tazetta L. | (−)-9-O-methylpseudolycorine, (−)-narcissidine, (−)-pancratinine-C, (+)-9-O-demethyl-2-a-hydroxyhomolycorine | Inhibition of acetylcholine esterase AChE and butyrylcholine esterase (BChE) | (AChE inh, IC50) 0.67–32.51 μM | [26] |
| Embelia ribes and others | Embelin and others | Inhibition of AChE, BChE and Beta-secretase 1 (BACE-1); induction of P-glycoprotein 1 (P-gp) | (AChE inh, IC50) 2.50–6.98 μM | [27] |
| Rumex abyssinicus | Helminthosporin, emodin, chryso-phanol, physcion | Inhibition of AChE and BChE | (AChE inh, IC50) 2.63–33.7 μM | [28] |
| Oxalis corniculate L. | Flavonoids 1-9 | Inhibition of AChE, BChE and carbonic anhydrases II (CA-II) | (AChE inh, IC50) 49.52–109.55 μg/mL | [29] |
| Lichens | Atranorin, perlatolic acid, physodic acid, usnic acid and others | Neurotrophic activity and AChE inhibition | (AChE inh, IC50) 6.8–27.1 μM | [30] |
| Fungi and plants | Tenuazonic acid, epi-racidinol, mycophenolic acid, radicinin, visoltricin, 6-methoxymellein | Inhibition of AChE, BChE and Aβ-aggregation; antioxidant activity, metal chelation | (AChE inh, IC50) 6.86–11.4 μM | [31] |
| S. flavescens | (−)-maackian and others | Inhibition of monoamine oxidases (MAOs) | (MAO-B inh, IC50) 0.68–52.3 μM | [32] |
| Renealmia Alpinia | Desmethoxyangonin and others | Inhibition of MAOs | (MAO-B inh, Ki) 31–110 nM | [33] |
| Ginkgo biloba | Ginkgolic acid and anacardic acid | Decreased accumulation of α-synuclein (αSN) aggregates | (αSN aggr inh) 10–100 μM | [34] |
| Ampelopsis grossedentata | Dihydromyricetin | Neuroprotective activity and inhibition of αSN fibril formation | (αSN aggr inh) 50–100 μM | [35] |
| Source | Bioactive Compounds | Effects | Main Activities | Ref. |
|---|---|---|---|---|
| Acalypha fluticosa | 2-methyl-5,7-dihydroxychromone 5-O-b-d-glucopyranosid, acalyphin, apigenin, and Kaempferol 3-O-rutinoside and an acetylated derivate of chromone glucoside | Agonism of PPARα and PPARγ; anti-inflammatory properties | (PPARα act., FI) 1.16–2.25 at 50 μM | [70] |
| Talisia nervosa Radlk | (–)-catechin, methyl gallate, ethyl gallate, and β-d-glucopyranose,1,4,6-tris(3,4,5-trihydroxybenzoate) | Agonism of PPARα, PPARγ and liver X receptor (LXR); reduction of NO production | (PPARγ act., FI) 1.85–3.02 at 50 μM | [71] |
| Penicillium chrysogenum J08NF-4 | A new bile acid trifluoroacetate | Agonism of PPARγ, anti-inflammatory properties | (PPARγ act., FI) 2.0 at 50 μM | [72] |
| Cyanobium sp. LEGE 07,175 and Nodosilinea sp. LEGE 06001 | 132-hydroxy-pheopytin a and 132-hydroxy-pheofarnesin a | Increase of PPARγ mRNA expression | (Lipid-reducing Activity, EC50) 8.9–15.5 μM | [73] |
| Penicillium expansum Y32 | Communesin A, B, I, fumiquinazoline Q, protuboxepin A, B, E, and others | Mitigation of bradycardia, vasculo-genetic effect | (Acid sphingomyelinase mitigation) 20–100 μM | [58] |
| Lichens | Thirty-seven secondary metabolites and semisynthetic derivates | Anti-AGE activity, vasodilation | (Pentosidine-like AGEs formation, IC50) 0.08–0.70 mM | [74] |
| Schisandra chinensis | Acidic polysaccharide (SCAP) | Anti-diabetic and anti-apoptotic role | (H2O2-induced apoptosis inh) 15.6–62.5 μM | [75] |
| Sesbania grandiflora | Quercetin, kaempferol, vomifoliol, loliolide and others | Inhibition of α-amylase and α-glucosidase; antioxidant activity | (α-Glucosidase inh, IC50) 17.45–388.48 μM | [76] |
| Cassia bakeriana craib | Kaempeferol-3-O-rhamnoside and kaempferol | Inhibition of α-amylase and antioxidant activity | (α-Glucosidase inh, IC50) 0.36–0.61 mg/mL | [77] |
| Ocimum campechianum Mill. | Methyl rosmarinate, rosmarinic acid, 5-demethyl nobiletin, 5-demethyl sinensetin, luteolin | Inhibition of α-glucosidase, antihyperglycemic action | (α-Glucosidase inh) 12.86–82.77% at 0.75 mM | [78] |
| Aspergillus terreus MC751 | Butyrolactone I and II, three acetylated derivates of butyrolactone I | Inhibition of α-glucosidase, antioxidant activity | (α-Glucosidase inh, IC50) 52.17–175.18 μM | [79] |
| Ganoderma australe | Stella-steroid | Inhibition of α-glucosidase and Dipeptidyl peptidase 4 (DPP-4) | (α-Glucosidase inh, IC50) 314.54 μM | [80] |
| Aspergillus sydowii | Asperentin B | Inhibition of Protein-tyrosine phosphatase 1B (PTP1B) | (PTP1B inh, IC50) 2.05 μM | [81] |
| Moringa oleifera | Two sulfur-contained compounds | Anti-adipogenic activity | (Lipid accumulation, inh, IC50) 29.6 μM | [82] |
| Allium sativum L. | Three eugenol diglycosides and three β-carboline alkaloids | Inhibition of adipogenesis and lipid accumulation | (Lipid accumulation, inh) active at 20 μM | [83] |
| Curcuma amada | Two natural labdane diterpenes and one drimane sesquiterpene | Inhibition of lipase and α-glucosidase | (Lipase inh, IC50) 6.1–665.9 μM | [84] |
| Magnolia spp. | Honokiol | Inhibition of Histone deacetylase (HDAC)- mediated cystathionine γ-lyase degradation | (HDAC6 inh) active at 5 μM | [85] |
| Panax spp. (Ginseng) | Ginsenoside K | Promotion of macrophage and foam cell apoptosis | (Reduction of foam cell formation) 1.25 μg/mL | [86] |
| Unspecified | Lupeol | Promotion of macrophage development into the M2 anti-inflammatory phenotype | (Proinflammatory cytokine secretion, inh) active at 50 μM | [87] |
| Tripterygium Wilfordi | Cerastrol | Action as leptin sensitizer | (Leptin sensitization) active at 150 μg/kg | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases. Foods 2021, 10, 29. https://doi.org/10.3390/foods10010029
Leuci R, Brunetti L, Poliseno V, Laghezza A, Loiodice F, Tortorella P, Piemontese L. Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases. Foods. 2021; 10(1):29. https://doi.org/10.3390/foods10010029
Chicago/Turabian StyleLeuci, Rosalba, Leonardo Brunetti, Viviana Poliseno, Antonio Laghezza, Fulvio Loiodice, Paolo Tortorella, and Luca Piemontese. 2021. "Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases" Foods 10, no. 1: 29. https://doi.org/10.3390/foods10010029