Simulation and Non-Invasive Testing of Vinegar Storage Time by Olfaction Visualization System and Volatile Organic Compounds Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
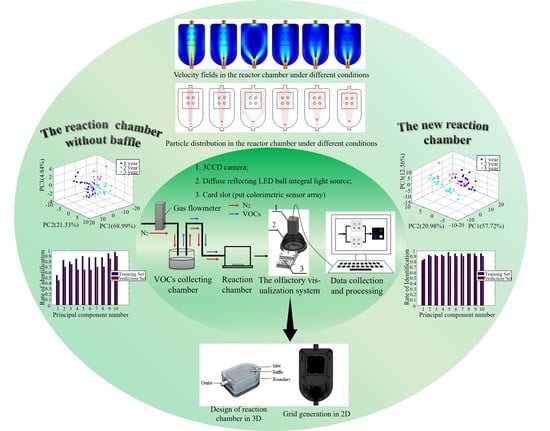
2.2. Olfactory Visualization System
2.3. Design of Reaction Chamber
2.4. Classification of Vinegar Samples
3. Results and Discussion
3.1. Simulation Results of COMSOL Software
3.1.1. The Effect of Baffle Curvature on the Gas Distribution in the Reaction Chamber
3.1.2. The Effect of the Position of the Baffle on the Gas Distribution in the Reaction Chamber
3.1.3. The Effect of Reaction Chambers with a Baffle and without a Baffle
3.1.4. Data Analysis of Experiment
3.2. The Determination Results and Analysis of Vinegar Storage
3.2.1. Principal Component Analysis (PCA) under Two Types of Reaction Chamber
3.2.2. Linear Discriminant Analysis (LDA) under Two Types of Reaction Chamber
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tesfaye, W.; Morales, M.L.; Garciaparrilla, M.C.; Troncoso, A.M. Technology, Wine vinegar: Technology, authenticity and quality evaluation. Trends Food Sci. Technol. 2002, 13, 12–21. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, J.; Wang, L.L.; Li, Z.G. Development of a SPME-GC-MS method for the determination of volatile compounds in Shanxi aged vinegar and its analytical characterization by aroma wheel. J. Food Sci. Technol. Mysore 2016, 53, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Fang, G.-Y.; Chai, L.-J.; Zhong, X.-Z.; Jiang, Y.-J. Deciphering the succession patterns of bacterial community and their correlations with environmental factors and flavor compounds during the fermentation of Zhejiang rosy vinegar. Int. J. Food Microbiol. 2021, 341, 109070. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-L.; Zheng, F.-J.; Lin, B.; Lao, S.-B.; He, J.; Huang, Z.; Zeng, Y.; Sun, J.; Verma, K.K. Phenolic and Volatile Compounds in the Production of Sugarcane Vinegar. ACS Omega 2020, 5, 30587–30595. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Xie, J.C.; Hou, L.; Zhao, M.Y.; Zhao, J.; Cheng, J.; Wang, S.; Sun, B.G. Aroma Constituents in Shanxi Aged Vinegar before and after Aging. J. Agric. Food Chem. 2016, 64, 7597–7605. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.L.; Silva, P.; Câmara, J.S. Impact of storage time and temperature on volatomic signature of Tinta Negra wines by LLME/GC-ITMS. Food Res. Int. 2018, 109, 99–111. [Google Scholar] [CrossRef]
- Bhandari, M.P.; Carmona, E.N.; Abbatangelo, M.; Sberveglieri, V.; Duina, G.; Malla, R.; Comini, E.; Sberveglieri, G.J.P. Discrimination of Quality and Geographical Origin of Extra Virgin Olive Oil by S3 Device with Metal Oxides Gas Sensors. Proceedings 2018, 2, 1061. [Google Scholar] [CrossRef] [Green Version]
- Rakow, N.A.; Suslick, K.S. A colorimetric sensor array for odour visualization. Nature 2000, 406, 710. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Yao, L.Y.; Han, F.K.; Guan, C. Rapid Non-destructive Testing for K Values of Silver Carps Based on the Olfactory Visualization Technique. Mod. Food Sci. Technol. 2014, 30, 233–237. [Google Scholar]
- Su-Yeon Kim, B.-S.K. A colorimetric sensor array-based classification of coffees. Sens. Actuators B Chem. 2018, 275, 277–283. [Google Scholar]
- Lin, H.; Yan, S.; Song, B.T.; Wang, Z.; Sun, L. Discrimination of aged rice using colorimetric sensor array combined with volatile organic compounds. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Narimani, K.; Nayeri, F.D.; Kolahdouz, M.; Ebrahimi, P. Fabrication, modeling and simulation of high sensitivity capacitive humidity sensors based on ZnO nanorods. Sens. Actuators B Chem. 2016, 224, 338–343. [Google Scholar] [CrossRef]
- Bogner, A.; Steiner, C.; Walter, S.; Kita, J.; Hagen, G.; Moos, R. Planar Microstrip Ring Resonators for Microwave-Based Gas Sensing: Design Aspects and Initial Transducers for Humidity and Ammonia Sensing. Sensors 2017, 17, 2422. [Google Scholar] [CrossRef]
- Guo, R.; Tang, W.; Shen, C.T.; Wang, X. High sensitivity and fast response graphene oxide capacitive humidity sensor with computer-aided design. Comput. Mater. Sci. 2016, 111, 289–293. [Google Scholar] [CrossRef]
- Rajavelu, M.; Sivakumar, D.; Daniel, R.J.; Sumangala, K. Perforated diaphragms employed piezoresistive MEMS pressure sensor for sensitivity enhancement in gas flow measurement. Flow Meas. Instrum. 2014, 35, 63–75. [Google Scholar] [CrossRef]
- Asmi, A.; Putra, J.C.P.; Rahman, I.A. Materials, Simulation of Room Airflow Using Comsol Multiphysics Software. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2014; pp. 571–577. [Google Scholar]
- Pásková, J.; Munk, V. A combined detecting reagent for the identification of organic acids on paper chromatograms. J. Chromatogr. A 1960, 4, 241–243. [Google Scholar] [CrossRef]
- Guan, B.B.; Zhao, J.W.; Cai, M.J.; Lin, H.; Yao, L.Y.; Sun, L.L. Analysis of volatile organic compounds from Chinese vinegar substrate during solid-state fermentation using a colorimetric sensor array. Anal. Methods 2014, 6, 9383–9391. [Google Scholar] [CrossRef]
- Joy, S.; Antony, J.K. Design Simulation a Micro Hotplate Using COMSOL Multiphysics for MEMS Based Gas Sensor. In Proceedings of the 2015 Fifth International Conference on Advances in Computing and Communications (ICACC), Kochi, India, 2–4 September 2015; pp. 465–468. [Google Scholar]
- Britz, D.; Chandra, S.; Strutwolf, J.; Wong, D.K. Diffusion-limited chronoamperometry at conical-tip microelectrodes. Electrochim. Acta 2010, 55, 1272–1277. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; LaGasse, M.K.; Suslick, K.S. Rapid Quantification of Trimethylamine. Anal. Chem. 2016, 88, 5615–5620. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Mu, N.; Chen, L.; Zhang, X.L. Hierarchical salient object detection model using contrast-based saliency and color spatial distribution. Multimed. Tools Appl. 2016, 75, 2667–2679. [Google Scholar] [CrossRef]
- Guan, B.B.; Zhao, J.W.; Jin, H.J.; Lin, H. Determination of Rice Storage Time with Colorimetric Sensor Array. Food Anal. Methods 2017, 10, 1054–1062. [Google Scholar] [CrossRef]
- Bhandari, M.P.; Carmona, E.N.; Galstyan, V.; Sberveglieri, V. Quality Evaluation of Parmigiano Reggiano Cheese by a Novel Nanowire Device S3 and Evaluation of the VOCs Profile. Procedia Eng. 2016, 168, 460–464. [Google Scholar] [CrossRef]
- De Luca, M.; Restuccia, D.; Clodoveo, M.L.; Puoci, F.; Ragno, G. Chemometric analysis for discrimination of extra virgin olive oils from whole and stoned olive pastes. Food Chem. 2016, 202, 432–437. [Google Scholar] [CrossRef]
- Gilbert, N.; Mewis, R.E.; Sutcliffe, O.B. Classification of fentanyl analogues through principal component analysis (PCA) and hierarchical clustering of GC-MS data. Forensic Chem. 2020, 21, 100287. [Google Scholar] [CrossRef]
- Banerjee, R.; Tudu, B.; Shaw, L.; Jana, A.; Bhattacharyya, N.; Bandyopadhyay, R. Instrumental testing of tea by combining the responses of electronic nose and tongue. J. Food Eng. 2012, 110, 356–363. [Google Scholar] [CrossRef]





| Sensor | Velocity (m/s) ± SD | |||||
|---|---|---|---|---|---|---|
| Large-Curvature | Small-Curvature | Close to Inlet | Far from Inlet | Optimization | Without Baffles | |
| 1 | 0.177 ± 0.03 | 0.029 ± 0.00 | 0.133 ± 0.02 | 0.102 ± 0.01 | 0.108 ± 0.01 | 0.214 ± 0.05 |
| 2 | 0.170 ± 0.03 | 0.031 ± 0.00 | 0.127 ± 0.02 | 0.098 ± 0.01 | 0.107 ± 0.01 | 0.201 ± 0.04 |
| 3 | 0.201 ± 0.04 | 0.029 ± 0.00 | 0.151 ± 0.02 | 0.121 ± 0.01 | 0.125 ± 0.02 | 0.239 ± 0.06 |
| 4 | 0.192 ± 0.04 | 0.030 ± 0.00 | 0.144 ± 0.02 | 0.116 ± 0.01 | 0.122 ± 0.01 | 0.219 ± 0.05 |
| Mean ± SD | 0.185 ± 0.035 | 0.030 ± 0.001 | 0.139 ± 0.019 | 0.109 ± 0.012 | 0.116 ± 0.013 | 0.218 ± 0.049 |
| PCs | New Reaction Chamber | The Free Gas Volatile Reaction Chamber | ||
|---|---|---|---|---|
| The Training Set | The Prediction Set | The Training Set | The Prediction Set | |
| 1 | 82.5 | 85 | 55 | 45 |
| 2 | 92.5 | 90 | 82.5 | 70 |
| 3 | 92.5 | 90 | 80 | 75 |
| 4 | 92.5 | 90 | 85 | 65 |
| 5 | 97.5 | 90 | 90 | 65 |
| 6 | 95 | 90 | 87.5 | 65 |
| 7 | 95 | 90 | 87.5 | 70 |
| 8 | 95 | 95 | 87.5 | 70 |
| 9 | 95 | 90 | 95 | 85 |
| 10 | 95 | 90 | 97.5 | 90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Lin, J.; Song, B.; Chen, Q. Simulation and Non-Invasive Testing of Vinegar Storage Time by Olfaction Visualization System and Volatile Organic Compounds Analysis. Foods 2021, 10, 532. https://doi.org/10.3390/foods10030532
Lin H, Lin J, Song B, Chen Q. Simulation and Non-Invasive Testing of Vinegar Storage Time by Olfaction Visualization System and Volatile Organic Compounds Analysis. Foods. 2021; 10(3):532. https://doi.org/10.3390/foods10030532
Chicago/Turabian StyleLin, Hao, Jinjin Lin, Benteng Song, and Quansheng Chen. 2021. "Simulation and Non-Invasive Testing of Vinegar Storage Time by Olfaction Visualization System and Volatile Organic Compounds Analysis" Foods 10, no. 3: 532. https://doi.org/10.3390/foods10030532