Optimal Dosing Regimen of Osteoporosis Drugs in Relation to Food Intake as the Key for the Enhancement of the Treatment Effectiveness—A Concise Literature Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
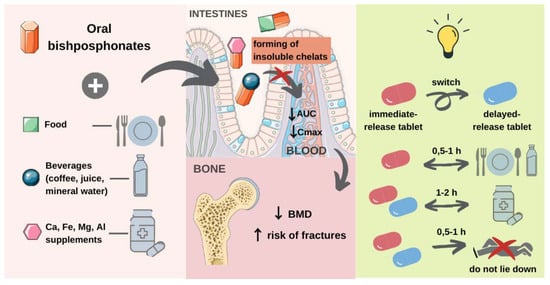
3.1. Oral Bisphosphonates
3.1.1. Chemical Characterization of the Bisphosphonates
3.1.2. Interaction with Di- and Trivalent Cations
3.1.3. Problem with Water Type
3.1.4. Alendronate
3.1.5. Risedronate
Immediate-Release (IR) Tablets
Delayed-Release (DR) Tablets
3.1.6. Ibandronate
3.1.7. Minodronate
3.1.8. Etidronate
3.2. Selective Estrogen Receptor Modulators (SERMs)
3.2.1. Raloxifene
3.2.2. Bazedoxifene
3.3. Recomendations
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cramer, J.A.; Roy, A.; Burrell, A.; Fairchild, C.J.; Fuldeore, M.J.; Ollendorf, D.A.; Wong, P.K. Medication compliance and persistence: Terminology and definitions. Value Health 2008, 11, 44–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Jung, K.J.; Nho, J.H.; Kim, J.H.; Won, S.H.; Chun, D.I.; Byun, D.W. Impact on bisphosphonate persistence and compliance: Daily postprandial administration. J. Bone Metab. 2019, 26, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Donato, P.; Pepe, J.; Colangelo, L.; Danese, V.; Cecchetti, V.; Minisola, S.; Cipriani, C. Adherence to bisphosphonates in the general population: A study in patients referred to a primary care service. Arch. Osteoporos. 2019, 14, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Kothawala, P.; Badamgarav, E.; Ryu, S.; Miller, R.M.; Halbert, R.J. Systematic Review and Meta-analysis of Real-World Adherence to Drug Therapy for Osteoporosis. Mayo Clin. Proc. 2007, 82, 1493–1501. [Google Scholar] [CrossRef]
- Yeam, C.T.; Chia, S.; Tan, H.C.C.; Kwan, Y.H.; Fong, W.; Seng, J.J.B. A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos. Int. 2018, 29, 2623–2637. [Google Scholar] [CrossRef]
- Cornelissen, D.; de Kunder, S.; Si, L.; Reginster, J.Y.; Evers, S.; Boonen, A.; Hiligsmann, M. Interventions to improve adherence to anti-osteoporosis medications: An updated systematic review. Osteoporos. Int. 2020, 31, 1645–1669. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, A.; Gajewska, D.; Paśko, P. Levothyroxine Interactions with Food and Dietary Supplements—A Systematic Review. Pharmaceuticals 2021, 14, 206. [Google Scholar] [CrossRef]
- Wilcock, M.; Pothecary, A.; Roberson, C. What do we know about the administration of oral bisphosphonates in a hospital setting? Pharmacoepidemiol. Drug Saf. 2018, 27, 3–14. [Google Scholar] [CrossRef]
- Paśko, P. Food-drug interactions among the elderly: Risk assessment and recommendations for patients. Encycl. Biomed. Gerontol. 2019, 107–114. [Google Scholar] [CrossRef]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological management of osteoporosis in postmenopausal women: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Health and Care Excellence (NICE). Bisphosphonates for Treating Osteoporosis; National Institute for Health and Care Excellence (NICE): London, UK, 2017; pp. 1–25. [Google Scholar]
- Ogura, Y.; Gonsho, A.; Cyong, J.C.; Orimo, H. Clinical trial of risedronate in Japanese volunteers: A study on the effects of timing of dosing on absorption. J. Bone Miner. Metab. 2004, 22, 120–126. [Google Scholar] [CrossRef]
- Qaseem, A.; Forciea, M.A.; McLean, R.M.; Denberg, T.D. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann. Intern. Med. 2017, 166, 818. [Google Scholar] [CrossRef] [PubMed]
- Pazianas, M.; Abrahamsen, B.; Ferrari, S.; Russell, R.G.G. Eliminating the need for fasting with oral administration of bisphosphonates. Ther. Clin. Risk Manag. 2013, 9, 395–402. [Google Scholar] [CrossRef] [Green Version]
- Byun, J.-H.; Jang, S.; Lee, S.; Park, S.; Yoon, H.K.; Yoon, B.-H.; Ha, Y.-C. The Efficacy of Bisphosphonates for Prevention of Osteoporotic Fracture: An Update Meta-analysis. J. Bone Metab. 2017, 24, 37. [Google Scholar] [CrossRef] [Green Version]
- Kinov, P.; Boyanov, M. Clinical utility of risedronate in postmenopausal osteoporosis: Patient considerations with delayed-release formulation. Int. J. Womens Health 2012, 4, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, A.P.; do Espírito Santo, R.F.; Line, S.R.P.; Pinto, M.d.G.F.; de Moura Santos, P.; Toralles, M.B.P.; do Espírito Santo, A.R. Bisphosphonates: Pharmacokinetics, bioavailability, mechanisms of action, clinical applications in children, and effects on tooth development. Environ. Toxicol. Pharmacol. 2016, 42, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wong, H. Food effects on oral drug absorption: Application of physiologically-based pharmacokinetic modeling as a predictive tool. Pharmaceutics 2020, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, J.; Betlejewska-Kielak, K.; Knłosíska-Szmurło, E.; Pluciński, F.A.; Mazurek, A.P. Prediction of bioavailability of selected bisphosphonates using in silico methods towards categorization into biopharmaceutical classification system. Acta Pol. Pharm. Drug Res. 2013, 70, 877–882. [Google Scholar]
- Reid, I.R.; Bolland, M.J. Calcium and/or vitamin D supplementation for the prevention of fragility fractures: Who needs it? Nutrients 2020, 12, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, N.C.; Biver, E.; Kaufman, J.M.; Bauer, J.; Branco, J.; Brandi, M.L.; Bruyère, O.; Coxam, V.; Cruz-Jentoft, A.; Czerwinski, E.; et al. The role of calcium supplementation in healthy musculoskeletal ageing: An expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundat. Osteoporos. Int. 2017, 28, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Gertz, B.J.; Holland, S.D.; Kline, W.F.; Matuszewski, B.K.; Freeman, A.; Quan, H.; Lasseter, K.C.; Mucklow, J.C.; Porras, A.G. Studies of the oral bioavailability of alendronate. Clin. Pharmacol. Ther. 1995, 58, 288–298. [Google Scholar] [CrossRef]
- Fosamax (Merck & Co., Inc.). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021575s017lbl.pdf (accessed on 5 February 2021).
- Actonel (Procter & Gamble Pharmaceuticals, Inc.). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020835s035lbl.pdf (accessed on 5 February 2021).
- Atelvia (Warner Chilcott (US), LLC). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022560s007lbl.pdf (accessed on 5 February 2021).
- Bonviva (Roche Therapeutics Inc.). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021455s009lbl.pdf (accessed on 6 February 2021).
- Didronel (Procter & Gamble Pharmaceuticals, Inc.). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/017831s055lbl.pdf (accessed on 6 February 2021).
- Ferracru (Norgine B.V.). Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/feraccru-epar-product-information_en.pdf (accessed on 6 February 2021).
- Ringe, J.D.; Van Der Geest, S.A.P.; Möller, G. Importance of calcium co-medication in bisphosphonate therapy of osteoporosis: An approach to improving correct intake and drug adherence. Drugs Aging 2006, 23, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Sakaue, T.; Yoneyama, E.; Aoyama, T. Influence of mineral water on absorption of oral alendronate in rats. Yakugaku Zasshi 2011, 131, 801–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, A.; Akagi, Y.; Shimomura, H.; Aoyama, T. Interaction between bisphosphonates and mineral water: Study of oral risedronate absorption in rats. Biol. Pharm. Bull. 2016, 39, 323–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, R. Which water for alendronate administration? Osteoporos. Int. 2009, 20, 1451. [Google Scholar] [CrossRef] [Green Version]
- Morr, S.; Cuartas, E.; Alwattar, B.; Lane, J.M. How much calcium is in your drinking water? A survey of calcium concentrations in bottled and tap water and their significance for medical treatment and drug administration. HSS J. 2006, 2, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Azoulay, A.; Garzon, P.; Eisenberg, M.J. Comparison of the mineral content of tap water and bottled waters. J. Gen. Intern. Med. 2001, 16, 168–175. [Google Scholar] [CrossRef]
- Binosto (Mission Pharmacal Company). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202344s011lbl.pdf (accessed on 7 February 2021).
- Imai, K. Alendronate sodium hydrate (oral jelly) for the treatment of osteoporosis: Review of a novel, easy to swallow formulation. Clin. Interv. Aging 2013, 8, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Gertz, B.J.; Holland, S.D.; Kline, W.F.; Matuszewski, B.K.; Porras, A.G. Clinical pharmacology of alendronate sodium. Osteoporos. Int. 1993, 3, 13–16. [Google Scholar] [CrossRef]
- Wagener, P. Safety and efficacy of alendronate when not taken in the morning. Results of an 8-week prospective study. Eur. J. Geriatr. 2000, 2, 35–37. [Google Scholar]
- Mitchell, D.Y.; Heise, M.A.; Pallone, K.A.; Clay, M.E.; Nesbitt, J.D.; Russell, D.A.; Melson, C.W. The effect of dosing regimen on the pharmacokinetics of risedronate. Br. J. Clin. Pharmacol. 1999, 48, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; He, X.; Li, H.; Ni, Y.; Xu, M.; Sattar, H.; Chen, H.; Li, W. Pharmacokinetics and Tolerability of Minodronic Acid Tablets in Healthy Chinese Subjects and Food and Age Effects on the Pharmacokinetics. Clin. Ther. 2015, 37, 869–876. [Google Scholar] [CrossRef]
- Kendler, D.L.; Ringe, J.D.; Ste-Marie, L.G.; Vrijens, B.; Taylor, E.B.; Delmas, P.D. Risedronate dosing before breakfast compared with dosing later in the day in women with postmenopausal osteoporosis. Osteoporos. Int. 2009, 20, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Krueger, D.C.; Engelke, J.A.; Nest, L.J.; Krause, P.F.; Drinka, P.J.; Binkley, N.C. Between-meal risedronate does not alter bone turnover in nursing home residents. J. Am. Geriatr. Soc. 2006, 54, 790–795. [Google Scholar] [CrossRef]
- Fukase, H.; Tanaka, S.; Ohishi, H.; Oikawa, I.; Furuie, H. Phase I clinical trial of NE-58095 DR, a risedronate delayed-release tablet: Dose escalation and food effect. Jpn. J. Clin. Pharmacol. Ther. 2018, 49, 113–124. [Google Scholar] [CrossRef]
- McClung, M.R.; Miller, P.D.; Brown, J.P.; Zanchetta, J.; Bolognese, M.A.; Benhamou, C.L.; Balske, A.; Burgio, D.E.; Sarley, J.; McCullough, L.K.; et al. Efficacy and safety of a novel delayed-release risedronate 35 mg once-a-week tablet. Osteoporos. Int. 2012, 23, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClung, M.R.; Balske, A.; Burgio, D.E.; Wenderoth, D.; Recker, R.R. Treatment of postmenopausal osteoporosis with delayed-release risedronate 35 mg weekly for 2 years. Osteoporos. Int. 2013, 24, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Soen, S.; Kishimoto, H.; Hagino, H.; Sone, T.; Ohishi, H.; Fujimoto, T.; Sasaki, E.; Tanaka, S.; Sugimoto, T. Phase II/III, randomized, double-blind, parallel-group study of monthly delayed-release versus daily immediate-release risedronate tablets in Japanese patients with involutional osteoporosis. J. Bone Miner. Metab. 2020, 38, 86–98. [Google Scholar] [CrossRef]
- Bondronat (Roche Therapeutic Inc.). Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/bondronat-epar-product-information_en.pdf (accessed on 7 February 2021).
- Nakai, K. The effect of fasting interval on oral bioavailability of ibandronate. Jpn. Pharmacol. Ther. 2015, 43, 1553–1560. [Google Scholar]
- Tankó, L.B.; McClung, M.R.; Schimmer, R.C.; Mahoney, P.; Christiansen, C. The efficacy of 48-week oral ibandronate treatment in postmenopausal osteoporosis when taken 30 versus 60 min before breakfast. Bone 2003, 32, 421–426. [Google Scholar] [CrossRef]
- Ohishi, T.; Matsuyama, Y. Minodronate for the treatment of osteoporosis. Ther. Clin. Risk Manag. 2018, 14, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, T.; Endo, I. Minodronate. Bone 2020, 137, 115432. [Google Scholar] [CrossRef]
- Papaioannou, A.; Morin, S.; Cheung, A.M.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hanley, D.A.; Hodsman, A.; Jamal, S.A.; Kaiser, S.M.; et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. CMAJ 2010, 182, 1864–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogelman, I.; Smith, L.; Mazesss, R.; Wilson, M.A.; Bevan, J.A. Absorption of Oral Diphosphonate in Normal Subjects. Clin. Endocrinol. 1986, 24, 57–62. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Blake, G.M.; Fogelman, I. The time of day that Etidronate is ingested does not influence its therapeutic effect in osteoporosis. Scand. J. Rheumatol. 2000, 29, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.J. Timing of cyclical etidronate. Clin. Exp. Rheumatol. 2000, 18, 609–612. [Google Scholar] [PubMed]
- Rosen, H. Selective Estrogen Receptor Modulators for Prevention and Treatment of Osteoporosis. Available online: https://www.uptodate.com/contents/selective-estrogen-receptor-modulators-for-prevention-and-treatment-of-osteoporosis (accessed on 23 January 2021).
- Whitaker Elam, R. What Is the Role of Raloxifene (Evista) in the Treatment of Osteoporosis? Available online: https://www.medscape.com/answers/330598-83093/what-is-the-role-of-raloxifene-evista-in-the-treatment-of-osteoporosis#:~:text=Raloxifene-may-be-most-useful-the-endometrium-was-not-stimulated (accessed on 23 January 2021).
- Jha, R.K.; Tiwari, S.; Mishra, B. Bioadhesive microspheres for bioavailability enhancement of raloxifene hydrochloride: Formulation and pharmacokinetic evaluation. AAPS PharmSciTech 2011, 12, 650–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morello, K.C.; Wurz, G.T.; DeGregorio, M.W. Pharmacokinetics of Selective Estrogen Receptor Modulators. Clin. Pharmacokinet. 2003, 42, 361–372. [Google Scholar] [CrossRef]
- Evista (SUBSTIPHARM). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/022042lbl.pdf (accessed on 9 February 2021).
- Conbriza (Pfizer Europe). Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/conbriza-epar-product-information_en.pdf (accessed on 9 February 2021).
- McKeand, W. Pharmacokinetics, Dose Proportionality, and Bioavailability of Bazedoxifene in Healthy Postmenopausal Women. Clin. Ther. 2017, 39, 1769–1779. [Google Scholar] [CrossRef]
- Duavee (Pfeizer Inc.). Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022247s002lbl.pdf (accessed on 9 February 2021).



| Type of Meal | Food Items | Nutrition Facts | Energy | References |
|---|---|---|---|---|
| Alendronate | ||||
| Breakfast Set No 1 | 2 pieces of white toast, jelly/marmalade (20 g), 1 fried egg, 2 strips of bacon, and orange juice (250 mL) | 70.6 g of carbohydrates, 6.3 g of fat, and 22.4 g of protein | Approx. 350 kcal | [22] |
| Breakfast Set No 2 | 1 piece of white toast, 2 pats of butter, 2 strips of bacon, 2 fried eggs, hash brown potatoes (2–4 oz), and whole milk (240 mL). | 40.3 g of carbohydrates, 29 g of fat, and 89.4 g of protein | Approx. 665 kcal | [22] |
| Risedronate | ||||
| Lunch Set No 3 | Smoked turkey, vegetable and beef soup with crackers, and whole wheat bread with lettuce (283 g), tossed salad (142 g) with light salad dressing (12 g), mayonnaise (15 mL), 2 canned peach halves, and skimmed milk (283 g) | 104 g of carbohydrates, 19 g of fat, and 38 g of protein | Approx. 716 kcal | [39] |
| Breakfast Set No 4 | 2 slices of white toast, 2 pats of butter, 2 slices of bacon, hash brown potatoes (57 g), 2 eggs fried in butter, and whole milk (226 g) | 50 g of carbohydrates, 46 g of fat, and 30 g of protein | Approx. 730 kcal | [39] |
| Dinner Set No 5 | baked chicken breast (113 g), 1 baked potato, light gravy (28 g), 1 pat of margarine, 0.5 cup of apple sauce, 0.5 cup of carrot rounds, 1 peanut butter cookie, and lemonade (283 g) | 103 g of carbohydrates, 16 g of fat, and 40 g of protein | Approx. 700 kcal | [39] |
| Minodronate | ||||
| Breakfast Set No 6 | chicken drumstick, fried egg, hamburger, and milk | 30% of carbohydrates, 55% of fat, 15% of protein | Approx. 900 kcal | [40] |
| Drug | Available Oral Formulations | Dosing Frequency | Recommendations with Regard to Food | Other Recommendations | References |
|---|---|---|---|---|---|
| Alendronate | tablets effervescent tablets oral solution jelly | daily or weekly | should be taken at least 30 min before breakfast should be taken at least 30 min before vitamins, mineral supplements, or antacids high in calcium, aluminum, or magnesium should be taken at least 2 h before iron preparations swallowing with coffee and juice should be avoided | tablets should be swallowed with a full glass of tap water effervescent tablets should be dissolved in a half a glass of tap water the oral solution should be taken with at least a quarter a glass of tap water jelly can be administered without water should be taken while standing or sitting in an upright position lying down for at least 30 min after administration should be avoided | [2,22,23,35,37,38] |
| Risedronate | immediate-release (IR) tablets | daily, weekly or monthly | should be taken at least 30 min before breakfast the flexible-dosing approach might be considered calcium, magnesium, and aluminum preparations should be administered at a different time of the day should be taken at least 2 h before iron preparations | should be swallowed with a full glass of tap water should be taken while standing or sitting in an upright position lying down for at least 30 min after administration should be avoided | [12,24,39,41,42] |
| delayed-release (DR) tablets | Weekly | should be taken immediately following breakfast administration at bedtime might be considered calcium, magnesium, and aluminum preparations should be administered at a different time of the day should be taken at least 2 h before iron preparations | should be swallowed with at least half a glass of tap water should be taken while standing or sitting in an upright position lying down for at least 30 min after administration should be avoided | [14,25,43,44,45,46] | |
| Ibandronate | tablets | Monthly | should be taken at least 1 h before breakfast should be taken at least 1 h before vitamins, mineral supplements, or antacids high in calcium, aluminum, or magnesium should be taken at least 2 h before iron preparations | should be swallowed with a full glass of tap water should be taken while standing or sitting in an upright position lying down for at least 60 min after administration should be avoided | [26,47,48,49] |
| Minodronate | tablets | Monthly | should be taken at least 30 min before breakfast | should be swallowed with a tap water should be taken while standing or sitting in an upright position lying down for at least 30 min after administration should be avoided | [40] |
| Etidronate | tablets | daily for 14 days, in 90-days cycles | should be taken at least 2 h before food (especially high-calcium products), early in the morning, or at bedtime should be taken at least 2 h before vitamins, mineral supplements, or antacids high in calcium, magnesium, aluminum, iron | should be swallowed with a full glass of tap water should be taken while standing or sitting in an upright position lying down immediately after administration should be avoided | [53,54,55] |
| Raloxifene | tablets | Daily | can be taken irrespectively of food | [60] | |
| Bazedoxifene | tablets | Daily | can be taken irrespectively of food | [61,62,63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiesner, A.; Szuta, M.; Galanty, A.; Paśko, P. Optimal Dosing Regimen of Osteoporosis Drugs in Relation to Food Intake as the Key for the Enhancement of the Treatment Effectiveness—A Concise Literature Review. Foods 2021, 10, 720. https://doi.org/10.3390/foods10040720
Wiesner A, Szuta M, Galanty A, Paśko P. Optimal Dosing Regimen of Osteoporosis Drugs in Relation to Food Intake as the Key for the Enhancement of the Treatment Effectiveness—A Concise Literature Review. Foods. 2021; 10(4):720. https://doi.org/10.3390/foods10040720
Chicago/Turabian StyleWiesner, Agnieszka, Mariusz Szuta, Agnieszka Galanty, and Paweł Paśko. 2021. "Optimal Dosing Regimen of Osteoporosis Drugs in Relation to Food Intake as the Key for the Enhancement of the Treatment Effectiveness—A Concise Literature Review" Foods 10, no. 4: 720. https://doi.org/10.3390/foods10040720