Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications
Abstract
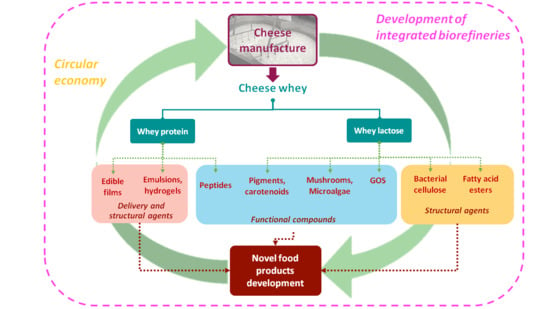
:1. Introduction
2. Bioprocess Development Using Whey Lactose
2.1. Enzymatic Bioprocesses
2.1.1. Galacto-Oligosaccharides
2.1.2. Lactose Fatty Acid Esters
2.2. Microbial Bioprocesses
2.2.1. Food Biocolorants and Aroma Compounds
2.2.2. Bacterial Cellulose
2.2.3. Functional Food Additives
3. Whey Proteins: Research Insights and Trends
3.1. Edible Films and Coatings
3.1.1. Recent Strategies for Improved Technical and Functional Properties
3.1.2. Delivery Agents of Bioactive Compounds
3.2. Whey Protein Hydrogels
3.2.1. Formulations and Structural Characteristics
3.2.2. Emerging Techniques for Food Applications
3.3. Whey Protein as a Source of Nutraceuticals
3.3.1. Nutritional Aspects of Whey Proteins
3.3.2. Generation of Bioactive Peptides
4. Current Integrated Biorefineries
5. Innovative Refining Processes of Cheese Whey and Future Perspectives in Food Applications
Author Contributions
Funding
Conflicts of Interest
References
- Lievore, P.; Simões, D.R.S.; Silva, K.M.; Drunkler, N.L.; Barana, A.C.; Nogueira, A.; Demiate, I.M. Chemical characterisation and application of acid whey in fermented milk. J. Food Sci. Technol. 2015, 52, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Masotti, F.; Cattaneo, S.; Stuknytė, M.; De Noni, I. Technological tools to include whey proteins in cheese: Current status and perspectives. Trends Food Sci. Technol. 2017, 64, 102–114. [Google Scholar] [CrossRef]
- Fernández-Gutiérrez, D.; Veillette, M.; Giroir-Fendler, A.; Ramirez, A.A.; Faucheux, N.; Heitz, M. Biovalorization of saccharides derived from industrial wastes such as whey: A review. Rev. Environ. Sci. BioTechnol. 2017, 16, 147–174. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445-446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef] [Green Version]
- Domingos, J.M.B.; Puccio, S.; Martinez, G.A.; Amaral, N.; Reis, M.A.M.; Bandini, S.; Fava, F.; Bertin, L. Cheese whey integrated valorisation: Production, concentration and exploitation of carboxylic acids for the production of polyhydroxyalkanoates by a fed-batch culture. Chem. Eng. J. 2018, 336, 47–53. [Google Scholar] [CrossRef]
- Valta, K.; Damala, P.; Angeli, E.; Antonopoulou, G.; Malamis, D.; Haralambous, K.J. Current Treatment Technologies of Cheese Whey and Wastewater by Greek Cheese Manufacturing Units and Potential Valorisation Opportunities. Waste Biomass Valorization 2017, 8, 1649–1663. [Google Scholar] [CrossRef]
- Remón, J.; Ruiz, J.; Oliva, M.; García, L.; Arauzo, J. Cheese whey valorisation: Production of valuable gaseous and liquid chemicals from lactose by aqueous phase reforming. Energy Convers. Manag. 2016, 124, 453–469. [Google Scholar] [CrossRef] [Green Version]
- Vasala, A.; Panula, J.; Neubauer, P. Efficient lactic acid production from high salt containing dairy by-products by Lactobacillus salivarius ssp. salicinius with pre-treatment by proteolytic microorganisms. J. Biotechnol. 2005, 117, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Banaszewska, A.; Cruijssen, F.; Claassen, G.D.H.; van der Vorst, J.G.A.J. Effect and key factors of byproducts valorization: The case of dairy industry. J. Dairy Sci. 2014, 97, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Panghal, A.; Patidar, R.; Jaglan, S.; Chhikara, N.; Khatkar, S.K.; Gat, Y.; Sindhu, N. Whey valorization: Current options and future scenario—A critical review. Nutr. Food Sci. 2018, 48, 520–535. [Google Scholar] [CrossRef]
- Corrochano, A.R.; Buckin, V.; Kelly, P.M.; Giblin, L. Invited review: Whey proteins as antioxidants and promoters of cellular antioxidant pathways. J. Dairy Sci. 2018, 101, 4747–4761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Królczyk, J.B.; Dawidziuk, T.; Janiszewska-Turak, E.; Sołowiej, B. Use of Whey and Whey Preparations in the Food Industry—A Review. Pol. J. Food Nutr. Sci. 2016, 66, 157. [Google Scholar] [CrossRef]
- Terpou, A.; Bosnea, L.; Kanellaki, M. Effect of Mastic Gum (Pistacia Lentiscus Via Chia) as a Probiotic Cell Encapsulation Carrier for Functional Whey Beverage Production. SCIOL Biomed. 2017, 1, 1–10. [Google Scholar]
- Skryplonek, K.; Dmytrów, I.; Mituniewicz-Małek, A. Probiotic fermented beverages based on acid whey. J. Dairy Sci. 2019, 102, 7773–7780. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Raychaudhuri, A.; Ghosh, S.K. Supply Chain of Bioethanol Production from Whey: A Review. Procedia Environ. Sci. 2016, 35, 833–846. [Google Scholar] [CrossRef]
- Dedenaro, G.; Costa, S.; Rugiero, I.; Pedrini, P.; Tamburini, E. Valorization of Agri-Food Waste via Fermentation: Production of l-lactic Acid as a Building Block for the Synthesis of Biopolymers. Appl. Sci. 2016, 6, 379. [Google Scholar] [CrossRef]
- Ganju, S.; Gogate, P.R. A review on approaches for efficient recovery of whey proteins from dairy industry effluents. J. Food Eng. 2017, 215, 84–96. [Google Scholar] [CrossRef]
- Uduwerella, G.; Chandrapala, J.; Vasiljevic, T. Preconcentration of yoghurt base by ultrafiltration for reduction in acid whey generation during Greek yoghurt manufacturing. Int. J. Dairy Technol. 2018, 71, 71–80. [Google Scholar] [CrossRef]
- Marx, M.; Kulozik, U. Thermal denaturation kinetics of whey proteins in reverse osmosis and nanofiltration sweet whey concentrates. Int. Dairy J. 2018, 85, 270–279. [Google Scholar] [CrossRef]
- Mawson, A.J. Bioconversions for whey utilization and waste abatement. Bioresour. Technol. 1994, 47, 195–203. [Google Scholar] [CrossRef]
- Pescuma, M.; de Valdez, G.F.; Mozzi, F. Whey-derived valuable products obtained by microbial fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 6183–6196. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Gialleli, A.-I.; Bekatorou, A.; Dimitrellou, D.; Ganatsios, V.; Barouni, E.; Koutinas, A.A.; Kanellaki, M. Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food Bioprod. Process. 2017, 101, 184–192. [Google Scholar] [CrossRef]
- Chandra, R.; Castillo-Zacarias, C.; Delgado, P.; Parra-Saldívar, R. A biorefinery approach for dairy wastewater treatment and product recovery towards establishing a biorefinery complexity index. J. Clean. Prod. 2018, 183, 1184–1196. [Google Scholar] [CrossRef]
- Villamiel, M.; Montilla, A.; Olano, A.; Corzo, N. Production and Bioactivity of Oligosaccharides Derived from Lactose. In Food Oligosaccharides: Production, Analysis and Bioactivity; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 135–167. [Google Scholar] [Green Version]
- Nooshkam, M.; Babazadeh, A.; Jooyandeh, H. Lactulose: Properties, techno-functional food applications, and food grade delivery system. Trends Food Sci. Technol. 2018, 80, 23–34. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loveren, H.v.; Sanz, Y.; Salminen, S. Health Claims in Europe: Probiotics and Prebiotics as Case Examples. Annu. Rev. Food Sci. Technol. 2012, 3, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.J.; Corzo, N.; Montilla, A.; Villamiel, M.; Olano, A. Current state and latest advances in the concept, production and functionality of prebiotic oligosaccharides. Curr. Opin. Food Sci. 2017, 13, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Sangwan, V.; Tomar, S.K.; Ali, B.; Singh, R.R.B.; Singh, A.K. Production of β-galactosidase from Streptococcus thermophilus for galactooligosaccharides synthesis. J. Food Sci. Technol. 2015, 52, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Hernández, O.; Ruiz-Matute, A.I.; Olano, A.; Moreno, F.J.; Sanz, M.L. Comparison of fractionation techniques to obtain prebiotic galactooligosaccharides. Int. Dairy J. 2009, 19, 531–536. [Google Scholar] [CrossRef]
- Rastall, R.A. Functional oligosaccharides: Application and manufacture. Annu. Rev. Food Sci. Technol. 2010, 1, 305–339. [Google Scholar] [CrossRef] [PubMed]
- Rastall, R.A. Galacto-Oligosaccharides as Prebiotic Food Ingredients. In Prebiotics: Development & Application; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 101–109. [Google Scholar]
- Adroit market research. Available online: https://www.adroitmarketresearch.com/industry-reports/galacto-oligosaccharides-gos-market (accessed on 15 June 2019).
- Anadón, A.; Martínez-Larrañaga, M.R.; Arés, I.; Martínez, M.A. Chapter 1—Prebiotics and Probiotics: An Assessment of Their Safety and Health Benefits. In Probiotics, Prebiotics, and Synbiotics; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 3–23. [Google Scholar]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Martins, G.N.; Ureta, M.M.; Tymczyszyn, E.E.; Castilho, P.C.; Gomez-Zavaglia, A. Technological Aspects of the Production of Fructo and Galacto-Oligosaccharides. Enzymatic Synthesis and Hydrolysis. Front. Nutr. 2019, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Aït-Aissa, A.; Aïder, M. Lactulose: Production and use in functional food, medical and pharmaceutical applications. Practical and critical review. Int. J. Food Sci. Technol. 2014, 49, 1245–1253. [Google Scholar] [CrossRef]
- Contesini, F.J.; de Lima, E.A.; Mandelli, F.; Borin, G.P.; Alves, R.F.; Terrasan, C.R.F. Carbohydrate Active Enzymes Applied in the Production of Functional Oligosaccharides. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 30–34. [Google Scholar]
- Albayrak, N.; Yang, S.T. Production of galacto-oligosaccharides from lactose by Aspergillus oryzae beta-galactosidase immobilized on cotton cloth. Biotechnol. Bioeng. 2002, 77, 8–19. [Google Scholar] [CrossRef]
- Guerrero, C.; Vera, C.; Acevedo, F.; Illanes, A. Simultaneous synthesis of mixtures of lactulose and galacto-oligosaccharides and their selective fermentation. J. Biotechnol. 2015, 209, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wichienchot, S.; Ishak, W.R.B.W. Prebiotics and Dietary Fibers from Food Processing By-Products. In Food Processing By-Products and Their Utilization; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 137–174. [Google Scholar]
- Splechtna, B.; Nguyen, T.-H.; Zehetner, R.; Lettner, H.P.; Lorenz, W.; Haltrich, D. Process development for the production of prebiotic galacto-oligosaccharides from lactose using β-galactosidase from Lactobacillus sp. Biotechnol. J. 2007, 2, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sen, D.; Sarkar, A.; Bhattacharyya, S.; Bhattacharjee, C. A Comparative Study on the Production of Galacto-oligosaccharide from Whey Permeate in Recycle Membrane Reactor and in Enzymatic Batch Reactor. Ind. Eng. Chem. Res. 2011, 50, 806–816. [Google Scholar] [CrossRef]
- Díez-Municio, M.; Montilla, A.; Jimeno, M.L.; Corzo, N.; Olano, A.; Moreno, F.J. Synthesis and Characterization of a Potential Prebiotic Trisaccharide from Cheese Whey Permeate and Sucrose by Leuconostoc mesenteroides Dextransucrase. J. Agric. Food Chem. 2012, 60, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Corzo-Martinez, M.; Luscher, A.; de Las Rivas, B.; Muñoz, R.; Moreno, F.J. Valorization of Cheese and Tofu Whey through Enzymatic Synthesis of Lactosucrose. PLoS ONE 2015, 10, e0139035. [Google Scholar] [CrossRef] [PubMed]
- Foda, M.I.; Lopez-Leiva, M. Continuous production of oligosaccharides from whey using a membrane reactor. Process Biochem. 2000, 35, 581–587. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of galactooligosaccharides using sweet and acid whey as a substrate. Int. Dairy J. 2015, 48, 15–22. [Google Scholar] [CrossRef]
- Geiger, B.; Nguyen, H.-M.; Wenig, S.; Nguyen, H.A.; Lorenz, C.; Kittl, R.; Mathiesen, G.; Eijsink, V.G.H.; Haltrich, D.; Nguyen, T.-H. From by-product to valuable components: Efficient enzymatic conversion of lactose in whey using β-galactosidase from Streptococcus thermophilus. BioChem. Eng. J. 2016, 116, 45–53. [Google Scholar] [CrossRef]
- Scott, F.; Vera, C.; Conejeros, R. Chapter 7—Technical and Economic Analysis of Industrial Production of Lactose-Derived Prebiotics with Focus on Galacto-Oligosaccharides. In Lactose-Derived Prebiotics; Illanes, A., Guerrero, C., Vera, C., Wilson, L., Conejeros, R., Scott, F., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 261–284. [Google Scholar]
- Market Research. Available online: https://www.marketresearch.com/MarketsandMarkets-v3719/Sucrose-Esters-Application-Food-Detergents-9762838/ (accessed on 15 June 2019).
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.G.; Cerqueira Pinto, M.C.; Kopsahelis, N.; Freire, D.M.G.; Mandala, I.; Koutinas, A.A. Development of Microbial Oil Wax-Based Oleogel with Potential Application in Food Formulations. Food Bioprocess Technol. 2019, 12, 899–909. [Google Scholar] [CrossRef]
- Papadaki, A.; Fernandes, K.V.; Chatzifragkou, A.; Aguieiras, E.C.G.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Papanikolaou, S.; Koutinas, A.; Freire, D.M.G. Bioprocess development for biolubricant production using microbial oil derived via fermentation from confectionery industry wastes. Bioresour. Technol. 2018, 267, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Mallouchos, A.; Efthymiou, M.-N.; Gardeli, C.; Kopsahelis, N.; Aguieiras, E.C.G.; Freire, D.M.G.; Papanikolaou, S.; Koutinas, A.A. Production of wax esters via microbial oil synthesis from food industry waste and by-product streams. Bioresour. Technol. 2017, 245, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.V.; Papadaki, A.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Koutinas, A.A.; Freire, D.M.G. Enzymatic esterification of palm fatty-acid distillate for the production of polyol esters with biolubricant properties. Ind. Crop. Prod. 2018, 116, 90–96. [Google Scholar] [CrossRef]
- Scholnick, F.; Sucharski, M.K.; Linfield, W.M. Lactose-derived surfactants (I) fatty esters of lactose. J. Am. Oil Chem. Soc. 1974, 51, 8–11. [Google Scholar] [CrossRef]
- Lay, L.; Panza, L.; Riva, S.; Khitri, M.; Tirendi, S. Regioselective acylation of disaccharides by enzymatic transesterification. Carbohydr. Res. 1996, 291, 197–204. [Google Scholar] [CrossRef]
- Riva, S.; Chopineau, J.; Kieboom, A.P.G.; Klibanov, A.M. Protease-catalyzed regioselective esterification of sugars and related compounds in anhydrous dimethylformamide. J. Am. Chem. Soc. 1988, 110, 584–589. [Google Scholar] [CrossRef]
- Enayati, M.; Gong, Y.; Goddard, J.M.; Abbaspourrad, A. Synthesis and characterization of lactose fatty acid ester biosurfactants using free and immobilized lipases in organic solvents. Food Chem. 2018, 266, 508–513. [Google Scholar] [CrossRef]
- Lee, S.-M.; Wagh, A.; Sandhu, G.; Walsh, M.K. Emulsification Properties of Lactose Fatty Acid Esters. Food Nutr. Sci. 2018, 09, 17. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Carotenoids: Health Effects. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 292–297. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Méndez, M.Á.; Aguilar-Machado, D.E.; Balagurusamy, N.; Montañez, J.; Morales-Oyervides, L. Agro-industrial wastes for the synthesis of carotenoids by Xanthophyllomyces dendrorhous: Mesquite pods-based medium design and optimization. BioChem. Eng. J. 2019, 150, 107260. [Google Scholar] [CrossRef]
- Barredo, J.L.; García-Estrada, C.; Kosalkova, K.; Barreiro, C. Biosynthesis of Astaxanthin as a Main Carotenoid in the Heterobasidiomycetous Yeast Xanthophyllomyces dendrorhous. J. Fungi 2017, 3, 44. [Google Scholar] [CrossRef]
- Roukas, T.; Varzakakou, M.; Kotzekidou, P. From Cheese Whey to Carotenes by Blakeslea trispora in a Bubble Column Reactor. Appl. Biochem. Biotechnol. 2015, 175, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Khodaiyan, F.; Razavi, S.H.; Mousavi, S.M. Optimization of canthaxanthin production by Dietzia natronolimnaea HS-1 from cheese whey using statistical experimental methods. BioChem. Eng. J. 2008, 40, 415–422. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Zhang, J.-F.; Zheng, Y.-G.; Shen, Y.-C. Improvement of astaxanthin production by a newly isolated Phaffia rhodozyma mutant with low-energy ion beam implantation. J. Appl. Microbiol. 2008, 104, 861–872. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Li, Y.-T.; Huang, J.-C.; Chen, F. Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process Biochem. 2008, 43, 1288–1292. [Google Scholar] [CrossRef]
- Aksu, Z.; Eren, A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agricultural wastes as a carbon source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Tsimidou, M.Z.; Roukas, T. Performance of Crude Olive Pomace Oil and Soybean Oil during Carotenoid Production by Blakeslea trispora in Submerged Fermentation. J. Agric. Food Chem. 2006, 54, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Varzakakou, M.; Roukas, T. Identification of carotenoids produced from cheese whey by Blakeslea trispora in submerged fermentation. Prep. Biochem. Biotechnol. 2009, 40, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Varzakakou, M.; Roukas, T.; Kotzekidou, P. Effect of the ratio of (+) and (−) mating type of Blakeslea trispora on carotene production from cheese whey in submerged fermentation. World J. Microbiol. Biotechnol. 2010, 26, 2151–2156. [Google Scholar] [CrossRef]
- Roukas, T.; Mantzouridou, F.; Boumpa, T.; Vafiadou, A.; Goksungur, Y. Production of β-Carotene from Beet Molasses and Deproteinized Whey by Blakeslea trispora. Food Biotechnol. 2007, 17, 203–215. [Google Scholar] [CrossRef]
- Psani, M.; Roukas, T.; Kotzekidou, P. Evaluation of cheese whey as substrate for carotenoids production by Blakeslea trispora. Australian J. Dairy Technol. 2006, 61, 222. [Google Scholar]
- Azmi, W.; Thakur, M.; Kumar, A. Production of beta-carotene from deproteinized waste whey filtrate using Mucor azygosporus MTCC 414 in submerged fermentation. Acta Microbiol. Immunol. Hung. 2011, 58, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Marova, I.; Carnecka, M.; Halienova, A.; Certik, M.; Dvorakova, T.; Haronikova, A. Use of several waste substrates for carotenoid-rich yeast biomass production. J. Environ. Manag. 2012, 95, S338–S342. [Google Scholar] [CrossRef]
- Frengova, G.; Simova, E.; Beshkova, D. Use of whey ultrafiltrate as a substrate for production of carotenoids by the yeast Rhodotorula rubra. Appl. Biochem. Biotechnol. 2004, 112, 133–141. [Google Scholar] [CrossRef]
- Frengova, G.; Simova, E.; Pavlova, K.; Beshkova, D.; Grigorova, D. Formation of carotenoids by Rhodotorula glutinis in whey ultrafiltrate. Biotechnol. Bioeng. 1994, 44, 888–894. [Google Scholar] [CrossRef]
- Simova, E.D.; Frengova, G.I.; Beshkova, D.M. Synthesis of carotenoids by Rhodotorula rubra GED8 co-cultured with yogurt starter cultures in whey ultrafiltrate. J. Ind. Microbiol. Biotechnol. 2004, 31, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Valduga, E.; Tatsch, P.; Vanzo, L.T.; Rauber, F.; Di Luccio, M.; Treichel, H. Assessment of hydrolysis of cheese whey and use of hydrolysate for bioproduction of carotenoids by Sporidiobolus salmonicolor CBS 2636. J. Sci. Food Agric. 2009, 89, 1060–1065. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Cai, D.; Zhan, Y.; Wang, Q.; Chen, S. Identification and High-level Production of Pulcherrimin in Bacillus licheniformis DW2. Appl. Biochem. Biotechnol. 2017, 183, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Turkel, S.; Korukluoglu, M.; Yavuz, M. Biocontrol Activity of the Local Strain of Metschnikowia pulcherrima on Different Postharvest Pathogens. Biotechnol. Res. Int. 2014, 2014, 397167. [Google Scholar] [CrossRef]
- Türkel, S.; Ener, B. Isolation and Characterization of New Metschnikowia pulcherrima Strains as Producers of the Antimicrobial Pigment Pulcherrimin. Z. Naturforsch. C 2009, 64, 405–410. [Google Scholar] [CrossRef]
- Savini, V.; Hendrickx, M.; Sisti, M.; Masciarelli, G.; Favaro, M.; Fontana, C.; Pitzurra, L.; Arzeni, D.; Astolfi, D.; Catavitello, C.; et al. An atypical, pigment-producing Metschnikowia strain from a leukaemia patient. Med. Mycol. 2013, 51, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Chreptowicz, K.; Sternicka, M.K.; Kowalska, P.D.; Mierzejewska, J. Screening of yeasts for the production of 2-phenylethanol (rose aroma) in organic waste-based media. Lett. Appl. Microbiol. 2018, 66, 153–160. [Google Scholar] [CrossRef]
- Chantasuban, T.; Santomauro, F.; Gore-Lloyd, D.; Parsons, S.; Henk, D.; Scott, R.J.; Chuck, C. Elevated production of the aromatic fragrance molecule, 2-phenylethanol, using Metschnikowia pulcherrima through both de novo and ex novo conversion in batch and continuous modes. J. Chem. Technol. Biotechnol. 2018, 93, 2118–2130. [Google Scholar] [CrossRef]
- Izawa, N.; Kudo, M.; Nakamura, Y.; Mizukoshi, H.; Kitada, T.; Sone, T. Production of aroma compounds from whey using Wickerhamomyces pijperi. AMB Express 2015, 5, 23. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef] [PubMed]
- Andritsou, V.; de Melo, E.M.; Tsouko, E.; Ladakis, D.; Maragkoudaki, S.; Koutinas, A.A.; Matharu, A.S. Synthesis and Characterization of Bacterial Cellulose from Citrus-Based Sustainable Resources. ACS Omega 2018, 3, 10365–10373. [Google Scholar] [CrossRef] [Green Version]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in bacterial cellulose matrices for biotechnological applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Tsouko, E.; Papadaki, A.; Papapostolou, H.; Ladakis, D.; Natsia, A.; Koutinas, A.; Kampioti, A.; Eriotou, E.; Kopsahelis, N. Valorization of Zante currant side-streams for the production of phenolic-rich extract and bacterial cellulose: A novel biorefinery concept. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More Than Meets the Eye in Bacterial Cellulose: Biosynthesis, Bioprocessing, and Applications in Advanced Fiber Composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Dahman, Y.; Jayasuriya, K.E.; Kalis, M. Potential of Biocellulose Nanofibers Production from Agricultural Renewable Resources: Preliminary Study. Appl. Biochem. Biotechnol. 2010, 162, 1647–1659. [Google Scholar] [CrossRef]
- Son, H.-J.; Kim, H.-G.; Kim, K.-K.; Kim, H.-S.; Kim, Y.-G.; Lee, S.-J. Increased production of bacterial cellulose by Acetobacter sp. V6 in synthetic media under shaking culture conditions. Bioresour. Technol. 2003, 86, 215–219. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Flanagan, B.M.; Dykes, G.A.; Gidley, M.J. Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. J. Appl. Microbiol. 2009, 107, 576–583. [Google Scholar] [CrossRef]
- Thompson, D.N.; Hamilton, M.A. Production of bacterial cellulose from alternate feedstocks. Appl. Biochem. Biotechnol. 2001, 91, 503. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef] [PubMed]
- Battad-Bernardo, E.; McCrindle, S.L.; Couperwhite, I.; Neilan, B.A. Insertion of an E. coli lacZ gene in Acetobacter xylinus for the production of cellulose in whey. FEMS Microbiol. Lett. 2004, 231, 253–260. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Patel, S.; Kim, S.-K. Anticancer and other therapeutic relevance of mushroom polysaccharides: A holistic appraisal. Biomed. Pharmacother. 2018, 105, 377–394. [Google Scholar] [CrossRef]
- Borthakur, M.; Joshi, S.R. Chapter 1—Wild Mushrooms as Functional Foods: The Significance of Inherent Perilous Metabolites. In New and Future Developments in Microbial Biotechnology Bioengineering; Gupta, V.K., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–12. [Google Scholar]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom Polysaccharides and Lipids Synthesized in Liquid Agitated and Static Cultures. Part I: Screening Various Mushroom Species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef]
- Hereher, F.; ElFallal, A.; Toson, E.; Abou-Dobara, M.; Abdelaziz, M. Pilot study: Tumor suppressive effect of crude polysaccharide substances extracted from some selected mushroom. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 767–775. [Google Scholar] [CrossRef]
- Giavasis, I. Polysaccharides from Medicinal Mushrooms for Potential Use as Nutraceuticals. In Polysaccharides Natural Fibers in Food and Nutrition; Benkeblia, N., Ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Velez, M.E.V.; da Luz, J.M.R.; da Silva, M.d.C.S.; Cardoso, W.S.; Lopes, L.d.S.; Vieira, N.A.; Kasuya, M.C.M. Production of bioactive compounds by the mycelial growth of Pleurotus djamor in whey powder enriched with selenium. LWT 2019, 114, 108376. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Guha, A.K. A comprehensive analysis of the nutritional quality of edible mushroom Pleurotus sajor-caju grown in deproteinized whey medium. LWT Food Sci. Technol. 2015, 61, 339–345. [Google Scholar] [CrossRef]
- Wu, X.J. Proximate Composition of Pleurotus ostreatus Grown in Whey Permeate Based Medium. Trans. ASABE 2009, 52, 1249–1254. [Google Scholar]
- Bhak, G.; Song, M.; Lee, S.; Hwang, S. Response Surface Analysis of Solid State Growth of Pleurotus ostreatus Mycelia utilizing Whey Permeate. Biotechnol. Lett. 2005, 27, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Israilides, C.; Philippoussis, A. Bio-technologies of Recycling Agro-industrial Wastes for the Production of Commercially Important Fungal Polysaccharides and Mushrooms. Biotechnol. Genet. Eng. Rev. 2003, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Hansen, C. Effects of Whey Permeate-Based Medium on the Proximate Composition of Lentinus edodes in the Submerged Culture. J. Food Sci. 2006, 71, M174–M179. [Google Scholar] [CrossRef]
- Wu, X.J.; Hansen, C. Antioxidant Capacity, Phenolic Content, and Polysaccharide Content of Lentinus edodes Grown in Whey Permeate-Based Submerged Culture. J. Food Sci. 2008, 73, M1–M8. [Google Scholar] [CrossRef] [PubMed]
- Inglet, B.S.; Song, M.; Hansen, C.L.; Hwang, S. Short Communication: Cultivation of Lentinus edodes Mycelia Using Whey Permeate as an Alternative Growth Substrate. J. Dairy Sci. 2006, 89, 1113–1115. [Google Scholar] [CrossRef]
- Song, M.; Kim, N.; Lee, S.; Hwang, S. Use of Whey Permeate for Cultivating Ganoderma lucidum Mycelia. J. Dairy Sci. 2007, 90, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Song, M.; Hwang, S. Optimizing bioconversion of deproteinated cheese whey to mycelia of Ganoderma lucidum. Process Biochem. 2003, 38, 1685–1693. [Google Scholar] [CrossRef]
- Lee, H.; Song, M.; Yu, Y.; Hwang, S. Production of Ganoderma lucidum mycelium using cheese whey as an alternative substrate: Response surface analysis and biokinetics. BioChem. Eng. J. 2003, 15, 93–99. [Google Scholar] [CrossRef]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.; Bisen, P.S. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef]
- Shao, P.; Xuan, S.; Wu, W.; Qu, L. Encapsulation efficiency and controlled release of Ganoderma lucidum polysaccharide microcapsules by spray drying using different combinations of wall materials. Int. J. Biol. Macromol. 2019, 125, 962–969. [Google Scholar] [CrossRef]
- Papadaki, A.; Diamantopoulou, P.; Papanikolaou, S.; Philippoussis, A. Evaluation of Biomass and Chitin Production of Morchella Mushrooms Grown on Starch-Based Substrates. Foods 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Kosaric, N.; Miyata, N. Growth of morel mushroom mycelium in cheese whey. J. Dairy Res. 1981, 48, 149–162. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Beheshtipour, H.; Mortazavian, A.M.; Mohammadi, R.; Sohrabvandi, S.; Khosravi-Darani, K. Supplementation of Spirulina platensis and Chlorella vulgaris Algae into Probiotic Fermented Milks. Compr. Rev. Food Sci. Food Saf. 2013, 12, 144–154. [Google Scholar] [CrossRef]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kulandaivel, S.; Prakash, R.; Anitha, R.; Arunnagendran, N. Comparative studies on biochemical profile of Spirulina platensis and Oscillatoria sp. on synthetic medium and dairy effluent. J. Pure Appl. Microbiol. 2007, 1, 109–112. [Google Scholar]
- Vieira Salla, A.C.; Margarites, A.C.; Seibel, F.I.; Holz, L.C.; Brião, V.B.; Bertolin, T.E.; Colla, L.M.; Costa, J.A.V. Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour. Technol. 2016, 209, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.-M.; Roy, M.-L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.-S. Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El Baroty, G.S.; El Baz, F.K.; Abd El Baky, H.H.; Murad, S.A. Scenedesmus obliquus: Antioxidant and antiviral activity of proteins hydrolyzed by three enzymes. J. Genet. Eng. Biotechnol. 2018, 16, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey protein layer applied on biodegradable packaging film to improve barrier properties while maintaining biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Tarhan, O.; Spotti, M.J.; Schaffter, S.; Corvalan, C.M.; Campanella, O.H. Rheological and structural characterization of whey protein gelation induced by enzymatic hydrolysis. Food Hydrocoll. 2016, 61, 211–220. [Google Scholar] [CrossRef]
- Fu, W.; Nakamura, T. Explaining the texture properties of whey protein isolate/starch co-gels from fracture structures. Biosci. Biotechnol. Biochem. 2017, 81, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, I.; Nie, R.; Ge, Z.; Li, K.; Li, C. Understanding the shielding effects of whey protein on mulberry anthocyanins: Insights from multispectral and molecular modelling investigations. Int. J. Biol. Macromol. 2018, 119, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Andoyo, R.; Dianti Lestari, V.; Mardawati, E.; Nurhadi, B. Fractal Dimension Analysis of Texture Formation of Whey Protein-Based Foods. Int. J. Food Sci. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Barba, C.; Eguinoa, A.; Maté, J.I. Preparation and characterization of β-cyclodextrin inclusion complexes as a tool of a controlled antimicrobial release in whey protein edible films. LWT Food Sci. Technol. 2015, 64, 1362–1369. [Google Scholar] [CrossRef]
- Boyacı, D.; Korel, F.; Yemenicioğlu, A. Development of activate-at-home-type edible antimicrobial films: An example pH-triggering mechanism formed for smoked salmon slices using lysozyme in whey protein films. Food Hydrocoll. 2016, 60, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Merzbacher, S.; Brzoska, N.; Müller, K.; Jesdinszki, M. Improvement of Food Packaging-Related Properties of Whey Protein Isolate-Based Nanocomposite Films and Coatings by Addition of Montmorillonite Nanoplatelets. Front. Mater. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Jiang, S.-J.; Zhang, T.; Song, Y.; Qian, F.; Tuo, Y.; Mu, G. Mechanical properties of whey protein concentrate based film improved by the coexistence of nanocrystalline cellulose and transglutaminase. Int. J. Biol. Macromol. 2019, 126, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Qazanfarzadeh, Z.; Kadivar, M. Properties of whey protein isolate nanocomposite films reinforced with nanocellulose isolated from oat husk. Int. J. Biol. Macromol. 2016, 91, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Shahabi-Ghahfarrokhi, I. Development of ecofriendly bionanocomposite: Whey protein isolate/pullulan films with nano-SiO2. Int. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Wang, H. Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr. Polym. 2016, 138, 59–65. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effects of carbohydrate/protein ratio on the microstructure and the barrier and sorption properties of wheat starch–whey protein blend edible films. J. Sci. Food Agric. 2017, 97, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Weng, Y.-M. Novel edible composite films fabricated with whey protein isolate and zein: Preparation and physicochemical property evaluation. LWT 2019, 101, 567–574. [Google Scholar] [CrossRef]
- Oymaci, P.; Altinkaya, S.A. Improvement of barrier and mechanical properties of whey protein isolate based food packaging films by incorporation of zein nanoparticles as a novel bionanocomposite. Food Hydrocoll. 2016, 54, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Bahram, S.; Rezaei, M.; Soltani, M.; Kamali, A.; Ojagh, S.M.; Abdollahi, M. Whey Protein Concentrate Edible Film Activated with Cinnamon Essential Oil. J. Food Process. Preserv. 2014, 38, 1251–1258. [Google Scholar] [CrossRef]
- Khanzadi, M.; Jafari, S.M.; Mirzaei, H.; Chegini, F.K.; Maghsoudlou, Y.; Dehnad, D. Physical and mechanical properties in biodegradable films of whey protein concentrate–pullulan by application of beeswax. Carbohydr. Polym. 2015, 118, 24–29. [Google Scholar] [CrossRef]
- Rantamäki, P.; Loimaranta, V.; Vasara, E.; Latva-Koivisto, J.; Korhonen, H.; Tenovuo, J.; Marnila, P. Edible films based on milk proteins release effectively active immunoglobulins. Food Qual. Saf. 2019, 3, 23–34. [Google Scholar] [CrossRef]
- Piccirilli, G.N.; Soazo, M.; Pérez, L.M.; Delorenzi, N.J.; Verdini, R.A. Effect of storage conditions on the physicochemical characteristics of edible films based on whey protein concentrate and liquid smoke. Food Hydrocoll. 2019, 87, 221–228. [Google Scholar] [CrossRef]
- Pereira, R.C.; de Deus Souza Carneiro, J.; Borges, S.V.; Assis, O.B.G.; Alvarenga, G.L. Preparation and Characterization of Nanocomposites from Whey Protein Concentrate Activated with Lycopene. J. Food Sci. 2016, 81, E637–E642. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, J.P.; Spotti, M.J.; Piagentini, A.M.; Milt, V.G.; Carrara, C.R. Development of edible films obtained from submicron emulsions based on whey protein concentrate, oil/beeswax and brea gum. Food Sci. Technol. Int. 2017, 23, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro-Santos, R.; de Melo, N.R.; Andrade, M.; Azevedo, G.; Machado, A.V.; Carvalho-Costa, D.; Sanches-Silva, A. Whey protein active films incorporated with a blend of essential oils: Characterization and effectiveness. Packag. Technol. Sci. 2018, 31, 27–40. [Google Scholar] [CrossRef]
- Pereira, R.C.; Carneiro, J.d.D.S.; Assis, O.B.; Borges, S.V. Mechanical and structural characterization of whey protein concentrate/montmorillonite/lycopene films. J. Sci. Food Agric. 2017, 97, 4978–4986. [Google Scholar] [CrossRef]
- Pérez, L.M.; Piccirilli, G.N.; Delorenzi, N.J.; Verdini, R.A. Effect of different combinations of glycerol and/or trehalose on physical and structural properties of whey protein concentrate-based edible films. Food Hydrocoll. 2016, 56, 352–359. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Costa Bonito, M.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; dos Santos, F.R.; Neves, I.d.A.; de Carvalho, M.G.; Sanches-Silva, A. Biological activities and major components determination in essential oils intended for a biodegradable food packaging. Ind. Crop. Prod. 2017, 97, 201–210. [Google Scholar] [CrossRef]
- Nicolai, T. Formation and functionality of self-assembled whey protein microgels. Colloids Surf. B Biointerfaces 2016, 137, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Abaee, A.; Madadlou, A. Niosome-loaded cold-set whey protein hydrogels. Food Chem. 2016, 196, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bhattacharya, S. Food Gels: Gelling Process and New Applications. Crit. Rev. Food Sci. Nutr. 2012, 52, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Chassenieux, C.; Nicolai, T.; Schmitt, C. Effect of the pH and NaCl on the microstructure and rheology of mixtures of whey protein isolate and casein micelles upon heating. Food Hydrocoll. 2017, 70, 114–122. [Google Scholar] [CrossRef]
- Kharlamova, A.; Chassenieux, C.; Nicolai, T. Acid-induced gelation of whey protein aggregates: Kinetics, gel structure and rheological properties. Food Hydrocoll. 2018, 81, 263–272. [Google Scholar] [CrossRef]
- Lam, C.W.Y.; Ikeda, S. Physical Properties of Heat-induced Whey Protein Aggregates Formed at pH 5.5 and 7.0. Food Sci. Technol. Res. 2017, 23, 595–601. [Google Scholar] [CrossRef]
- Lazidis, A.; Hancocks, R.D.; Spyropoulos, F.; Kreuß, M.; Berrocal, R.; Norton, I.T. Whey protein fluid gels for the stabilisation of foams. Food Hydrocoll. 2016, 53, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Alavi, F.; Momen, S.; Emam-Djomeh, Z.; Salami, M.; Moosavi-Movahedi, A.A. Radical cross-linked whey protein aggregates as building blocks of non-heated cold-set gels. Food Hydrocoll. 2018, 81, 429–441. [Google Scholar] [CrossRef]
- Kharlamova, A.; Nicolai, T.; Chassenieux, C. Calcium-induced gelation of whey protein aggregates: Kinetics, structure and rheological properties. Food Hydrocoll. 2018, 79, 145–157. [Google Scholar] [CrossRef]
- Ren, F.; Dong, D.; Yu, B.; Hou, Z.-h.; Cui, B. Rheology, thermal properties, and microstructure of heat-induced gel of whey protein–acetylated potato starch. Starch Stärke 2017, 69, 1600344. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z. Interaction between lactoferrin and whey proteins and its influence on the heat-induced gelation of whey proteins. Food Chem. 2018, 252, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.J.; Dar, B.N.; Kierulf, A.; Ravanfar, R.; Rizvi, S.S.H.; Abbaspourrad, A. Modulation of whey protein-kappa carrageenan hydrogel properties via enzymatic protein modification. Food Funct. 2018, 9, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Sogut, E.; Ili Balqis, A.M.; Nur Hanani, Z.A.; Seydim, A.C. The properties of κ-carrageenan and whey protein isolate blended films containing pomegranate seed oil. Polym. Test. 2019, 77. [Google Scholar] [CrossRef]
- Protte, K.; Weiss, J.; Hinrichs, J.; Knaapila, A. Thermally stabilised whey protein-pectin complexes modulate the thermodynamic incompatibility in hydrocolloid matrixes: A feasibility-study on sensory and rheological characteristics in dairy desserts. LWT 2019, 105, 336–343. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT Food Sci. Technol. 2015, 60, 773–780. [Google Scholar] [CrossRef]
- Su, J.; Wang, X.; Li, W.; Chen, L.; Zeng, X.; Huang, Q.; Hu, B. Enhancing the Viability of Lactobacillus plantarum as Probiotics through Encapsulation with High Internal Phase Emulsions Stabilized with Whey Protein Isolate Microgels. J. Agric. Food Chem. 2018, 66, 12335–12343. [Google Scholar] [CrossRef]
- Kwiecień, I.; Kwiecień, M. Application of Polysaccharide-Based Hydrogels as Probiotic Delivery Systems. Gels 2018, 4, 47. [Google Scholar] [CrossRef]
- O’Neill, G.J.; Egan, T.; Jacquier, J.C.; O’Sullivan, M.; Dolores O’Riordan, E. Whey microbeads as a matrix for the encapsulation and immobilisation of riboflavin and peptides. Food Chem. 2014, 160, 46–52. [Google Scholar] [CrossRef]
- Abbasi, A.; Emam-Djomeh, Z.; Mousavi, M.A.E.; Davoodi, D. Stability of vitamin D3 encapsulated in nanoparticles of whey protein isolate. Food Chem. 2014, 143, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Moreno, S.; Osorio-Revilla, G.; Gallardo-Velázquez, T.; Cárdenas-Bailón, F.; Meza-Márquez, G. Effect of the cross-linking agent and drying method on encapsulation efficiency of orange essential oil by complex coacervation using whey protein isolate with different polysaccharides. J. Microencapsul. 2018, 35, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z. Fabrication of curcumin-loaded whey protein microgels: Structural properties, antioxidant activity, and in vitro release behavior. LWT 2019, 103, 94–100. [Google Scholar] [CrossRef]
- Raei, M.; Shahidi, F.; Farhoodi, M.; Jafari, S.M.; Rafe, A. Application of whey protein-pectin nano-complex carriers for loading of lactoferrin. Int. J. Biol. Macromol. 2017, 105, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Maghsoudlou, Y.; Jafari, S.-M.; Ghorbani, M.; Aalami, M. Optimization of folic acid nano-emulsification and encapsulation by maltodextrin-whey protein double emulsions. Int. J. Biol. Macromol. 2016, 86, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Bao, H.; Ni, Y.; Choijilsuren, N.; Liang, L. Partition and digestive stability of α-tocopherol and resveratrol/naringenin in whey protein isolate emulsions. Int. Dairy J. 2019, 93, 116–123. [Google Scholar] [CrossRef]
- Nourbakhsh, H.; Madadlou, A.; Emam-Djomeh, Z.; Wang, Y.-C.; Gunasekaran, S.; Mousavi, M.E. One-Pot Procedure for Recovery of Gallic Acid from Wastewater and Encapsulation within Protein Particles. J. Agric. Food Chem. 2016, 64, 1575–1582. [Google Scholar] [CrossRef]
- Jain, A.; Sharma, G.; Ghoshal, G.; Kesharwani, P.; Singh, B.; Shivhare, U.S.; Katare, O.P. Lycopene loaded whey protein isolate nanoparticles: An innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int. J. Pharm. 2018, 546, 97–105. [Google Scholar] [CrossRef]
- O’Neill, G.J.; Egan, T.; Jacquier, J.C.; O’Sullivan, M.; Dolores O’Riordan, E. Kinetics of immobilisation and release of tryptophan, riboflavin and peptides from whey protein microbeads. Food Chem. 2015, 180, 150–155. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Yarmand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold gelation of curcumin loaded whey protein aggregates mixed with k-carrageenan: Impact of gel microstructure on the gastrointestinal fate of curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Mohammadian, M.; Madadlou, A. Cold-set hydrogels made of whey protein nanofibrils with different divalent cations. Int. J. Biol. Macromol. 2016, 89, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Salami, M.; Emam-Djomeh, Z.; Momen, S.; Moosavi-Movahedi, A.A. Gelation of oil-in-water emulsions stabilized by heat-denatured and nanofibrillated whey proteins through ion bridging or citric acid-mediated cross-linking. Int. J. Biol. Macromol. 2018, 120, 2247–2258. [Google Scholar] [CrossRef]
- Mantovani, R.A.; Fattori, J.; Michelon, M.; Cunha, R.L. Formation and pH-stability of whey protein fibrils in the presence of lecithin. Food Hydrocoll. 2016, 60, 288–298. [Google Scholar] [CrossRef]
- Hashemi, B.; Madadlou, A.; Salami, M. Functional and in vitro gastric digestibility of the whey protein hydrogel loaded with nanostructured lipid carriers and gelled via citric acid-mediated crosslinking. Food Chem. 2017, 237, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, X.; Wang, S.; Xu, Y.; Wang, D. Formation of nanocomplexes comprising whey proteins and fucoxanthin: Characterization, spectroscopic analysis, and molecular docking. Food Hydrocoll. 2017, 63, 391–403. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.-T.; Xu, W.; Xu, G. Coencapsulation of Polyphenols and Anthocyanins from Blueberry Pomace by Double Emulsion Stabilized by Whey Proteins: Effect of Homogenization Parameters. Molecules 2018, 23, 2525. [Google Scholar] [CrossRef] [PubMed]
- Chotiko, A.; Sathivel, S. Releasing characteristics of anthocyanins extract in pectin–whey protein complex microcapsules coated with zein. J. Food Sci. Technol. 2017, 54, 2059–2066. [Google Scholar] [CrossRef]
- Rocha, J.D.C.G.; Viana, K.W.C.; Mendonca, A.C.; Neves, N.D.A.; Carvalho, A.F.D.; Minim, V.P.R.; Barros, F.A.R.D.; Stringheta, P.C. Protein beverages containing anthocyanins of jabuticaba. Food Sci. Technol. 2019, 39, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Zhao, C.; Lu, J.; Guo, M. Physicochemical Properties of Whey-Protein-Stabilized Astaxanthin Nanodispersion and Its Transport via a Caco-2 Monolayer. J. Agric. Food Chem. 2018, 66, 1472–1478. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, R.N.; Martins, A.; Rodrigues, R.; Fuciños, C.; Teixeira, J.A.; Pastrana, L.; Malcata, F.X.; Vicente, A.A. Design of whey protein nanostructures for incorporation and release of nutraceutical compounds in food. Crit. Rev. Food Sci. Nutr. 2017, 57, 1377–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.-W.; Yu, S.-J.; Zeng, X.-A.; Yang, X.-Q.; Jia, X. Properties of whey protein isolate–dextran conjugate prepared using pulsed electric field. Food Res. Int. 2011, 44, 1052–1058. [Google Scholar] [CrossRef]
- Perusko, M.; Al-Hanish, A.; Cirkovic Velickovic, T.; Stanic-Vucinic, D. Macromolecular crowding conditions enhance glycation and oxidation of whey proteins in ultrasound-induced Maillard reaction. Food Chem. 2015, 177, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Díaz, O.; Candia, D.; Cobos, Á. Effects of ultraviolet radiation on properties of films from whey protein concentrate treated before or after film formation. Food Hydrocoll. 2016, 55, 189–199. [Google Scholar] [CrossRef]
- Kutzli, I.; Gibis, M.; Baier, S.K.; Weiss, J. Formation of Whey Protein Isolate (WPI)–Maltodextrin Conjugates in Fibers Produced by Needleless Electrospinning. J. Agric. Food Chem. 2018, 66, 10283–10291. [Google Scholar] [CrossRef]
- Zhong, J.; Mohan, S.D.; Bell, A.; Terry, A.; Mitchell, G.R.; Davis, F.J. Electrospinning of food-grade nanofibres from whey protein. Int. J. Biol. Macromol. 2018, 113, 764–773. [Google Scholar] [CrossRef]
- Colín-Orozco, J.; Zapata-Torres, M.; Rodríguez-Gattorno, G.; Pedroza-Islas, R. Properties of Poly (ethylene oxide)/whey Protein Isolate Nanofibers Prepared by Electrospinning. Food Biophys. 2015, 10, 134–144. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocoll. 2018, 77, 726–735. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Vieira da Silva, S.; Sogari Picolotto, R.; Wagner, R.; dos Santos Richards, N.S.P.; Smanioto Barin, J. Elemental (Macro- and Microelements) and Amino Acid Profile of Milk Proteins Commercialized in Brazil and Their Nutritional Value. J. Food Nutr. Res. 2015, 3, 430–436. [Google Scholar]
- Devries, M.C.; Phillips, S.M. Supplemental Protein in Support of Muscle Mass and Health: Advantage Whey. J. Food Sci. 2015, 80, A8–A15. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.-D.V.; Huang, P. Redox regulation of cell survival. Antioxid Redox Signal 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.B.; Zampieri, T.T.; Donato, J. Reviewing the Effects of l-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients 2015, 7, 3914–3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Risso, E.M.; Amaya-Farfan, J. Bioactivity of food peptides: Biological response of rats to bovine milk whey peptides following acute exercise. Food Nutr. Res. 2017, 61, 1290740. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.A.; Hardy, G. Clinical and nutritional benefits of cysteine-enriched protein supplements. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 562–568. [Google Scholar] [CrossRef]
- Winter, A.N.; Ross, E.K.; Daliparthi, V.; Sumner, W.A.; Kirchhof, D.M.; Manning, E.; Wilkins, H.M.; Linseman, D.A. A Cystine-Rich Whey Supplement (Immunocal(R)) Provides Neuroprotection from Diverse Oxidative Stress-Inducing Agents In Vitro by Preserving Cellular Glutathione. Oxid. Med. Cell. Longev. 2017, 2017, 3103272. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine restriction targets one carbon metabolism in humans and produces broad therapeutic responses in cancer. bioRxiv 2019, 627364. [Google Scholar] [CrossRef]
- Zheng, G.; Liu, H.; Zhu, Z.; Zheng, J.; Liu, A. Selenium modification of β-lactoglobulin (β-Lg) and its biological activity. Food Chem. 2016, 204, 246–251. [Google Scholar] [CrossRef]
- Sah, B.N.P.; McAinch, A.J.; Vasiljevic, T. Modulation of bovine whey protein digestion in gastrointestinal tract: A comprehensive review. Int. Dairy J. 2016, 62, 10–18. [Google Scholar] [CrossRef]
- Corrêa, A.P.F.; Daroit, D.J.; Fontoura, R.; Meira, S.M.M.; Segalin, J.; Brandelli, A. Hydrolysates of sheep cheese whey as a source of bioactive peptides with antioxidant and angiotensin-converting enzyme inhibitory activities. Peptides 2014, 61, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Desouky, W.I.; Mahmoud, A.H.; Abbas, M.M. Antioxidant potential and hypolipidemic effect of whey protein against gamma irradiation induced damages in rats. Appl. Radiat. Isot. 2017, 129, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review article: Insights into colonic protein fermentation, its modulation and potential health implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.P.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing resveratrol: Characterization and antioxidant activity. J. Drug Deliv. Sci. Technol. 2017, 39, 147–155. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagrange, V.; Clark, D.C. Chapter 15—Nutritive and Therapeutic Aspects of Whey Proteins. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 549–577. [Google Scholar]
- Tong, X.; Li, W.; Xu, J.-Y.; Han, S.; Qin, L.-Q. Effects of whey protein and leucine supplementation on insulin resistance in non-obese insulin-resistant model rats. Nutrition 2014, 30, 1076–1080. [Google Scholar] [CrossRef]
- Akhavan, T.; Luhovyy, B.L.; Panahi, S.; Kubant, R.; Brown, P.H.; Anderson, G.H. Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J. Nutr. Biochem. 2014, 25, 36–43. [Google Scholar] [CrossRef]
- Bamdad, F.; Bark, S.; Kwon, C.H.; Suh, J.-W.; Sunwoo, H. Anti-Inflammatory and Antioxidant Properties of Peptides Released from β-Lactoglobulin by High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis. Molecules 2017, 22, 949. [Google Scholar] [CrossRef]
- Alvarado, Y.; Muro, C.; Illescas, J.; Díaz, M.d.C.; Riera, F. Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions. Biomolecules 2019, 9, 164. [Google Scholar] [CrossRef]
- Tahavorgar, A.; Vafa, M.; Shidfar, F.; Gohari, M.; Heydari, I. Whey protein preloads are more beneficial than soy protein preloads in regulating appetite, calorie intake, anthropometry, and body composition of overweight and obese men. Nutr. Res. 2014, 34, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Tahavorgar, A.; Vafa, M.; Shidfar, F.; Gohari, M.; Heydari, I. Beneficial effects of whey protein preloads on some cardiovascular diseases risk factors of overweight and obese men are stronger than soy protein preloads—A randomized clinical trial. J. Nutr. Intermed. Metab. 2015, 2, 69–75. [Google Scholar] [CrossRef]
- Lollo, P.C.B.; Amaya-Farfan, J.; Faria, I.C.; Salgado, J.V.V.; Chacon-Mikahil, M.P.T.; Cruz, A.G.; Oliveira, C.A.F.; Montagner, P.C.; Arruda, M. Hydrolysed whey protein reduces muscle damage markers in Brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention. Int. Dairy J. 2014, 34, 19–24. [Google Scholar] [CrossRef]
- Cheung, L.K.Y.; Aluko, R.E.; Cliff, M.A.; Li-Chan, E.C.Y. Effects of exopeptidase treatment on antihypertensive activity and taste attributes of enzymatic whey protein hydrolysates. J. Funct. Foods 2015, 13, 262–275. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Kobayashi, T. Whey Peptides Prevent Chronic Ultraviolet B Radiation–Induced Skin Aging in Melanin-Possessing Male Hairless Mice. J. Nutr. 2013, 144, 27–32. [Google Scholar] [CrossRef]
- Dalziel, J.E.; Anderson, R.C.; Bassett, S.A.; Lloyd-West, C.M.; Haggarty, N.W.; Roy, N.C. Influence of Bovine Whey Protein Concentrate and Hydrolysate Preparation Methods on Motility in the Isolated Rat Distal Colon. Nutrients 2016, 8, 809. [Google Scholar] [CrossRef]
- Nilaweera, K.N.; Cabrera-Rubio, R.; Speakman, J.R.; O’Connor, P.M.; McAuliffe, A.; Guinane, C.M.; Lawton, E.M.; Crispie, F.; Aguilera, M.; Stanley, M.; et al. Whey protein effects on energy balance link the intestinal mechanisms of energy absorption with adiposity and hypothalamic neuropeptide gene expression. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E1–E11. [Google Scholar] [CrossRef]
- Hwang, J.S.; Han, S.G.; Lee, C.H.; Seo, H.G. Whey Protein Attenuates Angiotensin II-Primed Premature Senescence of Vascular Smooth Muscle Cells through Upregulation of SIRT1. Korean J. Food Sci. Anim. Resour. 2017, 37, 917–925. [Google Scholar] [CrossRef]
- Garg, G.; Singh, S.; Singh, A.K.; Rizvi, S.I. Whey protein concentrate supplementation protects rat brain against aging-induced oxidative stress and neurodegeneration. Appl. Physiol. Nutr. Metab. 2017, 43, 437–444. [Google Scholar] [CrossRef]
- Flaim, C.; Kob, M.; Di Pierro, A.M.; Herrmann, M.; Lucchin, L. Effects of a whey protein supplementation on oxidative stress, body composition and glucose metabolism among overweight people affected by diabetes mellitus or impaired fasting glucose: A pilot study. J. Nutr. Biochem. 2017, 50, 95–102. [Google Scholar] [CrossRef]
- Ney, D.M.; Blank, R.D.; Hansen, K.E. Advances in the nutritional and pharmacological management of phenylketonuria. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Whey protein hydrolysate supplementation accelerates recovery from exercise-induced muscle damage in females. Appl. Physiol. Nutr. Metab. 2017, 43, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Shirato, M.; Tsuchiya, Y.; Sato, T.; Hamano, S.; Gushiken, T.; Kimura, N.; Ochi, E. Effects of combined β-hydroxy-β-methylbutyrate (HMB) and whey protein ingestion on symptoms of eccentric exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2016, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Nisishima, L.H.; Carneiro, E.M.; Amaya-Farfan, J. Whey Protein Hydrolysate Enhances HSP90 but Does Not Alter HSP60 and HSP25 in Skeletal Muscle of Rats. PLoS ONE 2014, 9, e83437. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Muro Urista, C.; Alvarez Fernandez, R.; Riera Rodriguez, F.; Arana Cuenca, A.; Tellez Jurado, A. Review: Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef]
- Welsh, G.; Ryder, K.; Brewster, J.; Walker, C.; Mros, S.; Bekhit, A.E.-D.A.; McConnell, M.; Carne, A. Comparison of bioactive peptides prepared from sheep cheese whey using a food-grade bacterial and a fungal protease preparation. Int. J. Food Sci. Technol. 2017, 52, 1252–1259. [Google Scholar] [CrossRef]
- Rocha, G.F.; Kise, F.; Rosso, A.M.; Parisi, M.G. Potential antioxidant peptides produced from whey hydrolysis with an immobilized aspartic protease from Salpichroa origanifolia fruits. Food Chem. 2017, 237, 350–355. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Hsieh, C.-C.; Martínez-Villaluenga, C. Food Bioactive Compounds against Diseases of the 21st Century 2016. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Brandelli, A.; Daroit, D.J.; Corrêa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Brumini, D.; Criscione, A.; Bordonaro, S.; Vegarud, G.E.; Marletta, D. Whey proteins and their antimicrobial properties in donkey milk: A brief review. Dairy Sci. Technol. 2016, 96, 1–14. [Google Scholar] [CrossRef]
- Mohan, A.; Udechukwu, M.C.; Rajendran, S.R.C.K.; Udenigwe, C.C. Modification of peptide functionality during enzymatic hydrolysis of whey proteins. RSC Adv. 2015, 5, 97400–97407. [Google Scholar] [CrossRef]
- Adams, R.L.; Broughton, K.S. Insulinotropic Effects of Whey: Mechanisms of Action, Recent Clinical Trials, and Clinical Applications. Ann. Nutr. Metab. 2016, 69, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey protein hydrolysates as a source of bioactive peptides for functional foods—Biotechnological facilitation of industrial scale-up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Iltchenco, S.; Preci, D.; Bonifacino, C.; Fraguas, E.F.; Steffens, C.; Panizzolo, L.A.; Colet, R.; Fernandes, I.A.; Abirached, C.; Valduga, E.; et al. Whey protein concentration by ultrafiltration and study of functional properties. Ciênc. Rural 2018, 48. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; FitzGerald, R.J. Strategies for the discovery and identification of food protein-derived biologically active peptides. Trends Food Sci. Technol. 2017, 69, 289–305. [Google Scholar] [CrossRef] [Green Version]
- Dupont, D. Peptidomic as a tool for assessing protein digestion. Curr. Opin. Food Sci. 2017, 16, 53–58. [Google Scholar] [CrossRef]
- Valdés, A.; Cifuentes, A.; León, C. Foodomics evaluation of bioactive compounds in foods. TrAC Trends Anal. Chem. 2017, 96, 2–13. [Google Scholar] [CrossRef]
- Agyei, D.; Tsopmo, A.; Udenigwe, C.C. Bioinformatics and peptidomics approaches to the discovery and analysis of food-derived bioactive peptides. Anal. Bioanal. Chem. 2018, 410, 3463–3472. [Google Scholar] [CrossRef]
- de Jong, E.; Higson, A.; Walsh, P.; Wellissch, M. Bio-based chemicals: Value added products from biorefineries. 2012. Available online: http://www.iea-bioenergy.task42-biorefineries.com/publications/reports (accessed on 15 June 2019).
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review. Renew. Sustain. Energy Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- Iriondo-DeHond, M.; Miguel, E.; Del Castillo, M.D. Food Byproducts as Sustainable Ingredients for Innovative and Healthy Dairy Foods. Nutrients 2018, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Fermoso, F.G.; Serrano, A.; Alonso-Fariñas, B.; Fernández-Bolaños, J.; Borja, R.; Rodríguez-Gutiérrez, G. Valuable Compound Extraction, Anaerobic Digestion, and Composting: A Leading Biorefinery Approach for Agricultural Wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef] [PubMed]
- Popa, V.I. 1—Biomass for Fuels and Biomaterials. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Popa, V., Volf, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–37. [Google Scholar]
- Fu, W.; Mathews, A.P. Lactic acid production from lactose by Lactobacillus plantarum: Kinetic model and effects of pH, substrate, and oxygen. BioChem. Eng. J. 1999, 3, 163–170. [Google Scholar] [CrossRef]
- Petrov, K.K.; Yankov, D.S.; Beschkov, V.N. Lactic acid fermentation by cells of Lactobacillus rhamnosus immobilizedin polyacrylamide gel. World J. Microbiol. Biotechnol. 2006, 22, 337–345. [Google Scholar] [CrossRef]
- Sørensen, K.I.; Curic-Bawden, M.; Junge, M.P.; Janzen, T.; Johansen, E. Enhancing the Sweetness of Yoghurt through Metabolic Remodeling of Carbohydrate Metabolism in Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. Appl. Environ. Microbiol. 2016, 82, 3683–3692. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F.; Knill, C.J.; Kosseva, M. Production of L(+) lactic acid using Lactobacillus casei from whey. Braz. Arch. Biol. Technol. 2010, 53, 219–226. [Google Scholar] [CrossRef]
- Rama, G.R.; Kuhn, D.; Beux, S.; Maciel, M.J.; Volken de Souza, C.F. Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int. Dairy J. 2019, 98, 25–37. [Google Scholar] [CrossRef]
- Amaro, T.M.M.M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Production. Front. Microbiol. 2019, 10, 992. [Google Scholar] [CrossRef]
- Brown, K.; Harrison, J.; Bowers, K. Production of Oxalic Acid from Aspergillus niger and Whey Permeate. Water Air Soil Pollut. 2017, 229, 5. [Google Scholar] [CrossRef]
- Pasotti, L.; Zucca, S.; Casanova, M.; Micoli, G.; Cusella De Angelis, M.G.; Magni, P. Fermentation of lactose to ethanol in cheese whey permeate and concentrated permeate by engineered Escherichia coli. BMC Biotechnol. 2017, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hua, X.; Huang, L.; Xu, Y. Bio-utilization of cheese manufacturing wastes (cheese whey powder) for bioethanol and specific product (galactonic acid) production via a two-step bioprocess. Bioresour. Technol. 2019, 272, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, K. Technologies for the utilisation of biogenic waste in the bioeconomy. Food Chem. 2016, 198, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bekatorou, A.; Plioni, I.; Sparou, K.; Maroutsiou, R.; Tsafrakidou, P.; Petsi, T.; Kordouli, E. Bacterial Cellulose Production Using the Corinthian Currant Finishing Side-Stream and Cheese Whey: Process Optimization and Textural Characterization. Foods 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Kopsahelis, N.; Dimou, C.; Papadaki, A.; Xenopoulos, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Papanikolaou, S.; Koutinas, A.A. Refining of wine lees and cheese whey for the production of microbial oil, polyphenol-rich extracts and value-added co-products. J. Chem. Technol. Biotechnol. 2018, 93, 257–268. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y.; Kanellaki, M. Novel probiotic whey cheese with immobilized lactobacilli on casein. LWT 2017, 86, 627–634. [Google Scholar] [CrossRef]
- Paximada, P.; Koutinas, A.A.; Scholten, E.; Mandala, I.G. Effect of bacterial cellulose addition on physical properties of WPI emulsions. Comparison with common thickeners. Food Hydrocoll. 2016, 54, 245–254. [Google Scholar] [CrossRef]
- Gama, A.P.; Hung, Y.-C.; Adhikari, K. Optimization of Emulsifier and Stabilizer Concentrations in a Model Peanut-Based Beverage System: A Mixture Design Approach. Foods 2019, 8, 116. [Google Scholar] [CrossRef]
- Peng, J.; Calabrese, V.; Geurtz, J.; Velikov, K.P.; Venema, P.; van der Linden, E. Composite Gels Containing Whey Protein Fibrils and Bacterial Cellulose Microfibrils. J. Food Sci. 2019, 84, 1094–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavyko, O.; Bondarchuk, M. Ice-cream with functional properties as a means of commercial networks assortment extension and population feeding improving. J. Hyg. Eng. Des. 2019, 26, 127–133. [Google Scholar]
- Agustini, T.W.; Ma’ruf, W.F.; Widayat, W.; Suzery, M.; Hadiyanto, H.; Benjakul, S. Application of Spirulina platensis on ice cream and soft cheese with respect to their nutritional and sensory perspectives. J. Teknol. 2016, 78, 245–251. [Google Scholar] [CrossRef]
- Moreira, J.B.; Lim, L.-T.; da Zavareze, R.E.; Dias, A.R.G.; Costa, J.A.V.; de Morais, M.G. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocoll. 2019, 93, 131–136. [Google Scholar] [CrossRef]
- Batista, A.P.; Nunes, M.C.; Fradinho, P.; Gouveia, L.; Sousa, I.; Raymundo, A.; Franco, J.M. Novel foods with microalgal ingredients—Effect of gel setting conditions on the linear viscoelasticity of Spirulina and Haematococcus gels. J. Food Eng. 2012, 110, 182–189. [Google Scholar] [CrossRef]
- Gouveia, L. Spirulina maxima and Diacronema vlkianum microalgae in vegetable gelled desserts. Nutr. Food Sci. 2008, 38, 492–501. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]




| Microorganism | Supplementation of CW Medium | Composition of Total Carotenoids | Concentration (mg/L) | Yield (mg/g) 1 | Reference |
|---|---|---|---|---|---|
| Blakeslea trispora ATCC 14271 & ATCC 14272 | Tween 80, Span 80, β-ionone | β-carotene, γ-carotene, lycopene | 1620.0 | 222.0 | [76] |
| Blakeslea trispora ATCC 14271 & ATCC 14272 | Tween 80, Span 80, β-ionone | β-carotene, γ-carotene, lycopene | 1360.0 | 175.0 | [83] |
| Blakeslea trispora ATCC 14271 & ATCC 14272 | Tween 80, Span 80, vegetable oils | β-carotene, γ-carotene, lycopene | ~672.0 | 16.0 | [82] |
| Blakeslea trispora ATCC 14271 & ATCC 14272 | Tween 80, Span 80, vegetable oils, antioxidants and other nutrients | β-carotene | 350.0 | 11.6 | [84] |
| Blakeslea trispora ATCC 14271 &ATCC 14272 | Tween 80, Span 80, vegetable oils | N.S. 2 | 376.0 | 8.0 | [85] |
| Mucor azygosporus MTCC 414 | Soluble starch | β-carotene | 3.5 | 0.38 | [86] |
| Rhodotorula mucilaginosa NRRL 2502 | N.S. 2 | 70.0 | 29.2 | [80] | |
| Rhodotorula mucilaginosa CCY 20-7-31 | β-carotene | 11.3 | 0.38 | [87] | |
| Rhodotorula glutinis CCY 20-2-26 | β-carotene | 51.2 | 1.48 | [87] | |
| Rhodotorula rubra GED5 co-culture with Kluyveromyces lactis MP11 | Torularhodin, β-carotene, torulene | 10.2 | 0.42 | [88] | |
| Rhodotorula glutinis 22P co-culture with Lactobacillus helveticus | β-carotene, torularhodin, torulene | 8.09 | 0.27 | [89] | |
| Rhodotorula rubra GED8 co-culture with Lactobacillus bulbaricus, Streptococcus thermophilus | β-carotene, torulene, torularhodin | 13.1 | 0.50 | [90] | |
| Sporidiobolus salmonicor CBS 2636 | N.S. 2 | 0.91 | 0.25 | [91] | |
| Sporobolomyces roseus CCY 19-4-8 | β-carotene | 29.4 | 2.89 | [87] | |
| Dietzia natronolimnaea HS-1 | canthaxanthin (2.87 mg/L) | 3.06 | ~0.9 | [77] |
| Microorganism | Carbon Source | BC (g/L) | Reference |
|---|---|---|---|
| K. sucrofermentans DSM 15973 | Synthetic lactose | 1.6 | [100] |
| G. xylinus ATCC 53524 | Synthetic galactose | 0.1 | [108] |
| A. xylinum 10821 | Cheese whey | 0.04 | [109] |
| A. xylinum 23770 | Cheese whey | 1.13 | [109] |
| G. sacchari | Cheese whey | 0.15 | [110] |
| A. xylinum mutant | Cheese whey | 1.82 | [111] |
| G. xylinus PTCC 1734 | Hydrolyzed cheese whey | 3.55 | [112] |
| G. sucrofermentans B-11267 | Cheese whey | 5.4 | [113] |
| Substrate | Promoting Compound | Functionality | Reference |
|---|---|---|---|
| WPI | Almonds, walnut oil | Water barrier improvement | [148] |
| β-cyclodextrin/eugenol, carvacrol | Antimicrobial component delivery | [149] | |
| Lysozyme | Antimicrobial component delivery | [150] | |
| Montmorillonite nanoplatelets | Oxygen barriers improvement | [151] | |
| Montmorillonite clay nanoparticles | Thermal stability, water vapor permeability | [152] | |
| Nanocrystalline cellulose, transglutaminase | Improved mechanical properties | [153] | |
| Oat husk nanocellulose | Enhanced tensile strength, solubility, decreased elongation at break and moisture content, decreased transparency and water vapor permeability | [154] | |
| Pullulan, montmorillonite | Improve the mechanical properties, thermal properties, and water resistance | [155] | |
| Sodium laurate-modified TiO2 nanoparticles | Water vapor permeability decreased, tensile strength increase, decreased transparency | [156] | |
| Starch | Water vapor permeability, microstructure | [157] | |
| Zein | Enhanced water solubility and heat-sealablity | [158] | |
| Zein nanoparticles | Improved moisture barrier and mechanical properties | [159] | |
| WPC | Cinnamon essential oil | Antimicrobial | [160] |
| Glucerol, pullulan, beeswax | Improved color indices, diminished water solubility and water vapor permeability, and increased tensile strength | [161] | |
| Immunoglobulins | Increase stickiness, adhesion, and tensile strength of the films | [162] | |
| Liquid smoke | Antimicrobial/improved mechanical properties | [163] | |
| Montmorilonite, lycopene | Antioxidant activity and UV-vis light protection/mechanical properties improvement | [164] | |
| Rosmarinic acid, carnosol, carnosic acid | |||
| Sodium alginate, pectin, carrageenan, locust been gum/L. rhamnosus | Enhanced survival during drying and storage, reduced film water vapor permeability | [165] | |
| Sunflower, beeswax | Water vapor permeability | [166] |
| Biological Function | Formulation | Test Model | References |
|---|---|---|---|
| Anti-diabetic | Whey protein hydrolysate | Insulin-resistant rats | [239] |
| Whey protein | Human | [240] | |
| Anti-inflammatory | β-lactoglobulin hydrolysate | In vitro | [241] |
| Anti-hypertensive | Whey protein concentrate | In vitro | [242] |
| Anti-obesity | Whey protein concentrate | Obese human | [243] |
| Whey protein concentrate | Obese human | [244] | |
| Antitumor | β-lactoglobulin hydrolysate | In vitro | [231] |
| Benefit in resistant exercise | Hydrolyzed whey protein | Human | [245] |
| Blood pressure lowering | Whey protein hydrolysate | Rats | [246] |
| Dermatoprotective | Whey peptide | Mice | [247] |
| GI motility | Whey protein concentrate, Whey protein Hydrolysate | Mice | [248] |
| Gut and energy homeostasis | Whey protein isolate | Mice | [249] |
| Hypolipidemic | Whey protein | Mice | [234] |
| Muscle protein synthesis/glycogen content | Whey protein hydrolysate | Mice | [226] |
| Osteroprotection | Whey protein derived dipeptide Glu-Glu | In vitro | [250] |
| Oxidative stress | Whey protein concentrate | Mice | [251] |
| Oxidative stress/Glucose metabolism | Whey protein isolate | Overweight/obese patients | [252] |
| Phenylketonouria therapy | Whey protein glycomacropeptide | Human/mice | [253] |
| Recovery of muscle functions | Whey protein hydrolysate | Human | [254] |
| Whey protein | Human | [255] | |
| Sceletical muscle protection | Whey protein hydrolysate | Rats | [256] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. https://doi.org/10.3390/foods8080347
Lappa IK, Papadaki A, Kachrimanidou V, Terpou A, Koulougliotis D, Eriotou E, Kopsahelis N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods. 2019; 8(8):347. https://doi.org/10.3390/foods8080347
Chicago/Turabian StyleLappa, Iliada K., Aikaterini Papadaki, Vasiliki Kachrimanidou, Antonia Terpou, Dionysios Koulougliotis, Effimia Eriotou, and Nikolaos Kopsahelis. 2019. "Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications" Foods 8, no. 8: 347. https://doi.org/10.3390/foods8080347