Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing
Abstract
:1. Introduction
2. Bibliographic Research Methodology
3. Pesticides in Fruits and Vegetables
3.1. Classification and Properties
3.2. Toxicity and Maximal Allowed Concentration
3.3. Fruits and Vegetables with the Highest Presence of Pesticides
3.4. Pesticide Application and Physical Location in Fruits and Vegetables
3.5. Pesticide Analytical Determination
4. Fruit and Vegetable Waste (FVW)
4.1. Common Types of Fruit and Vegetable Wastes
4.2. Potential Applications of Fruit and Vegetable Wastes
4.3. FVW by-Products Processing
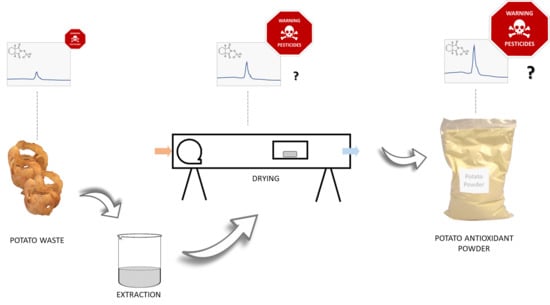
5. Drying and Extraction in FVW by-Products Processing
5.1. Process Intensification (PI)
5.1.1. Pulsed Electric Field (PEF)
5.1.2. Ultrasound (US)
5.1.3. Microwaves (MW)
6. Processing on Pesticide Residues Reduction
6.1. General Food Processing
6.2. Changes in Pesticide Residues during Drying
6.3. Change in Pesticide Residues during Extraction
6.4. Impact of Intensification Technologies on Pesticide Reduction
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [Green Version]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- Kjørstad, E. These Are the Fruits and Vegetables We Waste the Most. Available online: http://sciencenordic.com/these-are-fruits-and-vegetables-we-waste-most (accessed on 25 September 2018).
- Lokesh, K.; Ladu, L.; Summerton, L. Bridging the gaps for a “circular” bioeconomy: Selection criteria, bio-based value chain and stakeholder mapping. Sustainability 2018, 10, 1695. [Google Scholar] [CrossRef] [Green Version]
- Shokrzadeh, M.; Saravi, S.S.S. Pesticides in Agricultural Products: Analysis, Reduction, Prevention. In Pesticides-Formulations, Effects, Fate; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2012; pp. 225–242. [Google Scholar]
- Roberts, J.R.; Reigart, R.J. Organophosphate Insecticides. In Recognition and Management of Pesticide Poisonings; Roberts, J.R., Reigart, R.J., Eds.; The Environmental Protection Agency’s Office of Pesticide Programs: Washington, DC, USA, 2013; pp. 43–55. ISBN 9780470699010. [Google Scholar]
- EWG Dirty DozenTM EWG’s 2019 Shopper’s Guide to Pesticides in ProduceTM. Available online: https://www.ewg.org/foodnews/dirty-dozen.php (accessed on 30 April 2019).
- Rawn, D.F.K.; Quade, S.C.; Sun, W.F.; Fouguet, A.; Bélanger, A.; Smith, M. Captan residue reduction in apples as a result of rinsing and peeling. Food Chem. 2008, 109, 790–796. [Google Scholar] [CrossRef]
- Sharma, R.; Oberoi, H.S.; Dhillon, G.S. Fruit and Vegetable Processing Waste: Renewable Feed Stocks for Enzyme Production. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Dhillon, G.S., Kaur, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 23–59. ISBN 9780128026120. [Google Scholar]
- Yadav, Y.-C.; Devi, N.-L. Pesticides Classification and Its Impact on Human and Environment. In Environmental Science and Engineering; Chandra, R., Gurjar, B.R., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2017; Volume 6, pp. 140–158. ISBN 1-62699-094-8. [Google Scholar]
- Suwalsky, M.; Rodríguez, C.; Villena, F.; Sotomayor, C.P. Human erythrocytes are affected by the organochloride insecticide chlordane. Food Chem. Toxicol. 2005, 43, 647–654. [Google Scholar] [CrossRef]
- Xu, D.; Liang, D.; Guo, Y.; Sun, Y. Endosulfan causes the alterations of DNA damage response through ATM-p53 signaling pathway in human leukemia cells. Environ. Pollut. 2018, 238, 1048–1055. [Google Scholar] [CrossRef]
- Zacharia, J.T. Identity, Physical and Chemical Properties of Pesticides. In Pesticides in the Modern World-Trends in Pesticides Analysis; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 1–20. ISBN 978-953-307-437-5. [Google Scholar]
- Edwards, F.L.; Tchounwou, P.B. Environmental toxicology and health effects associated with methyl parathion exposure—A scientific review. Int. J. Environ. Res. Public Health 2005, 2, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Krstic, D.Z.; Colovic, M.; Bavcon Kralj, M.; Franko, M.; Krinulovic, K.; Trebse, P.; Vasic, V. Inhibition of AChE by malathion and some structurally similar compounds. J. Enzyme Inhib. Med. Chem. 2008, 23, 562–573. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Chen, J.; Qiu, Y.; Hong, X.; Xia, Y.; Feng, T.; Liu, J.; Song, L.; Zhang, Z.; Wang, X. Carbaryl inhibits basal and FSH-induced progesterone biosynthesis of primary human granulosa-lutein cells. Toxicology 2006, 220, 37–45. [Google Scholar] [CrossRef]
- Dias, E.; Morais, S.; Ramalheira, E.; Pereira, M.L. Characterization of the toxicological effects of aminocarb on rats: Hematological, biochemical, and histological analyses. J. Toxicol. Environ. Heal-Part A Curr. Issues 2014, 77, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, P.C.; Luchmann, K.H.; Ribeiro, A.B.; Veras, M.M.; Correa, J.R.M.B.; Nogueira, A.J.; Bainy, A.C.D.; Carvalho, P.S.M. Cholinesterase inhibition and behavioral toxicity of carbofuran on Oreochromis niloticus early life stages. Aquat. Toxicol. 2011, 105, 312–320. [Google Scholar] [CrossRef]
- Tiwari, S.; Tiwari, R.; Singh, A. Impact of cypermethrin on fingerlings of common edible carp (Labeo rohita). Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paravani, E.V.; Simoniello, M.F.; Poletta, G.L.; Casco, V.H. Cypermethrin induction of DNA damage and oxidative stress in zebrafish gill cells. Ecotoxicol. Environ. Saf. 2019, 173, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Yadav, S.; Nehra, R.; Pundir, C.S. An amperometric hypoxanthine biosensor based on Au@FeNPs for determination of hypoxanthine in meat samples. Int. J. Biol. Macromol. 2013, 62, 629–635. [Google Scholar] [CrossRef]
- Jayaraj, R.; Megha, P.; Sreedev, P. Review Article. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Fenik, J.; Tankiewicz, M.; Biziuk, M. Properties and determination of pesticides in fruits and vegetables. TrAC-Trends Anal. Chem. 2011, 30, 814–826. [Google Scholar] [CrossRef]
- Sood, C.; Jaggi, S.; Kumar, V.; Ravindranath, S.D.; Shanker, A. How manufacturing processes affect the level of pesticide residues in tea. Sci. Food Agric. 2004, 84, 2123–2127. [Google Scholar] [CrossRef]
- Karthika, C.; Muraleedharan, N. Influence of manufacturing process on the residues of certain fungicides used on tea. Toxicol. Environ. Chem. 2010, 92, 1249–1257. [Google Scholar] [CrossRef]
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576. [Google Scholar] [CrossRef]
- PCPA Maximum Residue Limits for Pesticides. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/pesticides-pest-management/public/protecting-your-health-environment/pesticides-food/maximum-residue-limits-pesticides.html (accessed on 8 April 2019).
- EFSA. 2008 Annual Report on Pesticide Residues according to Article 32 of Regulation (EC) No 396/2005; EFSA: Parma, Italy, 2010; Volume 8. [Google Scholar]
- CEC North American Regional Action Plan on Lindane and other Hexachlorocyclohexane Isomers: Final Evaluation Report; Commission for Environmental Cooperation: Montreal, QC, Canada, 2013.
- Pesticide Action Network-What’s on My Food. Available online: http://www.whatsonmyfood.org/index.jsp (accessed on 15 April 2019).
- Knoche, M.; Lang, A. Ongoing growth challenges fruit skin integrity. CRC Crit. Rev. Plant Sci. 2017, 36, 190–215. [Google Scholar] [CrossRef]
- Keikotlhaile, B.M.; Spanoghe, P.; Mebdoua, S.; Keikotlhaile, B.M.; Spanoghe, P. Pesticide residues in fruits and vegetables. In Bioactive Molecules in Food; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; p. 39. ISBN 978-953-307-532-7. [Google Scholar]
- Taube, J.; Vorkamp, K.; Förster, M.; Herrmann, R. Pesticide residues in biological waste. Chemosphere 2002, 49, 1357–1365. [Google Scholar] [CrossRef]
- Soliman, K.M. Changes in concentration of pesticide residues in potatoes during washing and home preparation. Food Chem. Toxicol. 2001, 39, 887–891. [Google Scholar] [CrossRef]
- Kong, Z.; Dong, F.; Xu, J.; Liu, X.; Zhang, C.; Li, J.; Li, Y.; Chen, X.; Shan, W.; Zheng, Y. Determination of difenoconazole residue in tomato during home canning by UPLC-MS/MS. Food Control 2012, 23, 542–546. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.K. Behavior of pesticides in tomatoes during commercial and home preparation. Food Chem. 1999, 65, 509–514. [Google Scholar] [CrossRef]
- Saber, A.N.; Malhat, F.M.; Badawy, H.M.A.; Barakat, D.A. Dissipation dynamic, residue distribution and processing factor of hexythiazox in strawberry fruits under open field condition. Food Chem. 2015, 196, 1108–1116. [Google Scholar] [CrossRef]
- Prodhan, M.D.; Alam, S.N.; Uddin, M.J. Analytical Methods in Measuring Pesticides in Foods. In Pesticide Residue in Foods: Sources, Management, and Control; Springer International Publishing: Cham, Switzerland, 2017; pp. 135–145. ISBN 9783319526836. [Google Scholar]
- Berk, Z. Chapter 11—Extraction. In Food Science and Technology; Berk, Z., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 287–309. ISBN 978-0-12-415923-5. [Google Scholar]
- de Pinho, G.P.; Neves, A.A.; de Queiroz, M.E.L.R.; Silvério, F.O. Optimization of the liquid-liquid extraction method and low temperature purification (LLE-LTP) for pesticide residue analysis in honey samples by gas chromatography. Food Control 2010, 21, 1307–1311. [Google Scholar] [CrossRef]
- Goulart, S.M.; Alves, R.D.; Neves, A.A.; de Queiroz, J.H.; de Assis, T.C.; de Queiroz, M.E.L.R. Optimization and validation of liquid-liquid extraction with low temperature partitioning for determination of carbamates in water. Anal. Chim. Acta 2010, 671, 41–47. [Google Scholar] [CrossRef]
- Hennion, M.-C. Solid-phase extraction: Method development, sorbents, and coupling with liquid chromatography. J. Chromatogr. A 1999, 856, 3–54. [Google Scholar] [CrossRef]
- Torreti, L.; Simonella, A.; Dossena, A.; Torreti, E. Determination of organochlorine pesticide residues by solid phase extraction and dual-column HRGC. J. High. Resolut. Chromatogr. 1992, 15, 99–101. [Google Scholar] [CrossRef]
- Štajnbaher, D.; Zupančič-Kralj, L. Multiresidue method for determination of 90 pesticides in fresh fruits and vegetables using solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 1015, 185–198. [Google Scholar] [CrossRef]
- Sivaperumal, P.; Anand, P.; Riddhi, L. Rapid determination of pesticide residues in fruits and vegetables, using ultra-high-performance liquid chromatography/time-of-flight mass spectrometry. Food Chem. 2015, 168, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, M.J.; Pawliszyn, J. Solid-Phase Microextraction: A solvent-free alternative for sample preparation. Anal. Chem. 1994, 66, 844A–853A. [Google Scholar] [CrossRef]
- Zambonin, C.G.; Quinto, M.; De Vietro, N.; Palmisano, F. Solid-phase microextraction—Gas chromatography mass spectrometry: A fast and simple screening method for the assessment of organophosphorus pesticides residues in wine and fruit juices. Food Chem. 2004, 86, 269–274. [Google Scholar] [CrossRef]
- Menezes Filho, A.; dos Santos, F.N.; de Paula Pereira, P.A. Development, validation and application of a methodology based on solid-phase micro extraction followed by gas chromatography coupled to mass spectrometry (SPME/GC-MS) for the determination of pesticide residues in mangoes. Talanta 2010, 81, 346–354. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, S.; Iqbal, M.M.; Javed, M.; Bahadur, A.; Yasien, S.; Hurr, A.; Ahmad, N.; Raheel, M.; Liu, G. Modified QuEChERS extraction method followed by simultaneous quantitation of nine multi-class pesticides in human blood and urine by using GC-MS. J. Chromatogr. B 2020, 1152, 122227. [Google Scholar] [CrossRef]
- Viera, M.S.; Rizzetti, T.M.; de Souza, M.P.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Multiresidue determination of pesticides in crop plants by the quick, easy, cheap, effective, rugged, and safe method and ultra-high-performance liquid chromatography tandem mass spectrometry using a calibration based on a single level standard addition in the sample. J. Chromatogr. A 2017, 1526, 119–127. [Google Scholar]
- Sojka, M.; Miszczak, A.; Sikorski, P.; Zagibajlo, K.; Karlinska, E.; Kosmala, M. Pesticide residue levels in strawberry processing by-products that are rich in ellagitannins and an assessment of their dietary risk to consumers. NFS J. 2015, 1, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Trevisan, M.T.S.; Owen, R.W.; Calatayud-Vernich, P.; Breuer, A.; Picó, Y. Pesticide analysis in coffee leaves using a quick, easy, cheap, effective, rugged and safe approach and liquid chromatography tandem mass spectrometry: Optimization of the clean-up step. J. Chromatogr. A 2017, 1512, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Shin, Y.; Lee, J.; Lee, J.; Kim, B.J.; Kim, J.H. Simultaneous analysis of 310 pesticide multiresidues using UHPLC-MS/MS in brown rice, orange, and spinach. Chemosphere 2018, 207, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Afshar Mogaddam, M.R.; Rezaee Aghdam, S.; Nouri, N.; Bamorrowat, M. Application of elevated temperature-dispersive liquid-liquid microextraction for determination of organophosphorus pesticides residues in aqueous samples followed by gas chromatography-flame ionization detection. Food Chem. 2016, 212, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Khoshmaram, L.; Nabil, A.A.A. Determination of pyrethroid pesticides residues in vegetable oils using liquid-liquid extraction and dispersive liquid-liquid microextraction followed by gas chromatography-flame ionization detection. J. Food Compos. Anal. 2014, 34, 128–135. [Google Scholar] [CrossRef]
- Balinova, A.M.; Mladenova, R.I.; Shtereva, D.D. Effects of processing on pesticide residues in peaches intended for baby food. Food Addit. Contam. 2006, 23, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Sapahin, H.A.; Makahleh, A.; Saad, B. Determination of organophosphorus pesticide residues in vegetables using solid phase micro-extraction coupled with gas chromatography-flame photometric detector. Arab. J. Chem. 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mac Loughlin, T.M.; Peluso, M.L.; Etchegoyen, M.A.; Alonso, L.L.; de Castro, M.C.; Percudani, M.C.; Marino, D.J.G. Pesticide residues in fruits and vegetables of the argentine domestic market: Occurrence and quality. Food Control 2018, 93, 129–138. [Google Scholar] [CrossRef]
- Stoytcheva, M. Pesticides-Formulations, Effects, Fate; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; ISBN 9789533075327. [Google Scholar]
- Bagheri, H.; Yamini, Y.; Safari, M.; Asiabi, H.; Karimi, M.; Heydari, A. Simultaneous determination of pyrethroids residues in fruit and vegetable samples via supercritical fluid extraction coupled with magnetic solid phase extraction followed by HPLC-UV. J. Supercrit. Fluids 2016, 107, 571–580. [Google Scholar] [CrossRef]
- Timofeeva, I.; Shishov, A.; Kanashina, D.; Dzema, D.; Bulatov, A. On-line in-syringe sugaring-out liquid-liquid extraction coupled with HPLC-MS/MS for the determination of pesticides in fruit and berry juices. Talanta 2017, 167, 761–767. [Google Scholar] [CrossRef]
- Martins, M.L.; Kemmerich, M.; Prestes, O.D.; Maldaner, L.; Jardim, I.C.S.F.; Zanella, R. Evaluation of an alternative fluorinated sorbent for dispersive solid-phase extraction clean-up of the quick, easy, cheap, effective, rugged, and safe method for pesticide residues analysis. J. Chromatogr. A 2017, 1514, 36–43. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- De Souza, V.B.; Thomazini, M.; Balieiro, J.C.D.C.; Fávaro-Trindade, C.S. Effect of spray drying on the physicochemical properties and color stability of the powdered pigment obtained from vinification byproducts of the Bordo grape (Vitis labrusca). Food Bioprod. Process. 2015, 93, 39–50. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Sepelev, I.; Galoburda, R. Industrial potato peel waste application in food production: A Review. Res. Rural Dev. 2015, 1, 130–136. [Google Scholar]
- Martins, M.P.; Cortés, E.J.; Eim, V.; Mulet, A.; Cárcel, J.A. Stabilization of apple peel by drying. Influence of temperature and ultrasound application on drying kinetics and product quality. Dry. Technol. 2019, 37, 559–568. [Google Scholar] [CrossRef]
- Do Nascimento, E.M.G.C.; Mulet, A.; Ascheri, J.L.R.; De Carvalho, C.W.P.; Cárcel, J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2015, 170, 108–118. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Proanthocyanidin composition and antioxidant potential of the stem winemaking byproducts from 10 different grape varieties (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 11850–11858. [Google Scholar] [CrossRef]
- Garau, M.C.; González-Centeno, M.R.; Luna, J.M.; Negre, A.; Rosselló, C.; Femenia, A. Potential of landrace winery byproducts (Vitis vinifera L.) as a source of phenolic compounds with antioxidant properties. Journal International des Sciences de la Vigne et du Vin 2015, 49, 241–251. [Google Scholar]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica-Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Rosales Soto, M.U.; Brown, K.; Ross, C.F. Antioxidant activity and consumer acceptance of grape seed flour-containing food products. Int. J. Food Sci. Technol. 2012, 47, 592–602. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Meza-Velázquez, J.A.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydr. Polym. 2014, 106, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Candelas-Cadillo, M.G.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of hemicelluloses from grape pomace using response surface methodology. Carbohydr. Polym. 2016, 138, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Umaña, M.; Eim, V.; Garau, C.; Rosselló, C.; Simal, S. Ultrasound-assisted extraction of ergosterol and antioxidant components from mushroom by-products and the attainment of a β-glucan rich residue. Food Chem. 2020, 332, 127390. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, F.; Lan, X.; Gong, S.; Wang, Z. Characteristics of pectin from black cherry tomato waste modified by dynamic high-pressure microfluidization. J. Food Eng. 2018, 216, 90–97. [Google Scholar] [CrossRef]
- Četojević-Simin, D.; Djilas, S.; Stajčić, S.; Ćetković, G.; Čanadanović-Brunet, J.; Mandić, A. Tomato waste: Carotenoids content, antioxidant and cell growth activities. Food Chem. 2014, 172, 225–232. [Google Scholar]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef] [Green Version]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Sójka, M.; Klimczak, E.; Macierzynski, J.; Kołodziejczyk, K. Nutrient and polyphenolic composition of industrial strawberry press cake. Eur Food Res. Technol 2013, 237, 995–1007. [Google Scholar] [CrossRef] [Green Version]
- Kosmala, M.; Zduńczyk, Z.; Kołodziejczyk, K.; Klimczak, E.; Juskiewicz, J.; Zduńczyk, P. Chemical composition of polyphenols extracted from strawberry pomace and their effect on physiological properties of diets supplemented with different types of dietary fibre in rats. Eur. J. Nutr. 2014, 53, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Tovar, A.K.; Godínez, L.A.; Espejel, F.; Ramírez-Zamora, R.M.; Robles, I. Optimization of the integral valorization process for orange peel waste using a design of experiments approach: Production of high-quality pectin and activated carbon. Waste Manag. 2019, 85, 202–213. [Google Scholar] [CrossRef]
- Rezaei, K.; Hosseini, S.S.; Raji, Z.; Khodaiyan, F.; Kiani, H. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromol. 2017, 98, 709–716. [Google Scholar]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of pectin extracted from banana peels of different varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Qiu, L.P.; Zhao, G.L.; Wu, H.; Jiang, L.; Li, X.F.; Liu, J.J. Investigation of combined effects of independent variables on extraction of pectin from banana peel using response surface methodology. Carbohydr. Polym. 2010, 80, 326–331. [Google Scholar] [CrossRef]
- Pagán, J.; Ibarz, A.; Llorca, M.; Pagán, A.; Barbosa-Cánovas, G.V. Extraction and characterization of pectin from stored peach pomace. Food Res. Int. 2001, 34, 605–612. [Google Scholar] [CrossRef]
- Bélafi-Bakó, K.; Cserjési, P.; Beszédes, S.; Csanádi, Z.; Hodúr, C. Berry pectins: Microwave-assisted extraction and rheological properties. Food Bioprocess. Technol. 2012, 5, 1100–1105. [Google Scholar] [CrossRef] [Green Version]
- Khattak, K.F.; Rahman, T.U. Analysis of vegetable’s peels as a natural source of vitamins and minerals. Int. Food Res. J. 2017, 24, 292–297. [Google Scholar]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat flour replacement by wine grape pomace powder positively affects physical, functional and sensory properties of cookies. Proc. Natl. Acad. Sci. USA 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, M.; Shi, T.; Guo, C.; Huang, Y.; Chen, Y.; Xie, M. Recovery of dietary fiber and polyphenol from grape juice pomace and evaluation of their functional properties and polyphenol compositions. Food Funct. 2017, 8, 341–351. [Google Scholar] [CrossRef]
- Garcia-Perez, J.V.; García-Alvarado, M.A.; Carcel, J.A.; Mulet, A. Extraction kinetics modeling of antioxidants from grape stalk (Vitis vinifera var. Bobal): Influence of drying conditions. J. Food Eng. 2010, 101, 49–58. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, H.; Ren, S. Antioxidant activity and HPLC analysis of polyphenol-enriched extracts from industrial apple pomace. J. Sci. Food Agric. 2013, 93, 2502–2506. [Google Scholar] [CrossRef]
- Constenla, D.; Ponce, A.G.; Lozano, J.E. Effect of pomace drying on apple pectin. LWT-Food Sci. Technol. 2002, 35, 216–221. [Google Scholar] [CrossRef]
- Silva-Beltrán, N.P.; Chaidez-Quiroz, C.; López-Cuevas, O.; Ruiz-Cruz, S.; López-Mata, M.A.; Del-Toro-sánchez, C.L.; Marquez-Rios, E.; Ornelas-Paz, J.D.J. Phenolic compounds of potato peel extracts: Their antioxidant activity and protection against human enteric viruses. J. Microbiol. Biotechnol. 2017, 27, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Salim, N.S.M.; Singh, A.; Raghavan, V. Potential utilization of fruit and vegetable wastes for food through drying or extraction techniques. Nov. Tech. Nutr. Food Sci. 2017, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Nair, G.R.; Liplap, P.; Gariepy, Y.; Orsat, V.; Raghavan, V. Effect of dielectric properties of a solvent-water mixture used in microwave-assisted extraction of antioxidants from potato peels. Antioxidants 2014, 3, 99–113. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the pectin production process using novel extraction techniques: A review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Araya-Farias, M.; Ratti, C. Dehydration of Foods: General Concepts. In Advances in Food Dehydration; Ratti, C., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 19–54. [Google Scholar]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Brennan, J.G. Food Processing Handbook; Wiley-VCH Verlag &, Co. KGaA: Weinheim, Germany, 2006; Volume 1, ISBN 9783527307197. [Google Scholar]
- Toledo, R.T. Fundamentals of Food Process. In Engineering, 3rd ed.; Toledo, R.T., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; ISBN 0878495223. [Google Scholar]
- Carrín, M.E.; Crapiste, G.H. Convective Drying of Foods. In Advances in Food Dehydration; Ratti, C., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 123–151. [Google Scholar]
- Krishna, R.; Wesselingh, J.A. The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Izák, P.; Bartovská, L.; Friess, K.; Šípek, M.; Uchytil, P. Comparison of various models for transport of binary mixtures through dense polymer membrane. Polymer 2003, 44, 2679–2687. [Google Scholar] [CrossRef]
- Thurner, F.; Schlünder, E.U. Progress towards understanding the drying of porous materials wetted with binary mixtures. Chem. Eng. Process. 1986, 20, 33–41. [Google Scholar] [CrossRef]
- Wolf, H. Selective drying of hygroscopic materials wetted with binary mixtures. Dry. Technol. 1994, 12, 1445–1470. [Google Scholar] [CrossRef]
- Ho, C.K.; Udell, K.S. Mass transfer limited drying of porous media containing an immobile binary liquid mixture. Int. J. Heat Mass Transf. 1995, 38, 339–350. [Google Scholar] [CrossRef]
- Wulsten, E.; Kiekens, F.; van Dycke, F.; Voorspoels, J.; Lee, G. Levitated single-droplet drying: Case study with itraconazole dried in binary organic solvent mixtures. Int. J. Pharm. 2009, 378, 116–121. [Google Scholar] [CrossRef]
- Heintz, A.; Stephan, W. A generalized solution-diffusion model of the pervaporation process through composite membranes Part II. Concentration polarization, coupled diffusion and the influence of the porous support layer. J. Memb. Sci. 1994, 89, 153–169. [Google Scholar] [CrossRef]
- Aguilera, J.M. Solid-Liquid Extraction. In Extraction Optimization in Food Engineering; Tzia, C., Ed.; Marcel Dekker: New York, NY, USA, 2003; p. 438. ISBN 0824747127. [Google Scholar]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process. Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Wu, J.Y. Kinetic models and process parameters for ultrasound-assisted extraction of water-soluble components and polysaccharides from a medicinal fungus. Biochem. Eng. J. 2013, 79, 214–220. [Google Scholar] [CrossRef]
- Jokić, S.; Velić, D.; Bilić, M.; Bucić-Kojić, A.; Planinić, M.; Tomas, S. Modelling of the process of solid-liquid extraction of total polyphenols from soybeans. Czech. J. Food Sci. 2010, 28, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Natolino, A.; Da Porto, C. Kinetic models for conventional and ultrasound assistant extraction of polyphenols from defatted fresh and distilled grape marc and its main components skins and seeds. Chem. Eng. Res. Des. 2020, 156, 1–12. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (Vitis vinifera L.): Experimental kinetics and modeling. Ultrason. Sonochem. 2015, 22, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Van Ngo, T.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Van Vuong, Q. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J. Food Qual. 2017, 2017, 9305047. [Google Scholar] [CrossRef] [Green Version]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Positive impact of pulsed electric field on lactic acid removal, demineralization and membrane scaling during acid whey electrodialysis. Int. J. Mol. Sci. 2019, 20, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallespir, F.; Rodríguez, Ó.; Cárcel, J.A.; Rosselló, C.; Simal, S. Ultrasound assisted low-temperature drying of kiwifruit: Effects on drying kinetics, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2019, 99, 2901–2909. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mustaffar, A.; Phan, A.N.; Zivkovic, V.; Reay, D.; Law, R.; Boodhoo, K. A review of process intensification applied to solids handling. Chem. Eng. Process. Process. Intensif. 2017, 118, 78–107. [Google Scholar] [CrossRef]
- Wiktor, A.; Witrowa-Rajchert, D. Pulsed Electric Fields as Pretreatment for Subsequent Food Process Operations. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–16. ISBN 978-3-319-26779-1. [Google Scholar]
- Barbosa-Cánovas, G.V.; Góngora-Nieto, M.M.; Pothakamury, U.R.; Swanson, B.G. Fundamentals of high-intensity pulsed electric fields (PEF). In Preservation of Foods with Pulsed Electric Fields; Barbosa-Cánovas, G.V., Ed.; Academic Press: San Diego, CA, USA, 1999; pp. 1–19. [Google Scholar]
- Wouters, P.C.; Alvarez, I.; Raso, J. Critical factors determining inactivation kinetics by pulsed electric field food processing. Trends Food Sci. Technol. 2001, 12, 112–121. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Chen, F.; Zeng, L.; Zhang, Y.; Liao, X.; Ge, Y.; Hu, X.; Jiang, L. Degradation behaviour of methamidophos and chlorpyrifos in apple juice treated with pulsed electric fields. Food Chem. 2009, 112, 956–961. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Sayanfar, S.; Toepfl, S. Effect of pulsed electric field on texture and drying time of apple slices. J. Food Sci. Technol. 2018, 55, 2251–2258. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Wiktor, A.; Siemer, C.; Toepfl, S.; Mykhailyk, V.; Gondek, E.; Rybak, K.; Witrowa-Rajchert, D.; Parniakov, O. The effects of pulsed electric fields on the quality parameters of freeze-dried apples. J. Food Eng. 2019, 252, 36–43. [Google Scholar] [CrossRef]
- Telfser, A.; Gómez Galindo, F. Effect of reversible permeabilization in combination with different drying methods on the structure and sensorial quality of dried basil (Ocimum basilicum L.) leaves. LWT 2019, 99, 148–155. [Google Scholar] [CrossRef]
- Ostermeier, R.; Giersemehl, P.; Siemer, C.; Töpfl, S.; Jäger, H. Influence of pulsed electric field (PEF) pre-treatment on the convective drying kinetics of onions. J. Food Eng. 2018, 237, 110–117. [Google Scholar] [CrossRef]
- Lebovka, N.I.; Shynkaryk, N.V.; Vorobiev, E. Pulsed electric field enhanced drying of potato tissue. J. Food Eng. 2007, 78, 606–613. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Z.; Aila, R.; Hou, Y.; Carne, A.; Bekhit, A.E.D.A. Effect of pulsed electric fields (PEF) on physico-chemical properties, β-carotene and antioxidant activity of air-dried apricots. Food Chem. 2019, 291, 253–262. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, T.Z.; Xiao, G. Effects of pulsed electric fields pretreatment and drying method on drying characteristics and nutritive quality of blueberries. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Peiro, S.; Luengo, E.; Segovia, F.; Raso, J.; Pilar Almajano, M. Improving polyphenol extraction from lemon residues by pulsed electric fields. Waste Biomass Valor 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Jeyakondan, S.; Jayas, D.S.; Holley, R.A. Pulsed electric field processing of foods: A review. J. Food Prot. 2016, 62, 1088–1096. [Google Scholar] [CrossRef]
- Mason, T.J.; Chemat, F.; Vinatoru, M. The extraction of natural products using ultrasound or microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Eim, V.; Rosselló, C.; Femenia, A.; Cárcel, J.A.; Simal, S. Application of power ultrasound on the convective drying of fruits and vegetables: Effects on quality. J. Sci. Food Agric. 2018, 98, 1660–1673. [Google Scholar] [CrossRef]
- Cárcel, J.A.; García-Pérez, J.V.; Benedito, J.; Mulet, A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012, 110, 200–207. [Google Scholar] [CrossRef]
- Cárcel, J.A.; García-Pérez, J.V.; Riera, E.; Mulet, A. Influence of high-intensity ultrasound on drying kinetics of persimmon. Dry. Technol. 2007, 25, 185–193. [Google Scholar] [CrossRef]
- Ratti, C. Novel Food Dryers and Future Perspectives. In Advances in Food Dehydration; Ratti, C., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 447–458. ISBN 9780429147296. [Google Scholar]
- Cárcel, J.A.; Castillo, D.; Simal, S.; Mulet, A. Influence of temperature and ultrasound on drying kinetics and antioxidant properties of red pepper. Dry. Technol. 2019, 37, 486–493. [Google Scholar] [CrossRef]
- Carrión, C.; Mulet, A.; García-Pérez, J.V.; Cárcel, J.A. Ultrasonically assisted atmospheric freeze-drying of button mushroom. Drying kinetics and product quality. Dry. Technol. 2018, 36, 1814–1823. [Google Scholar] [CrossRef]
- Brines, C.; Mulet, A.; García-Pérez, J.V.; Riera, E.; Cárcel, J.A. Influence of the ultrasonic power applied on freeze drying kinetics. Phys. Procedia 2015, 70, 850–853. [Google Scholar] [CrossRef] [Green Version]
- Ozuna, C.; Álvarez-Arenas, T.G.; Riera, E.; Cárcel, J.A.; Garcia-Perez, J.V. Influence of material structure on air-borne ultrasonic application in drying. Ultrason. Sonochem. 2014, 21, 1235–1243. [Google Scholar] [CrossRef]
- Rodríguez, Ó.; Santacatalina, J.V.; Simal, S.; Garcia-Perez, J.V.; Femenia, A.; Rosselló, C. Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. J. Food Eng. 2014, 129, 21–29. [Google Scholar] [CrossRef]
- Santacatalina, J.V.; Contreras, M.; Simal, S.; Cárcel, J.A.; Garcia-Perez, J.V. Impact of applied ultrasonic power on the low temperature drying of apple. Ultrason. Sonochem. 2016, 28, 100–109. [Google Scholar] [CrossRef]
- Musielak, G.; Mierzwa, D.; Kroehnke, J. Food drying enhancement by ultrasound – A review. Trends Food Sci. Technol. 2016, 56, 126–141. [Google Scholar] [CrossRef]
- Cárcel, J.A.; García-Pérez, J.V.; Riera, E.; Rosselló, C.; Mulet, A. Drying Assisted by Power Ultrasound. In Modern Drying Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 237–278. ISBN 9783527631704. [Google Scholar]
- Llavata, B.; García-Pérez, J.V.; Simal, S.; Cárcel, J.A. Innovative pre-treatments to enhance food drying: A current review. Curr. Opin. Food Sci. 2020, 35, 20–26. [Google Scholar] [CrossRef]
- Quintin, D.; Garcia-Gomez, P.; Ayuso, M.; Sanmartin, A.M. Active biocompounds to improve food nutritional value. Trends Food Sci. Technol. 2019, 84, 19–21. [Google Scholar] [CrossRef]
- Ribeiro, A.; Ruphuy, G.; Lopes, J.C.; Dias, M.M.; Barros, L.; Barreiro, F.; Ferreira, I.C.F.R. Spray-drying microencapsulation of synergistic antioxidant mushroom extracts and their use as functional food ingredients. Food Chem. 2015, 188, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Galviz-Quezada, A.; Ochoa-Aristizábal, A.M.; Arias Zabala, M.E.; Ochoa, S.; Osorio-Tobón, J.F. Valorization of iraca (Carludovica palmata, Ruiz & Pav.) infructescence by ultrasound-assisted extraction: An economic evaluation. Food Bioprod. Process. 2019, 118, 91–102. [Google Scholar]
- Machado, I.; Faccio, R.; Pistón, M. Characterization of the effects involved in ultrasound-assisted extraction of trace elements from artichoke leaves and soybean seeds. Ultrason. Sonochem. 2019, 59, 104752. [Google Scholar] [CrossRef]
- Polachini, T.C.; Mulet, A.; Telis-Romero, J.; Cárcel, J.A. Influence of high-intensity ultrasound application on the kinetics of sugar release from acid suspensions of artichoke (Cynara scolymus) biomass. Chem. Eng. Process.-Process. Intensif. 2019, 145. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Amiri-Rigi, A.; Abbasi, S.; Scanlon, M.G. Enhanced lycopene extraction from tomato industrial waste using microemulsion technique: Optimization of enzymatic and ultrasound pre-treatments. Innov. Food Sci. Emerg. Technol. 2016, 35, 160–167. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35. [Google Scholar] [CrossRef]
- Mello Jr, R.E.; Fontana, A.; Corrêa, J.; Karaağaç, E.; García-Pérez, J.V.; Carcel, J.A. Influence of the pretreatment with pulsed electric field on the hot air drying of orange peel. In Proceedings of the Eurodrying’2019, Torino, Italy, 10–12 July 2019. [Google Scholar]
- Menor-García, L.; García-Pérez, J.V.; Raso, J.; Benedito, J.; Cárcel, J.A. Enhancement of betanin extraction from red beetroot by ultrasounds and pulsed electric fields. In Proceedings of the 3rd World Congress on Electroporation, Toulouse, France, 3–6 September 2019. [Google Scholar]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Luque De Castro, M.D. Microwave-assisted extraction of phenolic compounds from wine lees and spray-drying of the extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process. Intensifi Cation 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; Keefe, S.O. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Hahn, T.; Lang, S.; Ulber, R.; Muffler, K. Novel procedures for the extraction of fucoidan from brown algae. Process. Biochem. 2012, 47, 1691–1698. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, Z.; Zhao, M.; Wu, C.; Wang, L.; Xu, Y. Microwave-assisted extraction of an acidic polysaccharide from Ribes nigrum L.: Structural characteristics and biological activities. Ind. Crops Prod. 2020, 147, 112249. [Google Scholar] [CrossRef]
- Arrutia, F.; Adam, M.; Calvo-Carrascal, M.Á.; Mao, Y.; Binner, E. Development of a continuous-flow system for microwave-assisted extraction of pectin-derived oligosaccharides from food waste. Chem. Eng. J. 2020, 395, 125056. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, F.; Ge, J.; Ma, L.; Wu, L.; Xue, X. Changes in eleven pesticide residues in jujube (Ziziphus jujuba Mill.) during drying processing. Dry. Technol. 2018, 36, 965–972. [Google Scholar] [CrossRef]
- Szadzińska, J.; Łechtańska, J.; Pashminehazar, R.; Kharaghani, A.; Tsotsas, E. Microwave- and ultrasound-assisted convective drying of raspberries: Drying kinetics and microstructural changes. Dry. Technol. 2019, 37, 1–12. [Google Scholar] [CrossRef]
- M’hiri, N.; Ghali, R.; Ben Nasr, I.; Boudhrioua, N. Effect of different drying processes on functional properties of industrial lemon byproduct. Process. Saf. Environ. Prot. 2018, 116, 450–460. [Google Scholar] [CrossRef]
- Santana, I.; Castelo-Branco, V.N.; Guimarães, B.M.; de, O. Silva, L.; Peixoto, V.O.D.S.; Cabral, L.M.C.; Freitas, S.P.; Torres, A.G. Hass avocado (Persea americana Mill.) oil enriched in phenolic compounds and tocopherols by expeller-pressing the unpeeled microwave dried fruit. Food Chem. 2019, 286, 354–361. [Google Scholar] [CrossRef]
- Rodriguez, A.; Rodriguez, M.M.; Lemoine, M.L.; Mascheroni, R.H. Study and comparison of different drying processes for dehydration of raspberries. Dry. Technol. 2017, 35, 689–698. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Tang, J.; Mujumdar, A.S.; Wang, S. Trends in microwave-related drying of fruits and vegetables. Trends Food Sci. Technol. 2006, 17, 524–534. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Chizoba Ekezie, F.-G.; Sun, D.-W.; Han, Z.; Cheng, J.-H. Microwave-assisted food processing technologies for enhancing product quality and process efficiency: A review of recent developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- FAO/WHO Updating the Principles and Methods of Risk Assessment: MRLs for Pesticides and Veterinary Drugs; FAO: Rome, Italy, 2006.
- Holland, P.T.; Hamilton, D.; Ohlin, B.; Skidmore, M.W. Effects of Storage and Processing on Pesticide Residues in Plant Products; IUPAC: London, UK, 1994; Volume 66. [Google Scholar]
- Kaushik, G.; Satya, S.; Naik, S.N. Food processing a tool to pesticide residue dissipation—A review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Kar, A.; Mandal, K.; Singh, B. Decontamination of chlorantraniliprole residues on cabbage and cauliflower through household processing methods. Bull. Environ. Contam. Toxicol. 2012, 88, 501–506. [Google Scholar] [CrossRef]
- Mergnat, T.; Fritsch, P.; Saint-Joly, C.; Truchot, E.; Saint-Blanquat, G. Reduction in phosalone residue levels during industrial dehydration of apples. Food Addit. Contam. 1995, 12, 759–767. [Google Scholar] [CrossRef]
- Lentza-Rizos, C.; Balokas, A. Residue levels of chlorpropham in individual tubers and composite samples of postharvest-treated potatoes. J. Agric. Food Chem. 2001, 49, 710–714. [Google Scholar] [CrossRef]
- Reiler, E.; Jørs, E.; Bælum, J.; Huici, O.; Alvarez Caero, M.M.; Cedergreen, N. The influence of tomato processing on residues of organochlorine and organophosphate insecticides and their associated dietary risk. Sci. Total Environ. 2015, 527–528, 262–269. [Google Scholar] [CrossRef]
- Chavarri, M.J.; Herrera, A.; Arino, A. Pesticide residues in field-sprayed and processed fruits and vegetables. J. Sci. Food Agric. 2004, 84, 1253–1259. [Google Scholar] [CrossRef]
- Hassanzadeh, N.; Bahramifar, N.; Esmaili-Sari, A. Residue content of carbaryl applied on greenhouse cucumbers and its reduction by duration of a pre-harvest interval and post-harvest household processing. J. Sci. Food Agric. 2010, 90, 2249–2253. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Fan, B.; Lu, J.; He, Y.; Kong, Z.; Zhu, Y.; Jian, Q.; Wang, F. A chemometric processing-factor-based approach to the determination of the fates of five pesticides during apple processing. LWT-Food Sci. Technol. 2015, 63, 1102–1109. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.; Satya, S.; Kumar, V.; Tewary, D.K. Dissipation of pesticides during bread-making. Chem. Health Saf. 2005, 12, 17–22. [Google Scholar] [CrossRef]
- Uygun, U.; Koksel, H.; Atli, A. Residue levels of malathion and its metabolites and fenitrothion in post-harvest treated wheat during storage, milling and baking. Food Chem. 2005, 92, 643–647. [Google Scholar] [CrossRef]
- Habiba, R.A.; Ismail, S.M.M.; Ali, H.M. Biochemical effects of profenofos residues in potatoes. J. Agric. Food Chem. 1992, 40, 1852–1855. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, P.E.; Pappas, C. Effects of fruit acidity and storage conditions on the rate of degradation of azinphos methyl on apples and lemons. Food Chem. 2000, 69, 69–72. [Google Scholar] [CrossRef]
- Pappas, C.J.; Athanasopoulos, P.E.; Kyriakidis, N.B. Degradation of azinphos-ethyl in apples stored in different conditions. J. Agric. Food Chem. 1998, 46, 2092–2095. [Google Scholar] [CrossRef]
- Noh, H.H.; Kim, D.K.; Lee, E.Y.; Chang, M.I.; Im, M.H.; Lee, Y.D.; Kyung, K.S. Effects of oven drying on pesticide residues in field-grown chili peppers. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 97–104. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Cabitza, F.; Pala, M. Pesticide residues in raisin processing. J. Agric. Food Chem. 1998, 46, 2309–2311. [Google Scholar] [CrossRef]
- Özbey, A.; Karagöz, Ş.; Cingöz, A. Effect of drying process on pesticide residues in grapes. J. Food 2017, 42, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Archer, T.E.; Toscano, R.A. Fate of kelthane residues on apple pomace exposed to drying in the dark, sunlight and ultraviolet light irradiation. Bull. Environ. Contam. Toxicol. 1972, 7, 353–357. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Cabitza, F.; Cubeddu, M. Pesticide residues on field-sprayed apricots and in apricot drying processes. J. Agric. Food Chem. 1998, 46, 2306–2308. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Minelli, E.V.; Cabitza, F.; Cubeddu, M. Residues of some pesticides in fresh and dried apricots. J. Agric. Food Chem. 1997, 45, 3221–3222. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef]
- Shabeer, T.P.A.; Banerjee, K.; Jadhav, M.; Girame, R.; Utture, S.; Hingmire, S.; Oulkar, D. Residue dissipation and processing factor for dimethomorph, famoxadone and cymoxanil during raisin preparation. Food Chem. 2015, 170, 180–185. [Google Scholar] [CrossRef]
- Lentza-Rizos, C.; Avramides, E.J.; Kokkinaki, K. Residues of azoxystrobin from grapes to raisins. J. Agric. Food Chem. 2006, 54, 138–141. [Google Scholar] [CrossRef]
- Shabeer, T.P.A.; Girame, R.; Hingmire, S.; Banerjee, K.; Sharma, A.K.; Oulkar, D.; Utture, S.; Jadhav, M. Dissipation pattern, safety evaluation, and generation of processing factor (PF) for pyraclostrobin and metiram residues in grapes during raisin preparation. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Pirisi, F.M.; Cabitza, F.; Pala, M.; Farris, G.A. Fate of quinoxyfen residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 6128–6131. [Google Scholar] [CrossRef]
- Fang, Q.; Shi, Y.; Cao, H.; Tong, Z.; Xiao, J.; Liao, M.; Wu, X.; Hua, R. Degradation dynamics and dietary risk assessments of two neonicotinoid insecticides during Lonicera japonica planting, drying, and tea brewing processes. J. Agric. Food Chem. 2017, 65, 1483–1488. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Jiao, B. Dissipation behavior of five organophosphorus pesticides in kumquat sample during honeyed kumquat candied fruit processing. Food Control. 2016, 66, 87–92. [Google Scholar] [CrossRef]
- Nath, G.; Jat, N.R.; Srivastava, B.P. Effect of washing, cooking and dehydration on the removal of some insecticides from Okra (Abelmoschus esculentus Moench.). J. Food Sci. Technol. 1975, 12, 127–130. [Google Scholar]
- Bajwa, U.; Sandhu, K.S. Effect of handling and processing on pesticide residues in food—A review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.; Tao, W.; Yao, X.; Wang, J.; Tang, F. Effects of processing on carbendazim residue in Pleurotus ostreatus. Food Sci. Nutr. 2016, 4, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Cabras, P.; Angioni, A.; Garau, V.L.; Pirisi, F.M.; Brandolini, V.; Cabitza, F.; Cubeddu, M. Pesticide residues in prune processing. J. Agric. Food Chem. 1998, 46, 3772–3774. [Google Scholar] [CrossRef]
- Alister, C.; Araya, M.; Becerra, K.; Volosky, C.; Saavedra, J.; Kogan, M. Industrial prune processing and its effect on pesticide residue concentrations. Food Chem. 2018, 268, 264–270. [Google Scholar] [CrossRef]
- Lee, M.G. Reduction of chlorpyrifos and fenitrothion residues in red pepper peel by washing and drying. Food Sci. Biotechnol. 2001, 10, 429–432. [Google Scholar]
- Liu, T.; Zhang, C.; Peng, J.; Zhang, Z.; Sun, X.; Xiao, H.; Sun, K.; Pan, L.; Liu, X.; Tu, K. Residual behaviors of six pesticides in shiitake from cultivation to postharvest drying process and risk assessment. J. Agric. Food Chem. 2016, 64, 8977–8985. [Google Scholar] [CrossRef]
- Hwang, K.W.; Bang, W.S.; Jo, H.W.; Moon, J.K. Dissipation and removal of the etofenprox residue during processing in spring onion. J. Agric. Food Chem. 2015, 63, 6675–6680. [Google Scholar] [CrossRef]
- Jaggi, S.; Sood, C.; Kumar, V.; Ravindranath, S.D.; Shanker, A. Leaching of pesticides in tea brew. J. Agric. Food Chem. 2001, 49, 5479–5483. [Google Scholar] [CrossRef]
- Chen, H.; Pan, M.; Pan, R.; Zhang, M.; Liu, X.; Lu, C. Transfer rates of 19 typical pesticides and the relationship with their physicochemical property. J. Agric. Food Chem. 2015, 63, 723–730. [Google Scholar] [CrossRef]
- Kumar, V.; Sood, C.; Jaggi, S.; Ravindranath, S.D.; Bhardwaj, S.P.; Shanker, A. Dissipation behavior of propargite––an acaricide residues in soil, apple (Malus pumila) and tea (Camellia sinensis). Chemosphere 2005, 58, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Kobara, Y.; Baba, K.; Eun, H. Reduction of hazardous organic solvent in sample preparation for hydrophilic pesticide residues in agricultural products with conventional liquid chromatography. J. Agric. Food Chem. 2013, 61, 4792–4798. [Google Scholar] [CrossRef]
- Iwafune, T.; Ogino, T.; Watanabe, E. Water-based extraction and liquid chromatography-tandem mass spectrometry analysis of neonicotinoid insecticides and their metabolites in green pepper/tomato samples. J. Agric. Food Chem. 2014, 62, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-W.; Pan, Z.; Deng, L.-Z.; El-Mashad, H.M.; Yang, X.-H.; Mujumdar, A.S.; Gao, Z.-J.; Zhang, Q. Recent developments and trends in thermal blanching—A comprehensive review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
- Ketata, M.; Desjadins, Y.; Ratti, C. Effect of liquid nitrogen pretreatments on osmotic dehydration of blueberries. J. Food Eng. 2013, 116, 202–212. [Google Scholar] [CrossRef]
- Bonnechère, A.; Hanot, V.; Jolie, R.; Hendrickx, M.; Bragard, C.; Bedoret, T.; Loco, J. Van Effect of household and industrial processing on levels of five pesticide residues and two degradation products in spinach. Food Control. 2012, 25, 397–406. [Google Scholar] [CrossRef]
- Krol, W.J.; Arsenault, T.L.; Pylypiw, H.M.; Incorvia Mattina, M.J. Reduction of pesticide residues on produce by rinsing. J. Agric. Food Chem. 2000, 48, 4666–4670. [Google Scholar] [CrossRef]
- Adou, K.; Bontoyan, W.R.; Sweeney, P.J. Multiresidue method for the analysis of pesticide residues in fruits and vegetables by accelerated solvent extraction and capillary gas chromatography. J. Agric. Food Chem. 2001, 49, 4153–4160. [Google Scholar] [CrossRef]
- García-Reyes, J.F.; Gilbert-López, B.; Molina-Díaz, A.; Fernández-Alba, A.R. Determination of pesticide residues in fruit-based soft drinks. Anal. Chem. 2008, 80, 8966–8974. [Google Scholar] [CrossRef]
- Ozbey, A.; Uygun, U. Behaviour of some organophosphorus pesticide residues in peppermint tea during the infusion process. Food Chem. 2007, 104, 237–241. [Google Scholar] [CrossRef]
- Al-Taher, F.; Chen, Y.; Wylie, P.; Cappozzo, J. Reduction of pesticide residues in tomatoes and other produce. J. Food Prot. 2013, 76, 510–515. [Google Scholar] [CrossRef]
- Ozbey, A.; Uygun, U. Behaviour of some organophosphorus pesticide residues in thyme and stinging nettle tea during infusion process. Int. J. Food Sci. Technol. 2007, 42, 380–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Liao, X.; Zhang, J.; Hou, Y.; Xiao, Z.; Chen, F.; Hu, X. Degradation of diazinon in apple juice by ultrasonic treatment. Ultrason. Sonochem. 2010, 17, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Chen, F.; Zhang, H.; Hu, X. Effect of sonication on eliminating of phorate in apple juice. Ultrason. Sonochem. 2012, 19, 43–48. [Google Scholar] [CrossRef] [PubMed]

| Examples | Physicochemical Characteristics | Health and Environment Risks |
|---|---|---|
| Dichlorodiphenyltrichloroethane (DDT) | Melting point: 108.5 °C Solubility in water: 25 × 10−3 mg/L (25 °C) Solubility in ethanol (20 × 103 mg/L) Solubility in ether (280 × 103 mg/L) Vapor pressure (20 °C): 2.53 × 10−5 Pa | Probable carcinogen Reproductive effect Liver and kidney problem Eye, nose, skin, throat irritant |
| Captan | Melting point: 178 °C Soluble in water: 3.3 mg/L (25 °C), in acetone: 21 g/L, chloroform: 70 g/L, cyclohexanone: 23 g/L, and in isopropanol: 1.7 g/L. Vapor pressure (20 °C): 13.3 × 10−5 Pa. | Probable carcinogen, allergen Induces hyperplasia of the crypt cells Potent eye irritant Mild skin irritant |
| Lindane | Melting point: 112.5 °C Solubility in water: insoluble. Moderately soluble in ethanol, ether, benzene acetone. Vapor pressure (20 °C): 125.32 × 10−5 Pa | Suspected carcinogen Affects central nervous system, and respiratory, reproductive systems. |
| Endosulfan | Melting point: 70 to 100 °C Solubility in water (22 °C): 0.33 mg/L. Vapor pressure (25 °C): 133.32 × 10−5 Pa | Causes DNA damage Potential correlation between endosulfan and leukemia. |
| Aldrin | Melting point: 104 °C Solubility in water: slightly soluble (0.003%) Vapor Pressure (20 °C): 100 × 10−4 Pa | Causes problems with the central nervous system (the brain and spinal cord), and the liver. Eye, skin and mucous membrane irritants. |
| Dieldrin | Melting point: 176 to 177 °C Solubility in water: 0.186 mg/L at 25–29 °C Vapor Pressure (20 °C): 2.37 × 10–5 Pa | Causes problems with the central nervous system (the brain and spinal cord) and the liver. Eye, skin and mucous membrane irritants. |
| Chlordane | Melting point: 102 to 106 °C Solubility in water: 1 × 10−3 mg/L (20 °C) Vapor Pressure (25 °C): 133.32 × 10−5 Pa | Chlordane interacts with the human erythrocyte membrane and change its morphology. |
| Examples | Physicochemical Characteristics | Health and Environment Risks |
|---|---|---|
| Parathion | Melting point: 6 °C Solubility in water: 6.54 mg/L at 24 °C. High solubility in xylene and butanol. Vapor Pressure (20 °C): 503.94 × 10−5 Pa | Depressed red blood cell cholinesterase activity, nausea, and headache. Affects central nervous system, blood, respiratory systems, eyes and skin. |
| Methyl parathion | Melting point: 37 °C.Solubility in water: 37.7 mg/L (20 °C)Vapor Pressure (25 °C): 46.7 × 10−5 Pa | Causes neuropsychiatric disorders in humans after chronic exposure as well as hematological and ocular alterations. Reduces cholinesterase levels in the brain, erythrocytes, and plasma. |
| Malathion | Melting point: 156 to 157 °C Solubility in water: 145 mg/L at 20 °C. Soluble in ethanol and acetone; very soluble in ethyl ether. Vapor Pressure (25 °C): 23.73 × 10−3 Pa | Inhibition of Acetylcholinesterase activity Affects central nervous system, respiratory systems. Eye, nose, skin irritant |
| Diazinon | Flash point: 82 °C. Solubility in water: 40 mg/L Vapor Pressure (20 °C): 111.99 × 10−4 Pa | Eye and skin irritant Causes gastrointestinal symptoms. |
| Glyphosate | Melting point: 184.5 °C Solubility in water: 12 × 103 mg/L (25 °C) Vapor Pressure (25 °C): <1 × 10−5 Pa | Probable carcinogen Eye and skin irritant |
| Examples | Physicochemical Characteristics | Health and Environment Risks |
|---|---|---|
| Carbaryl | Melting point: 142 °C Solubility in water: 0.1 mg/L Soluble in most popular organic solvents: dimethylformamide, dimethyl sulfoxide, acetone, cyclohexanone. Vapor Pressure (25 °C): 18.13 × 10−5 Pa | Inhibit progesterone biosynthesis of primary human granulose-lutein cells. |
| Propiconazole | Boiling point: 180 °C Soluble in water: 100 mg/L (25 °C), in hexane: 47 g/L, and in most organic solvents. Completely miscible with ethanol, and acetone. Vapor pressure (25 °C): 13.3 × 10−5 Pa. | Skin irritant Liver toxicity and central nervous system effects Adverse changes in erythrocytes |
| Carbofuran | Melting point: 151 °C Solubility in water: 320 mg/L Highly soluble in N-methyl-2-pyrrolidone, dimethylformamide, dimethyl sulfoxide, acetone, acetonitrile, methylene, chloride, cyclohexanone, benzene, and xylene. Vapor Pressure (33 °C): 2.7 × 10−3 Pa | Inhibit acetylcholinesterase. Toxicity affects to vision, growth and predator avoidance skills of fish early life stages. |
| Propoxur | Melting point: 86 to 92 °C Solubility in water: 1.75 × 103 mg/L (20 °C). Vapor Pressure (20 °C): 39.99 × 10−5 Pa | Inhibit of Acetylcholinesterase activity. Probable carcinogen after long-term oral or inhalation exposure. |
| Aminocarb | Melting point: 93 °C Solubility in water: 0.9 × 103 mg/L. Soluble in polar organic solvents. Moderately soluble in aromatic solvents. Vapor Pressure (20 °C): 2.3 × 10−3 Pa | Reduces in immune responsiveness in exposed animals Decrease in activity of acetylcholinesterase. degenerative changes in both liver and kidney |
| Examples | Physicochemical Characteristics | Health and Environment Risks |
|---|---|---|
| Permethrin | Melting point: 34 °C Solubility in water: 5.5 × 10−3 mg/L Vapor Pressure (20 °C): 2.87 × 10−6 Pa | Eye, skin, and respiratory irritant. Affects central nervous system. |
| Cypermethrin | Melting point: 81.3 °C Solubility in water (20 °C): 4 × 10−3 mg/L Soluble in ethanol: 337 × 103 mg/L, hexane 103 × 103 mg/L Vapor Pressure (20 °C): 2.27 × 10−7 Pa | Causes DNA damage and oxidative stress in gill cells of fish. |
| Deltamethrin | Melting point: 98 °C Solubility in water: Insoluble Vapor Pressure (25 °C): 2.0 × 10−6 Pa | Causes neurotoxicity and liver dysfunction accompanied by elevated reactive oxygen species (ROS) levels. |
| Types of Pesticide | Examples | MRLs (µg kg−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| European Commission 1 | US-FDA 2 | PCPA Canada 3 | |||||||||||
| Apple | Potato | Tomato | Strawberry | Apple | Potato | Tomato | Strawberry | Apple | Potato | Tomato | Strawberry | ||
| Organochlorines | DDT | 50 | 50 | 50 | 500 | 100 | --- | 50 | 100 | For fresh vegetable: 500 | |||
| Captan | 104 | 30 | 100 | 100 | 25 × 103 | 50 | 50 | 2 × 104 | 5000 | --- | 5000 | 5000 | |
| Lindane | 10 | 10 | 10 | 10 | --- | 500 | --- | 500 | Banned | ||||
| Endosulfan | 50 | 50 | 50 | 100 | --- | --- | --- | --- | 2000 | --- | 1000 | 1000 | |
| Aldrin | 10 | 10 | 10 | 10 | 30 | 100 | 50 | 50 | --- | --- | --- | --- | |
| Dieldrin | 10 | 10 | 10 | 10 | 30 | 100 | 50 | 50 | --- | --- | --- | --- | |
| Chlordane | 10 | 10 | 10 | 20 | 100 | 100 | 100 | 100 | --- | --- | --- | --- | |
| Organophosphates | Parathion | 50 | 50 | 50 | 100 | --- | --- | --- | --- | Banned | |||
| Methyl parathion | 10 | 10 | 10 | 50 | --- | --- | --- | --- | Banned | ||||
| Malathion | 20 | 20 | 20 | 20 | 8000 | 8000 | 8000 | 8000 | 2000 | 500 | 3000 | 8000 | |
| Diazinon | 10 | 10 | 10 | 50 | 500 | 100 | 750 | 500 | 750 | 750 | 750 | ||
| Glyphosate | 100 | 500 | 100 | 2000 | 200 | 200 | 100 | 200 | --- | --- | --- | --- | |
| Carbamates | Carbaryl | 10 | 10 | 10 | 50 | 12,000 | 2000 | 5000 | 4000 | 5000 | 200 | 5000 | 7000 |
| Propiconazole | 150 | 10 | 300 | 50 | --- | --- | 3000 | 1300 | --- | --- | 3000 | 1300 | |
| Carbofuran | 1 | 1 | 2 | 50 | --- | --- | --- | --- | --- | 500 | 400 | ||
| Propoxur | 50 | 50 | 50 | 100 | --- | --- | --- | --- | Banned | ||||
| Aminocarb | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | |
| Pyrethrins and pyrethroids | Permethrin | 50 | 50 | 50 | 100 | 50 | 50 | 2000 | 1000 | 50 | 500 | --- | |
| Cypermethrin | 1000 | 50 | 500 | 100 | --- | --- | --- | --- | 1000 | 100 | 300 | 200 | |
| Deltamethrin | 200 | 300 | 70 | --- | 200 | 40 | 200 | --- | 400 | 40 | 300 | 200 | |
| F&V Produce | Pesticide Compounds | Operation | Conditions | Results | Reference |
|---|---|---|---|---|---|
| Apple [193], apple pomace [207] | Phosalone [193], kelthane [207] | Rotating ‘Hatmacker’ drum dryer [193], natural drying [207] | Steam pressure (5 bars), discharge rate (150 L/h), rotation speed (5–76 cm/s) [193]. In the dark, under UV light or sunlight [207]. | Phosalone levels were reduced from 22 to 77%. Manufacturers should seek the total elimination of surface residues, i.e., peeling the fruit [193] to improve quality. The loss of kelthane residues was mainly due to volatility rather than photodecomposition [207]. | [193,207] |
| Apricot | Phosalone, iprodione, diazinon, procymidone, bitertanol [208], fenitrothion, dimethoate, omethoate, ziram [209] | Sun drying [208] and ventilated oven [208,209]. | Sunlight for 7 days [208] and ventilated oven at 100 °C for 30 min and at 70 °C for 12 h [208,209]. | Pesticide residues present in dried fruit were lower than in the fresh fruit (half after sun drying). The exception was phosalone, which increased by 50 (sun-drying) and by 3 times for oven-drying [208]. Omethoate and ziram residues almost doubled after drying, while fenitrothion disappeared and dimethoate remained constant [209]. | [208,209] |
| Chili pepper | Chlorfenapyr, clothianidin, diethofencarb, folpet, imidacloprid, indoxacarb, methomyl, methoxyfenozide and tetraconazole | Oven drying | 60 °C for 35 h | Large reductions (37–49%) in clothianidin, diethofencarb, imidacloprid, and tetraconazole. Moderate reductions (16 and 22%) in methomyl and methoxyfenozide, respectively. No effect of drying on chlorfenapyr, folpet, and indoxacarb levels. | [204] |
| Grape | Iprodione and procymidone [205] Benalaxyl, dimethoate, iprodione, metalaxyl, phosalone, procymidone, vinclozolin [210] Chlorpyrifos, diazinon, methidathion and dimethoate [206] Dimethomorph, famoxadone and cymoxanil [211] Azoxystrobin [212] Pyraclostrobin and metiram [213] Quinoxyfen [214] | Oven drying [205,214] Sun drying & oven drying [206,210] Natural drying [211,213] | 70 °C for 24 h [205,214]. No operating conditions [210]. Direct sunlight for 21 days and in an oven at 50 °C for 72 h, at 60 °C for 60 h, at 70 °C for 48 h, at 80°C for 36 h [206]. Shade and outdoors, for 15 days [211] or 25 days [213]. Direct sunlight for 15 days [212] | Iprodione and procymidone decreased by 57 and 41%, respectively [205]. Benalaxyl, phosalone, metalaxyl, and procymidone residues in sun-dried grapes were the same as those on fresh grapes, whereas those of iprodione were higher (1.6 times) and vinclozolin and dimethoate, lower. For the oven-drying process, benalaxyl, metalaxyl, and vinclozolin showed the same residue values in fresh and dried fruits, whereas iprodione and procymidone resides were lower in raisins [210]. Chlorpyrifos, diazinon, methidathion and dimethoate decreased by 73, 92, 82 and 39%, respectively [206]. PF values for raisin processing were 1.03 to 1.14 for dimethomorph, 1.95 to 2.09 for famoxadone, and 1.99 to 1.35 for cymoxanil [211]. Pre-treatment with alkali and sun drying effectively removed a substantial amount of azoxystrobin residues. Commercial production of raisins is, however, carried out with sun drying only [212]. PF values 1.01 to 1.31 for metiram and 1.34 to 1.10 for pyraclostrobin indicated residue concentration after drying [213]. The residue levels in oven dried raisins were comparable to fresh grapes. The lower degradation in the oven-dried sample could be explained by the absence of the degradation effect due to solar radiation [214]. | [205,206,210,211,212,213,214] |
| Honeysuckle (Lonicera japonica) | Thiamethoxam and thiacloprid | Three drying methods: sun-, natural (shade)-, and oven drying. | Oven-drying at 30, 40, 50, 60, and 70 °C | 59.4–81.0% residue reduction after sun- and oven-drying at 70 °C, higher than for shade- and oven-drying at lower temperatures (at 30 to 60 °C). | [215] |
| Jujube | Dichlorvos, malathion, chlorpyrifos, triadimefon, hexaconazole, myclobutanil, kresoxim-methyl, tebuconazole, epoxicona-zole, bifenthrin, and cyhalothrin | Microwave drying | Microwave oven (700 W) for 4 min | Degradation rates were from 67% to 93% | [181] |
| Kumquat candied fruit | Dimethoate, chlorpyrifos, malathion, methidathion and triazophos | Convective drying | 60–80 °C | PF of dimethoate, malathion and triazophos after drying were >1, which could be due to the water loss. | [216] |
| Okra | Malathion, carbaryl [217], endosulfan [217,218], bifenthrin and profenofos [218] | Convective drying [217], sun drying [218] | No specific conditions were found [217,218] | 91.8% malathion, 78% carbaryl and 57.4% endosulfan removal [217]. Sun drying helped to decrease endosulfan up to 5.5%, profenos up to 11% and bifenthrin, up to 75%. Bifenthrin was more affected by sun drying because it is hydrolyzed in the presence of UV rays [218]. | [217,218] |
| Pleurotus ostreatus mushroom | Carbendazim | Sun drying and freeze-drying | Direct sunlight (sun drying) and at −86 °C with vacuum of 0.06 mbar (freeze-drying) | Direct sun-drying removed higher carbendazim amounts than freeze-drying, with removal rates ranging between 70 and 97%. | [219] |
| Plum | Bitertanol, diazinon, iprodione, phosalone, procymidone, and vinclozolin [220] Buprofezin, L-cyhalothrin, spirodiclofen, indoxacarb, acetamiprid, imidacloprid, emamectin benzoate [221] | Oven drying [220] Sunlight drying [221] | Temperature: 30 min at 95 °C, 30 min at 90 °C, 16 h at 85 °C [220] Sunlight drying for 26 days with avg. air temp. 17.6 °C, relative hum. 67.3%, solar radiation 546.3 W m−2; no rain fell [221]. | PF factor was around 3, however pesticide residues were lower or similar in dried than in fresh fruits: phosalone showed the same value, while procymidone, iprodione, and bitertanol were lower (0.6, 2.3 and 3.2 times, respectively). [220]. The insecticide residue reductions during sunlight drying was variable and related to the pesticides’ physico-chemical properties. The whole industrial prune processing has an important reduction effect on pesticide residues [221]. | [220,221] |
| Red pepper | Chlorpyriphos and fenitrothion | Sun/hot air drying | ---- | Sun or hot air-drying eliminated a 20–30% of residues. | [218,222] |
| Shiitake mushroom | Carbendazim, thiabendazole, procymidone, bifenthrin, λ-cyhalothrin, and β-cyfluthri | Drying | Sunlight (26–33 °C, 20 days) and hot-air drying (30–53 °C in the first 10 h, 53–60 °C in the last 10 h) | Removal rate of pesticides by sunlight exposure drying (36.2–94.6%) was higher than that of hot-air drying (26.0–68.1%) | [223] |
| Spring onion | Etofenprox | Drying | Oven (80 °C for 24 h) and freeze-dried (3 days) | Removal rate by oven dried (85.5%) higher than freeze-dried (66.6%) | [224] |
| F&V Produce | Pesticide Compounds | Operation | Conditions | Results | Reference |
|---|---|---|---|---|---|
| Apples, asparagus, beets, cucumbers, green beans, lettuce, nectarines, peaches, peas, raspberries, spinach, strawberries, tomatoes | Captan, chlorothalonil, iprodione, vinclozolin, endosulfan, permethrin, methoxychlor, malathion, etc. | Water immersion (rinsing) | Ambient temperature for 15–30 s. | A short rinse in tap water reduces pesticide residues on many types of produce. Water solubility of pesticides did not play a significant. The majority of pesticide residue appears to reside on the surface. | [233] |
| Cabbage, cantaloupe, pear, white potato | Over 33 types of pesticides coming from different families | Accelerated solvent extraction | Dionex ASE 200 extractor; solvent acetone/dichloromethane; 110 °C; 1500 psi; 2 cycles | Accelerated solvent extraction with acetone/dicholoromethane was able to extract a wide range of pesticide residues | [234] |
| Fruit juice soft drinks (bottles and cans of different brands from 15 European countries) | Over 100 pesticide compounds from 8 different families | Solid-phase extraction (SPE) | HLB cartridges (200 mg) Soft drink samples passed through the cartridges at a flow rate of 3 mL min−1. | Carbendazim, thiabendazole, imazalil, prochloraz, malathion, and iprodione were detected in fruit soft drinks, which are mainly those applied to crops as postharvest treatment. The presence of these pesticides in fruit-based soft drinks could be attributed to the use of the peels in the extracts. Therefore, steps should be taken with the aim of removing any traces of pesticides in these products. | [235] |
| Green pepper, tomato, spinach | Acetamiprid, clothianidin, dinotefuran, flonicamid, imidacloprid, methomyl, pymetrozine, thiacloprid, and thiamethoxam | Water extraction | Please refer to reference. | Water extraction of downsized samples allow quantitative recovery of hydrophilic pesticides | [228] |
| Green pepper, tomato | CPMF, dinotefuran, CPMA, Nitenpyram, thiamethoxam, clothianidin, imidacloprid, thiaclopridamide, acetamiprid, thiacloprid | Water extraction | Please refer to reference. | This water-based extraction method is convenient to remove pesticides and could be utilized for regular monitoring of neonicotinoid insecticides and their metabolites in high water content crops. | [229] |
| Peppermint leaves | Malathion, fenitrothion, dimethoate, chlorpyrifos and pirimiphos-ethyl | Infusion | Boiling water (2 g in 100 mL), in 5, 10, 15 and 20 min. | Residues of dimethoate into the infusion was highest (91%), followed by malathion (62%) and fenitrothion (38%) | [236] |
| Spinach | Boscalid, mancozeb, iprodione, propamocarb, and deltamethrin | Blanching | Sample immersed in hot water (88 °C) for 5 min. | Decreased residue of propamocarb (70%), iprodione, and others by 10 to 58%. | [232] |
| Tea leaves | Phosphamidon, dimethoate, monocrotophos, malathion, methyl parathion, quinalphos, and chlorpyrifos [185], propargite [187] | Extraction (infusion) | Boiling water (5 g in 200 mL), for 2, 5 and 10 min [185] and for 2 min [187] | Residue to the tea brew: 33%, 26%, 20%, 12%, 10%, 8%, and 3%, respectively [185]; 24–40% in infusion media [187] | [225,227] |
| Tomato (cherry), and farm produce i.e., tomatoes, apples, green peppers, peaches, oranges, and lemons | Acephate, malathion, carbaryl, bifenthrin, cypermethrin, cyhalothrin, permethrin, chlorothalonil, and imidacloprid | Water immersion (washing) | Pure water (for all produce) or other washing solutions (for Cherry tomatoes) with added chemical compounds (600 rpm for 1 min, at 10 °C forwashing solutions while for pure water, at 5, 10, and 22 °C, w/wo sonication). | Cherry tomatoes washed at 22 °C presented the highest reduction. Residues in contaminated produce decreased from 40 to 90%. Sonication used with the washing process would increase pesticide removal from produce surfaces. | [237] |
| Thyme and stinging nettle leaves | Fenitrothion, dimethoate, chlorpyrifos and pirimiphos-ethyl | Infusion | Boiling water (2 g in 100 mL), in 5, 10, 15 and 20 min. | The residues of dimethoate (highest water solubility) transferred into the infusions (89–86%), followed by fenitrothion (27–29%), pirimiphos -ethyl (8–14%) and chlorpyrifos (8–8%) during 5 min infusion. | [238] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. https://doi.org/10.3390/foods9101468
Nguyen TT, Rosello C, Bélanger R, Ratti C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods. 2020; 9(10):1468. https://doi.org/10.3390/foods9101468
Chicago/Turabian StyleNguyen, Tri Thanh, Carmen Rosello, Richard Bélanger, and Cristina Ratti. 2020. "Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing" Foods 9, no. 10: 1468. https://doi.org/10.3390/foods9101468