Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems
Abstract
:1. Introduction
2. Short Historical Remarks
3. The Main Effects and Trends Characteristic of the Cryostructuring Processes
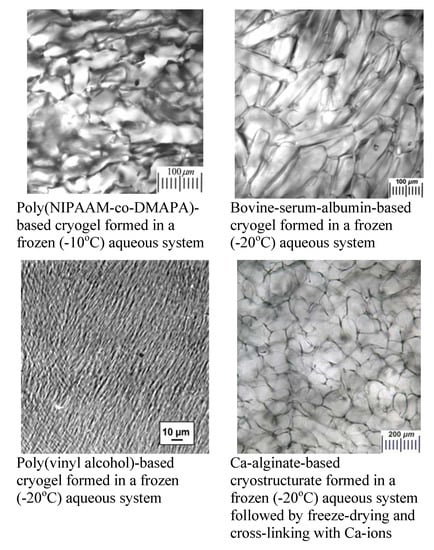
3.1. Specifics of the Macroporous Morphology of Polymeric Cryogels and Cryostructurates
3.2. Some Peculiarities of the Mechanisms of the Cryostructuring Processes in Different Polymeric Systems and the Influence of These Peculiarities on the Physico-Chemical Properties of the Resulting Cryogels and Cryostructurates
3.3. Filled (Composite) Cryogels and Cryostructurates
4. Applied Potential of Some Cryogenically-Structured Polymeric Materials Developed in the IOEC
4.1. Food-Related Systems
4.2. Cryogenically-Structured Materials of Biotechnological Purposes
4.3. Polymeric Cryogels and Cryostructurates of Biomedical Interests
4.4. Polymeric Cryogels and Cryostructurates as Technical Materials
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Available online: https://apps.webofknowledge.com/Search.do?product=UA&SID=E43ITX9UlkSonrz2QrP&search_mode=GeneralSearch&prID=45dc4303-5728-41fc-8784-2d027837d63c (accessed on 7 September 2020).
- Lozinsky, V.I. Cryostructuring of polymer systems. 50. Cryogels and cryotropic gel-formation: Terms and definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, Z.J.; Bencherif, S.A. Cryogelation and Cryogels. Gels 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, R.; Consolacion, F.; Jelen, P. Formation of structured protein foods by freeze texturization. Food Technol. 1986, 40, 77–90. [Google Scholar]
- Lozinsky, V.I. Cryogels on the basis of natural and synthetic polymers: Preparation, properties and areas of implementation. Russ. Chem. Rev. 2002, 71, 489–511. [Google Scholar] [CrossRef]
- Zhang, H.; Cooper, A.I. Aligned porous structures by directional freezing. Adv. Mater. 2007, 19, 1529–1533. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Ice-templated materials: Sophisticated structures exhibiting enhanced functionalities obtained after unidirectional freezing and ice-segregation-induced self-assembly. Chem. Mater. 2008, 20, 634–648. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, H. Controlled freezing and freeze drying: A versatile route for porous and micro-/nano-structured materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Okay, O. Basic principles of cryotropic gelation. Adv. Polym. Sci. 2014, 263, 49–102. [Google Scholar] [CrossRef]
- Okay, O.; Lozinsky, V.I. Synthesis, structure-property relationships of cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar] [CrossRef]
- Cheng, Q.; Huang, C.; Antoni, P.; Tomsia, A.P. Freeze casting for assembling bioinspired structural materials. Adv. Mater. 2017, 29, 1703155. [Google Scholar] [CrossRef]
- Okay, O. (Ed.) Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Springer: Cham, Switzerland, 2014; 330p, ISBN 978-3-319-05845-0. [Google Scholar]
- Kumar, A. (Ed.) Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2016; 480p, ISBN 978-1-4822-281-6. [Google Scholar]
- Nambu, M. Rubber-like poly(vinyl alcohol) gel. Kobunshi Ronbunshu 1990, 47, 695–703. (In Japanese) [Google Scholar] [CrossRef]
- Peppas, N.A.; Stauffer, S.R. Reinforced uncrosslinked poly (vinyl alcohol) gels produced by cyclic freezing-thawing processes: A short review. J. Control Release 1991, 16, 305–310. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryotropic gelation of poly(vinyl alcohol) solutions. Russ. Chem. Rev. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Kumakura, M. Preparation method of porous polymer materials by radiatation technique and its application. Polym. Adv. Technol. 2001, 12, 415–421. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous polysaccharide gels. In Macroporous Polymers: Production, Properties and Biotechnological/Biomedical Applications; Mattiasson, B., Kumar, A., Galaev, I.Y., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010; pp. 131–154. ISBN 978-1-4200-8561-0. [Google Scholar]
- Kumar, A.; Mishra, R.; Reinwald, Y.; Bhat, S. Cryogels: Freezing unveiled by thawing. Mater. Today 2010, 13, 42–44. [Google Scholar] [CrossRef]
- Kirsebom, H.; Mattiasson, B. Cryostructuration as a tool for preparing highly porous polymer materials. Polym. Chem. 2011, 2, 1059–1062. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterization. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Geidobler, R.; Winter, G. Controlled ice nucleation in the field of freeze-drying: Fundamentals and technology review. Eur. J. Pharm. Biopharm. 2013, 85, 214–222. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze-thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Tanthapanichakoon, W.; Tamon, H.; Nakagawa, K.; Charinpanitkul, T. Synthesis of porous materials and their microstructural control through ice templating. Eng. J. 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I. A breif history of polymeric cryogels. Adv. Polym. Sci. 2014, 263, 1–48. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Girolamo, R.D. Kinetic analysis of cryotropic gelation of poly(vinyl alcohol)/water solutions by small-angle neutron scattering. Adv. Polym. Sci. 2014, 263, 159–197. [Google Scholar] [CrossRef]
- Petrov, P.D.; Tsvetanov, C.B. Cryogels via UV irradiation technique. Adv. Polym. Sci. 2014, 263, 199–222. [Google Scholar] [CrossRef]
- Shlyakhtin, O.A. Inorganic cryogels. Adv. Polym. Sci. 2014, 263, 222–244. [Google Scholar] [CrossRef]
- Liu, C.; Tong, G.; Chen, C.; Tan, Z.; Quan, C.; Zhang, C. Polymeric cryogel: Preparation, properties and biomedical applications. Progr. Chem. 2014, 26, 1190–1201. (In Chinese) [Google Scholar] [CrossRef]
- Carvalho, B.M.A.; Da Silva, S.L.; Da Silva, L.H.M.; Minim, V.P.R.; Da Silva, M.C.H.; Carvalho, L.M.; Minim, L.A. Cryogel poly(acrylamide): Synthesis, structure and applications. Sep. Purif. Rev. 2014, 43, 241–262. [Google Scholar] [CrossRef]
- Reichelt, S.; Becher, J.; Weisser, J.; Prager, A.; Decker, U.; Möller, S.; Berg, A.; Schnabelrauch, M. Biocompatible polysaccharide-based cryogels. Mater. Sci. Eng. C 2014, 35, 164–170. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Dragan, E.S. Advances in interpenetrating polymer network hydrogels and their applications. Pure Appl. Chem. 2014, 86, 1707–1721. [Google Scholar] [CrossRef]
- Reichelt, S. Introduction to macroporous cryogels. Methods Mol. Biol. 2015, 1286, 173–181. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Thamomsilp, C.; Suwantong, O. A review: Starch-based composite foams. Compos. Part A Appl. Sci. Manuf. 2015, 78, 246–263. [Google Scholar] [CrossRef]
- Suzuki, A.; Sasaki, S. Swelling and mechanical properties of physically crosslinked poly(vinyl alcohol) hydrogels. J. Eng. Med. 2015, 229, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.M.; Nash, L.D.; Duncan, J.; Maitland, D.J. Porous shape memory polymers: Design and applications. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1300–1318. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Daud, W.M.A.W. Materials, preparation, and characterization of PVA/MMT nanocomposite hydrogels: A review. Polym. Compos. 2017, 38, 1086–1102. [Google Scholar] [CrossRef]
- Jiang, S.; Agarwal, S.; Greiner, A. Low-density open cellular sponges as functional materials. Angew. Chem. Int. Ed. 2017, 56, 15520–15538. [Google Scholar] [CrossRef]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of anisotropic hydrogels and their applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Non-conventional methods for gelation of alginates. Gels 2018, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Tripathi, A.; Melo, J.S. Cryostructurization of polymeric systems for developing macroporous cryogel as a foundational framework in bioengineering applications. J. Chem. Sci. 2019, 131, 92. [Google Scholar] [CrossRef] [Green Version]
- Moradi, Z.; Kalapour, N. Kefaran, a branched polysaccharide: Preparation, properties and applications: A review. Carbohydr. Polym. 2019, 223, 115100. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.D. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Kaetsu, I. Radiation synthesis of polymeric materials for biomedical and biochemical applications. Adv. Polym. Sci. 1993, 105, 81–97. [Google Scholar] [CrossRef]
- Suzuki, M.; Hirasa, O. An approach to artificial muscle using polymer gels formed by micro-phase separation. Adv. Polym. Sci. 1993, 110, 241–261. [Google Scholar] [CrossRef]
- Tomasik, P.; Zaranyika, M.F. Nonconventional methods of modification of starch. Adv. Carbohydr. Chem. Biochem. 1995, 51, 243–318. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, L. Progress in bioartificial polymeric materials. Trends Polym. Sci. 1996, 4, 249–252. [Google Scholar]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Jugur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine: Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Shoichet, M.S. Polymer scaffolds for biomaterials applications. Macromolecules 2010, 43, 581–591. [Google Scholar] [CrossRef]
- Van Vierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Greco, F.; Busulacchi, A.; Sollazo, V. Chitosan, hyaluronan and chodroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(vinyl alcohol) cryogels for biomedical applications. Adv. Polym. Sci. 2014, 263, 283–321. [Google Scholar] [CrossRef]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Yan, X.; Huang, C.; Melerzanov, A.; Du, Y. Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell 2015, 6, 638–653. [Google Scholar] [CrossRef] [Green Version]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef]
- Shakya, A.R.; Kandalam, U. Three-dimensional macroporous materials for tissue engineering of craniofacial bone. Brit. J. Oral Maxillofac. Surg. 2017, 55, 875–891. [Google Scholar] [CrossRef]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thaw synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of polymeric systems as a tool for the creation of innovative materials of biomedical purposes. In Synthesis and Functional Properties of Hydrid Nanoforms of Bioactive and Drug Substances; Melnikov, M.Y., Trakhtenberg, L.I., Eds.; Tekhnosfera Publishing House: Moscow, Russia, 2019; Chapter 3; pp. 68–100. ISBN 978-5-94836-561_3. (In Russian) [Google Scholar]
- Rodionov, I.A.; Sinitskaya, E.S.; Ivanov, R.V.; Tsiskarashvili, A.V.; Lozinsky, V.I. Proteinaceous cryogels and cryostructurates. In Synthesis and Functional Properties of Hydrid Nanoforms of Bioactive and Drug Substances; Melnikov, M.Y., Trakhtenberg, L.I., Eds.; Tekhnosfera Publishing House: Moscow, Russia, 2019; Chapter 4; pp. 101–135. ISBN 978-5-94836-561_4. (In Russian) [Google Scholar]
- Shabatina, T.I.; Vernaya, O.I.; Nuzhdina, A.V.; Shabatin, V.P.; Semenov, A.M.; Lozinsky, V.I.; Melnikov, M.Y. Hybrid nanoforms of antibacterial substances with metallic nanoparticles entrapped in the cryostuctured biopolymeric matices for target delivery. In Synthesis and Functional Properties of Hydrid Nanoforms of Bioactive and Drug Substances; Melnikov, M.Y., Trakhtenberg, L.I., Eds.; Tekhnosfera Publishing House: Moscow, Russia, 2019; Chapter 5; pp. 136–159. ISBN 978-5-94836-561_5. (In Russian) [Google Scholar]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehria, S.; Ahmadib, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Mahumane, G.D.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. 3D scaffolds for brain tissue regeneration: Architectural challenges. Biomater. Sci. 2018, 6, 2812–2837. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludağ, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- Li, P.; Yang, Z.; Jiang, S. Tissue mimicking materials in image-guided needle-based interventions: A review. Mater. Sci. Eng. C 2018, 93, 1116–1131. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, D.A.; Bowlin, G.L. Honey-based templates in wound healing and tissue engineering. Bioengineering 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of poly(vinyl alcohol) and natural polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Razavi, M.; Qiao, Y.; Thakor, A.S. Three-dimensional cryogels for biomedical applications. J. Biomed. Mater. Res. A 2019, 107, 2736–2755. [Google Scholar] [CrossRef]
- Saylan, S.; Denizli, A. Supermacroporous composite cryogels in biomedical applications. Gels 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, D.G.; Young, S.; Hartgerink, J.D. Advances in immunotherapy delivery from implantable and injectable biomaterials. Acta Biomater. 2019, 88, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Bakhshpour, M.; Idil, M.; Perçin, I.; Denizli, A. Biomedical applications of polymeric cryogels. Appl. Sci. 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Dragan, E.S.; Dinu, M.V. Polysaccharides constructed hydrogels as vehicles for proteins and peptides: A review. Carbohydr. Polym. 2019, 225, 115210. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Colombani, T.; Eggermont, L.J.; Rezaeeyazdi, M.; Steingold, J.; Rogers, Z.J.; Navare, K.J.; Mohammed, H.S.; Bencherif, S.A. Latest advances in cryogel technology for biomedical applications. Adv. Ther. 2019, 2, 1800114. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef]
- Varfolomeev, S.D.; Rainina, E.I.; Lozinsky, V.I.; Kalyuzhnyi, S.B.; Sinitsyn, A.P.; Makhlis, T.A.; Bachurina, G.P.; Bokova, I.G.; Sklyankina, O.A.; Agafonov, E.V. Application of poly(vinyl alcohol) cryogel for immobilization of mesophilic and thermophilic microorganisms. In Physiology of Immobilized Cells; de Bont, J.A.M., Visser, J., Mattiasson, B., Tramper, J., Eds.; Elsevier Science Publisher B.V.: Amsterdam, The Netherlands, 1990; pp. 325–330. ISBN 978-0-4444-2700-7. [Google Scholar]
- Sinitsyn, A.P.; Rainina, E.I.; Lozinsky, V.I.; Spasov, S.D. Immobilized Microbial Cells; St. Okhridsky University: Sofia, Bulgaria, 1991; 288p, ISBN 5-211-00907-X. (In Russian) [Google Scholar]
- Lozinsky, V.I.; Vakula, A.V.; Zubov, A.L. Application of poly(vinyl alcohol) cryogels in biotechnology. IV. Literature data overview. Sov. Biotechnol. 1992, 4, 4–17. [Google Scholar]
- Varfolomeev, S.D.; Rainina, E.I.; Lozinsky, V.I. Cryoimmobilized enzymes and cells in organic synthesis. Pure Appl. Chem. 1992, 64, 1193–1196. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Plieva, F.M. Poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. 3. Overview of recent research and developments. Enzym. Microb. Technol. 1998, 23, 227–242. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric cryogels as promising materials of biotechnological interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, N.N.; Gracheva, I.M. Physiological and biochemical characteristics of immobilized champagne yeasts and their participation in sparkling processes. Appl. Biochem. Microbiol. 2003, 39, 439–445. [Google Scholar] [CrossRef]
- Lozinsky, V.I. What new opportunities the use of diverse polymeric cryogels opens for the immobilization of molecules and cells. Chem. Ind. (Belgrade) 2004, 58, 111–115. [Google Scholar]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J. Separ. Sci. 2007, 30, 1657–1671. [Google Scholar] [CrossRef]
- Lozinsky, V.I. New generation of macroporous and supermacroporous materials of biotechnological interest—Polymeric cryogels. Russ. Chem. Bull. 2008, 57, 1015–1032. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Noppe, W.; Mattiasson, B. Cryogel applications in microbiology. Trends Microbiol. 2008, 16, 543–551. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Senko, O.V.; Zubaerova, D.H.; Podorozhko, E.A.; Lozinsky, V.I. Effective immobilized biocatalyst for the treatment of various foodwasters. In Biotechnology: State of the Art and Prospects for Development; Zaikov, G.E., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2008; Chapter 11; pp. 103–110. ISBN 978-1-60456-015-2. [Google Scholar]
- Stolarzewicz, I.; Bialecka-Florjańczyk, E.; Majewska, E.; Krzyczkowska, J. Immobilization of yeast on polymeric supports. Chem. Biochem. Eng. Q. 2011, 25, 135–144. [Google Scholar]
- Mattiasson, B. Cryogels for biotechnological applications. Adv. Polym. Sci. 2014, 263, 245–282. [Google Scholar] [CrossRef]
- Ismailov, A.D.; Alekserova, L.E. Photobiosensors containing luminescent bacteria. Biochemistry (Mosc.) 2015, 80, 733–744. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Sen’ko, O.V.; Maslova, O.V.; Stepanov, N.A.; Lozinsky, V.I.; Varfolomeev, S.D. Immobilized fungal cells: General trends in the studies development and regulation methods of functional activity in the precesses of the biologically-active compounds production. In Immobilized Cells: Biocatalysts and Processes; Efremenko, E.N., Ed.; RIOR: Moscow, Russia, 2018; pp. 123–160. ISBN 978-5-369-02004-3. (In Russian) [Google Scholar]
- Efremenko, E.N.; Lyagin, I.V.; Lozinsky, V.I. Biocatalysts immobilized on/in the cryogenically-structured polymeric matrices. In Synthesis and Functional Properties of Hydrid Nanoforms of Bioactive and Drug Substances; Melnikov, M.Y., Trakhtenberg, L.I., Eds.; Tekhnosfera Publishing House: Moscow, Russia, 2019; Chapter 6; pp. 160–210. ISBN 978-5-94836-561_6. (In Russian) [Google Scholar]
- Bagal-Kestwal, D.R.; Chiang, B.-H. Exploration of chitinous scaffold-based interfaces for glucose sensing assemblies. Polymers 2019, 11, 1958. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.C.; Rutt, B.K. Poly(vinyl alcohol) cryogel: An ideal phantom material for MR studies of arterial flow and elasticity. Magn. Reson. Med. 1997, 37, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, P.R. Simulation and validation of arterial ultrasound imagining and blood flow. Ultrasound Med. Biol. 2008, 34, 693–717. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, H.; Viatage, H.; Kidane, A.G.; Burriesci, G.; Tavakoli, M.; Seifalian, A.M. Polymeric heart valves: New materials, emerging hopes. Trends Biotechnol. 2009, 27, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Kundu, S.C. Osteogenesis of human stem cells in silk biomaterial for regenerative therapy. Prog. Polym. Sci. 2010, 35, 1116–1127. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwatz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B 2012, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Gajra, B.; Pandya, S.S.; Vidyasagar, G.; Rabari, H.; Dedania, R.R.; Rao, S. Poly(vinyl alcohol) hydrogel and its pharmaceutical and biomedical applications: A review. Int. J. Pharm. Res. 2012, 4, 20–26. [Google Scholar]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, L.S.M.; Patterson, J.; Luyten, F.P. Skeletal tissue regeneration: Where can hydogels play a role? Int. Orthop. 2014, 38, 1861–1876. [Google Scholar] [CrossRef]
- Beddoes, C.M.; Whitehouse, M.R.; Briscoe, W.H.; Su, B. Hydrogels as a replacement materials for damaged articular hyaline cartilage. Materials 2016, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The potential of polymeric cryogels in bioseparation. Bioseparation 2001, 10, 163–188. [Google Scholar] [CrossRef]
- Mallik, R.; Hage, D.S. Affinity monolith chromatography. J. Sep. Sci. 2006, 29, 1686–1704. [Google Scholar] [CrossRef] [PubMed]
- Daniak, M.B.; Kumar, A.; Galaev, I.Y.; Mattiasson, B. Methods in cell separations. Adv. Biochem. Eng. Biotechnol. 2007, 106, 1–18. [Google Scholar] [CrossRef]
- Daniak, M.B.; Galaev, I.Y.; Kumar, A.; Plieva, F.M.; Mattiasson, B. Chromatography of living cells using supermacroporous hydrogels, cryogels. Adv. Biochem. Eng. Biotechnol. 2007, 106, 101–127. [Google Scholar] [CrossRef]
- Josic, D.; Clifton, J.G. Use of monolithic supports in proteomics technology. J. Chromatogr. A 2007, 1144, 2–13. [Google Scholar] [CrossRef]
- Kumar, A.; Bhardwaj, A. Methods in cell separation for biomedical application: Cryogels as a new tool. Biomed. Mater. 2008, 3, 034008. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Hahn, R. Polymethacrylate monolith for preparative and industrial separation of biomolecular assemblies. J. Chromatorgr. A 2008, 1184, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Nordborg, A.; Hilder, E.F. Recent advances in polymer monoliths for ion-exchange chromatography. Anal. Bioanal. Chem. 2009, 394, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Svec, F. Porous polymer monoliths: Amazingly wide variety of techniques enabling their preparation. J. Chromatogr. A 2010, 1217, 902–924. [Google Scholar] [CrossRef] [Green Version]
- Tetala, K.K.R.; van Beek, T.A. Bioaffinity chromatography on monolithic supports. J. Sep. Sci. 2010, 33, 422–438. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A. Cell separation using cryogel-based affinity chromatography. Nat. Protoc. 2010, 5, 1737–1747. [Google Scholar] [CrossRef]
- Sproß, J.; Sinz, A. Monilithic media for applications in affinity chromatography. J. Sep. Sci. 2011, 34, 1958–1973. [Google Scholar] [CrossRef] [PubMed]
- Arrua, R.D.; Igarzabal, C.I.A. Macropoous monolithic supports for affinity chromatography. J. Sep. Sci. 2011, 34, 1974–1978. [Google Scholar] [CrossRef] [PubMed]
- Plieva, F.M.; Kirsebom, H.; Mattiasson, B. Preparation of macroporous cryostructurated gel monoliths, their characterization and main applications. J. Sep. Sci. 2011, 34, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Nordborg, A.; Hilder, E.F.; Haddad, P.R. Monolithic phases for ion chromatography. Annu. Rev. Anal. Chem. 2011, 4, 197–226. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Currivan, S.; Paull, B. Polymeric monolithic materials modified with nanoparticles for separation and detection of biomolecules: A review. Proteomics 2012, 12, 2904–2917. [Google Scholar] [CrossRef] [PubMed]
- Sousa, Â.; Sousa, F.; Queiroz, J.A. Advances in chromatographic supports for pharmaceutical-grade plasmid DNA purification. J. Sep. Sci. 2012, 35, 3046–3058. [Google Scholar] [CrossRef]
- Podgornik, A.; Krajnc, N.L. Application of monoliths for bioparticle isolation. J. Sep. Sci. 2012, 35, 3059–3072. [Google Scholar] [CrossRef]
- Gunasena, D.N.; El Rassi, Z. Organic monoliths for hydrophilic interaction electrochromatography/chromatography and immunoaffinity chromatography. Electrophoresis 2012, 33, 251–261. [Google Scholar] [CrossRef]
- Barroso, T.; Hussain, A.; Roque, A.C.A.; Aguiar-Ricardo, A. Functional monolithic platforms: Chromatographic tools for antibody purification. Biotechnol. J. 2013, 8, 671–681. [Google Scholar] [CrossRef]
- Pfaunmiller, E.L.; Paulemond, M.L.; Dupper, C.M.; Hage, D.S. Affinity monolith chromatography: A review of principles and recent analytical applications. Anal. Bioanal. Chem. 2013, 405, 2133–2145. [Google Scholar] [CrossRef] [Green Version]
- Ertürk, G.; Mattiasson, B. Cryogels—Versatile tools in bioseparation. J. Chromatogr. A 2014, 1357, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, S.; Mattiasson, B. Cryogels with affinity ligands as tools in protein purification. Methods Mol. Biol. 2015, 1286, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Connolly, D.; White, B. Supermacroporous polyHIPE and cryogel monolithic materials as stationary phases in separation science: A review. Anal. Methods 2015, 7, 6967–6982. [Google Scholar] [CrossRef]
- Vlakh, E.G.; Korzhikov, V.A.; Hubina, A.V.; Tennikova, T.B. Molecular imprinting: A tool of modern chemistry for the preparation of highly selective monolithic sorbents. Russ. Chem. Rev. 2015, 84, 952–980. [Google Scholar] [CrossRef]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Highly selective monitoring of metals using ion-imprinted polymers. Environ. Sci. Polut. Res. 2015, 22, 7375–7404. [Google Scholar] [CrossRef] [PubMed]
- Acquah, C.; Moy, C.K.S.; Michael, K.; Danquah, M.K.; Clarence, M.; Ongkudon, C.M. Development and characteristics of polymer monoliths for advanced LC bioscreening applications: A review. J. Chromatogr. B 2016, 1015–1016, 121–134. [Google Scholar] [CrossRef]
- Andaç, M.; Galaev, I.Y.; Denizli, A. Affinity based and molecularly imprinted cryogels: Applications in biomacromolecule purification. J. Chromatogr. B 2016, 1021, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rodriguez, E.; Azaria, S.; Pekarek, A.; Hage, D.S. Affinity monolith chromatography: A review of general principles and applications. Electrophoresis 2017, 38, 2837–2850. [Google Scholar] [CrossRef]
- Ganewatta, N.; El Rassi, Z. Organic polymer-based monolithic stationary phases with incorporated nanostructured materials for HPLC and CEC. Electrophoresis 2018, 39, 53–66. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E. Physicochemical, complexation and catalytic properties of polyampholyte cryogels. Gels 2019, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Shlyakhtin, O.A.; Lozinsky, V.I. Low-temperature methods in the synthesis of inorganic nanomaterials and biomaterials originating from the aqueous solutions and suspensions. In Synthesis and Functional Properties of Hydrid Nanoforms of Bioactive and Drug Substances; Melnikov, M.Y., Trakhtenberg, L.I., Eds.; Tekhnosfera Publishing House: Moscow, Russia, 2019; Chapter 7; pp. 211–244. ISBN 978-5-94836-561_7. (In Russian) [Google Scholar]
- Kudaibergenov, S.E.; Dzhardimalieva, G.I. Flow-through catalytic reactors based on metal nanoparticles immobilized within porous polymeric gels and surfaces/hollows of polymeric membranes. Polymers 2020, 12, 572. [Google Scholar] [CrossRef] [Green Version]
- Tolstoguzov, V.B.; Braudo, E.E. Fabricated foodstuffs as multicomponent gels. J. Texture Stud. 1983, 14, 183–212. [Google Scholar] [CrossRef]
- Cassanelli, M.; Norton, I.; Mills, T. Role of gellan gum microstructure in freeze drying and rehydration mechanisms. Food Hydrocolloids 2018, 75, 51–61. [Google Scholar] [CrossRef]
- Altunina, L.K.; Kuvshinov, V.A.; Dolgikh, S.N. Cryogels—A promising material for underground works in permafrost. In Advances in Geological Storage of Carbon Dioxide; NATO Science Series IV, Lombardi, S., Altunina, L.K., Beaubien, S.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 65, pp. 103–110. ISBN 978-1-4020-4469-4. [Google Scholar]
- Vasiliev, N.K.; Pronk, A.D.C.; Shatalina, I.N.; Janssen, F.H.M.E.; Houben, R.W.G. A review on the development of reinforced ice for use as a building material in cold regions. Cold Reg. Sci. Technol. 2015, 115, 56–63. [Google Scholar] [CrossRef]
- Altunina, L.K.; Fufaeva, M.S.; Filatov, D.A.; Scarovskaya, L.I.; Rozhdestvenskii, E.A.; Gan-Erdene, T. Effect of cryogel on soil properties. Euroasian Soil Sci. 2014, 47, 425–431. [Google Scholar] [CrossRef]
- Mastrangelo, R.; Montis, C.; Bonelli, N.; Tempesti, P.; Baglioni, P. Surface cleaning of artworks: Structure and dynamics of nanostructured fluids confined in polymeric hydrogel networks. Phys. Chem. Chem. Phys. 2017, 19, 23762–23772. [Google Scholar] [CrossRef]
- Bonelli, N.; Poggi, G.; Chelazzi, D.; Giorgi, R.; Baglioni, P. Poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels for the cleaning of art. J. Colloid Interface Sci. 2019, 536, 339–348. [Google Scholar] [CrossRef]
- Mastrangelo, R.; Chelazzi, D.; Poggia, G.; Fratinia, E.; Buemi, L.P.; Petruzzellis, M.L.; Baglioni, P. Twin-chain polymer hydrogels based on poly(vinyl alcohol) as new advanced tool for the cleaning of modern and contemporary art. Proc. Natl. Acad. Sci. USA 2020, 117, 7011–7020. [Google Scholar] [CrossRef] [Green Version]
- Manzhai, V.N.; Fufaeva, M.S. Poly(vinyl alcohol) cryogels as an efficient spent-oil utilization method. Chem. Technol. Fuels Oils 2015, 51, 487–492. [Google Scholar] [CrossRef]
- Glazunov, P.A.; Reschetilowski, V.P.; Manzhai, V.N.; Zyatikov, P.N.; Solov’ev, V.V. Physical modeling of briquetting processes on the basis of the wastes polydisperse coke particles and cryogels of a poly(vinyl alcohol). Tomsk State Univ. J. Math. Mech. 2018, 53, 73–83. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Glushkov, D.O.; Kuznetsov, G.V.; Nigay, A.G.; Yanovsky, V.A.; Yashutin, O.S. Ignition mechanism and characteristics of gel fuels based on oil-free and oil-filled cryogels with fine coal particles. Powder Technol. 2020, 360, 65–79. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Kuznetsov, G.V.; Tabakaev, R.B.; Dariga, B.; Altynbaeva, D.B.; Nigay, A.G. Kinetic properties of gas-phase combustion of gel fuels based on oil-filled cryogels. Thermochim. Acta 2020, 686, 178553. [Google Scholar] [CrossRef]
- Available online: https://ineos.ac.ru/en/history-background (accessed on 7 September 2020).
- Buttkus, H. Accelerated denaturation of myosin in frozen solution. J. Food Sci. 1970, 35, 558–562. [Google Scholar] [CrossRef]
- Labudzińska, A.; Ziabicki, A. Effect of composition and gelation conditions on structural changes accompanying the gelation of PAN, PVA and gelatin solutions. Kolloid-Z. Z. Polym. 1971, 243, 21–27. [Google Scholar] [CrossRef]
- Kaetsu, I.; Okubo, H.; Ito, A.; Hayashi, K. Radiation-induced polymerization of glass-forming systems. I. Effect of temperature on the initial polymerization rate. J. Polym. Sci. A-1 1972, 10, 2203–2214. [Google Scholar] [CrossRef]
- Smith, P.; Pennings, A.J. Eutectic crystallization of pseudo binary systems of polyethylene and high melting diluents. Polymer 1974, 15, 413–419. [Google Scholar] [CrossRef]
- Dianova, V.T.; Tolstoguzov, V.B.; Krokha, N.G.; Shtulboi, V.B.; Strashenko, E.S.; Bushueva, S.G.; Yaroshenko, Y.F.; Gonsales, R.S.; Kupriyanova, N.I. Methods for the preparation of protein gels. SU Patent 924,940; registered, 30 April 1982. [Google Scholar]
- Rogozhin, S.V.; Vainerman, E.S.; Burmistrova, L.M. Method for Producing Protein Jellies from Fishes and Crustaceans. U.S. Patent 4,167,590, 11 September 1979. [Google Scholar]
- Belikov, V.M.; Rogozhin, S.V.; Slonimskii, G.L.; Golovnya, R.V.; Tolstoguzov, V.B. Development of chemical investigations on artificial and synthetic foods. Russ. Chem. Rev. 1969, 38, 699–713. [Google Scholar] [CrossRef]
- Vainerman, E.S.; Lozinsky, V.I.; Rogozhin, S.V. Study of cryostructurization of polymer systems. I. Structure formation in solutions of thiol-containing polymers under freezing-thawing. Colloid Polym. Sci. 1981, 259, 1198–1201. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Method for the preparation of macroporous polymer materials. SU Patent 1,008,214, 1 December 1982. [Google Scholar]
- Rogozhin, S.V.; Vainerman, E.S.; Lozinsky, V.I. Formation of 3-dimentional cross-linked polymeric structures during freezing of the reacting system. Dokl. Akad. Nauk SSSR 1982, 263, 115–118. (In Russian) [Google Scholar]
- Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Study of cryostructurization of polymer systems. II. The influence of freezing of reacting mass on the properties of products in the preparation of covalently cross-linked gels. Colloid Polym. Sci. 1982, 260, 776–780. [Google Scholar] [CrossRef]
- Nikonorov, V.V.; Ivanov, R.V.; Kil’deeva, N.R.; Bulatnikova, L.N.; Lozinsky, V.I. Synthesis and characteristics of chitosan cryogels crosslinked by glutaric aldehyde. Polym. Sci. A 2010, 52, 828–834. [Google Scholar] [CrossRef]
- Nikonorov, V.V.; Ivanov, R.V.; Kil’deeva, N.R.; Lozinsky, V.I. Effect of polymer precursor molecular mass on the formation and properties of covalently crosslinked chitosan cryogels. Polym. Sci. A 2011, 53, 1150–1158. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Korneeva, M.N.; Vainerman, E.S.; Rogozhin, S.V. Structurization during freezing of the polymerizing system consisting of vinyl and divinyl monomers. Dokl. Akad. Nauk SSSR 1983, 270, 101–104. (In Russian) [Google Scholar]
- Lozinsky, V.I.; Vainerman, E.S.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. IV. Cryostructurization of the system: Solvent–vinyl monomer–divinyl monomer–initiator of polymerization. Colloid Polym. Sci. 1984, 262, 769–774. [Google Scholar] [CrossRef]
- Belavtseva, E.M.; Titova, E.F.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Study of cryostructurization of polymer systems. V. Electron microscopic studies of cross-linked polyacrylamide cryogels. Colloid Polym. Sci. 1984, 262, 775–779. [Google Scholar] [CrossRef]
- Rogozhin, S.V.; Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Ivanova, S.A.; Shtil’man, M.I.; Korshak, V.V. Noncovalent cryostructurization in polymer systems. Dokl. Akad. Nauk SSSR 1984, 278, 129–133. (In Russian) [Google Scholar]
- Lozinsky, V.I.; Vainerman, E.S.; Korotaeva, G.F.; Rogozhin, S.V. Study of cryostructurization of polymer systems. III. Cryostructurization in organic media. Colloid Polym. Sci. 1984, 262, 617–622. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Ivanova, S.A.; Titova, E.F.; Shtil’man, M.I.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. VI. The influence of the process temperature on the dynamics of formation and structure of cross-linked polyacrylamide cryogels. Acta Polym. 1986, 37, 142–146. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Domotenko, L.V.; Vainerman, E.S.; Mamtsis, A.M.; Rogozhin, S.V. On the possibility of mechanodestruction of poly(vinyl alcohol) molecules under moderate freezing of its concentrated water solutions. Polym. Bull. 1986, 15, 333–340. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. VII. Structure formation under freezing of poly(vinyl alcohol) aqueous solutions. Colloid Polym. Sci. 1986, 264, 19–24. [Google Scholar] [CrossRef]
- Domotenko, L.V.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Influence of freezing and thawing conditions of poly(vinyl alcohol) aqueous solutions on the properties of obtained cryogels. Polym. Sci. USSR A 1988, 30, 1758–1764. [Google Scholar] [CrossRef]
- Manolov, R.Z.; Tavobilov, I.M.; Lozinsky, V.I.; Vainerman, E.S.; Titova, E.F.; Belavtseva, E.M.; Bezborodova, S.I.; Rogozhin, S.V.; Bezborodov, A.M. A study of Aspergillus clavatus immobilized cells producing ribonuclease. Appl. Biochem. Microbiol. 1988, 24, 427–431. [Google Scholar]
- Lusta, K.A.; Starostina, N.G.; Gorkina, N.B.; Fikhte, B.A.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Immobilization of E. coli cells into macroporous cryogels on the poly(acrylamide) basis. Appl. Biochem. Microbiol. 1988, 24, 498–504. [Google Scholar]
- Slabova, O.I.; Nikitin, D.I.; Lozinsky, V.I.; Kulakova, V.K.; Vainerman, E.S.; Rogozhin, S.V. Hydrogen oxidation by olygotrophic bacterial cells immobilized in silica gel and cryosilica gel. Microbiology 1988, 57, 749–753. [Google Scholar]
- Lozinsky, V.I.; Morozova, S.A.; Vainerman, E.S.; Titova, E.F.; Shtil’man, M.I.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. VIII. Characteristics features of the formation of cross-linked poly(acrylamide) cryogels under different thermal conditions. Acta Polym. 1989, 40, 8–15. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Golovina, T.O.; Vainerman, E.S.; Rogozhin, S.V. Change of the amount of titrated SH-groups in the poly(acrylamide) thiol derivative during freezing of its aqueous solutions. Polym. Sci. USSR A 1989, 31, 367–372. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Golovina, T.O.; Gusev, D.G. Study of cryostructuration of polymer systems. 13. Some characteristic features of the behaviour of macromolecular thiols in frozen aqueous solutions. Polymer 2000, 41, 35–47. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Blumenfel’d, A.L.; Rogov, V.V.; Barkovskaya, E.N.; Fedin, E.I.; Rogozhin, S.V. Characteristic features of freezing of concentrated aqueous poly(vinyl alcohol) solutions: Correlation with properties of hydrogels obtained after thawing. Colloid J. USSR 1990, 51, 592–596. [Google Scholar]
- Rogozhin, S.V.; Cheverev, V.G.; Vainerman, E.S.; Lozinsky, V.I.; Gagarin, V.E.; Torbin, V.V.; Barkovskaya, E.N.; Panchenko, V.I.; Rogov, V.V. Method for the preparation of an artificial ice. SU Patent 1,649,219, 15 May 1991. [Google Scholar]
- Lozinsky, V.I.; Domotenko, L.V.; Vainerman, E.S.; Rogozhin, S.V. Some thermomechanical properties of poly(vinyl alcohol) cryogels. Polym. Sci. USSR A 1989, 31, 1983–1988. [Google Scholar] [CrossRef]
- Gusev, D.G.; Lozinsky, V.I.; Vainerman, E.S.; Bakhmutov, V.I. Study of the frozen water-poly(vinyl alcohol) system by 2H and 13C NMR spectroscopy. Magn. Res. Chem. 1990, 28, 651–655. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Zubov, A.L.; Kulakova, V.K.; Rogozhin, S.V. Application of poly(vinyl alcohol) cryogels in biotechnology. II. Variation of rheological properties of the gel matrix as a result of yeast cells entrapment. Sov. Biotechnol. 1990, 5, 43–46. [Google Scholar]
- Slabova, O.I.; Nikitin, D.I.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Some features of gas exchange in hydrogen bacteria immobilized into usual and cryogels of cross-linked poly(acrylamide). Microbiology 1991, 60, 14–18. [Google Scholar]
- Mikhalev, O.I.; Serpinski, M.; Lozinsky, V.I.; Kapanin, P.V.; Chkeidze, I.I.; Alfimov, M.V. Method for determination of liquid microphase volume: Application to the investigation of frozen H2O-poly(vinyl alcohol) system. Cryo-Letters 1991, 12, 197–206. [Google Scholar]
- Lozinsky, V.I.; Zubov, A.L. Method for the preparation of poly(vinyl alcohol) cryogels. Russian Patent 2,070,901, 27 December 1996. [Google Scholar]
- Lozinsky, V.I.; Solodova, E.V.; Zubov, A.L.; Simenel, I.A. Study of cryostructuration of polymer systems. 11. The formation of PVA cryogels by freezing-thawing the polymer aqueous solutions containing additives of some polyols. J. Appl. Polym. Sci. 1995, 58, 171–178. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Zubov, A.I.; Kulakova, V.K.; Titova, E.F.; Rogozhin, S.V. Study of cryostructurization of polymer systems. IX. Poly(vinyl alcohol) cryogels filled with particles of cross-linked dextran gel. J. Appl. Polym. Sci. 1992, 44, 1423–1435. [Google Scholar] [CrossRef]
- Gusev, D.G.; Lozinsky, V.I.; Bakhmutov, V.I. Study of cryostructurization of polymer systems. 10. 1H- and 2H-NMR studies of the formation of crosslinked polyacrylamide cryogels. Eur. Polym. J. 1993, 29, 49–56. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Simenel, I.A.; Chebyshev, A.V. Method for the production of porous polymeric materials. Russian Patent 2,035,476, 20 May 1995. [Google Scholar]
- Lozinsky, V.I.; Zubov, A.L. Method for the preparation of macroporous polymeric material. Russian Patent 2,078,099, 27 April 1997. [Google Scholar]
- Pavlova, L.A.; Kastelyanos-Dominges, O.M. Radical polymerization of 2-hydroxymethylmethacrylate in frozen aqueous solutions. Russ. J. Appl. Chem. 1996, 69, 738–741. [Google Scholar]
- Pavlova, L.A.; Kastelyanos-Dominges, O.M. Preparation of cryogels by polymerization of 2-hydroxyethyl methacrylate in the presence of sodium persulfate-N,N,N′,N′-tetramethylethylenediamine initiating system. Russ. J. Appl. Chem. 1996, 69, 1396–1400. [Google Scholar]
- Lozinsky, V.I.; Zubov, A.L.; Titova, E.F. Swelling behaviour of poly(vinyl alcohol) cryogels employed as matrices for cell immobilization. Enzym. Microb. Technol. 1996, 18, 561–569. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Domotenko, L.V.; Zubov, A.L.; Simenel, I.A. Study of cryostructuration of polymer systems. 12. Poly(vinyl alcohol) cryogels: Influence of low-molecular electrolytes. J. Appl. Polym. Sci. 1996, 61, 1991–1998. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kalinina, E.V.; Grinberg, V.Y.; Grinberg, N.V.; Chupov, V.A.; Plate, N.A. Thermoresponsive cryogels based on cross-linked poly(N,N-diethylacrylamide). Polym. Sci. Ser. A 1997, 39, 1300–1305. [Google Scholar]
- Lozinsky, V.I.; Zubov, A.L.; Titova, E.I. Poly(vinyl alcohol) cryogels which are used as matrices for cell immobilization. 2. Entrapped cells resemble porous fillers in their effects on the properties of PVA-cryogel carrier. Enzym. Microb. Technol. 1997, 20, 182–190. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Podorozhko, E.A.; Nikitina, Y.B.; Klabukova, L.F.; Vasil’iev, V.G.; Burmistrov, A.A.; Kondrashov, Y.G.; Vasiliev, N.K. A study of cryostructuring of polymeric systems. 45. Effect of porosity of dispersed filler on physicochemical characteristics of composite poly(vinyl alcohol) cryogels. Colloid J. 2017, 79, 497–507. [Google Scholar] [CrossRef]
- Konstantinova, N.R.; Lozinsky, V.I. Cryotropic gelation of ovalbumin solutions. Food Hydrocoll. 1997, 11, 113–123. [Google Scholar] [CrossRef]
- Golovnya, R.V.; Misharina, T.A.; Terenina, M.B. GC evaluation of flavour compound sorption from water solutions by corn starch cryotextures obtained by freezing. Nahrung/Food 1998, 42, 380–384. [Google Scholar] [CrossRef]
- Terenina, M.B.; Misharina, T.A.; Golovnya, R.V. Sorption of aliphatic alcohols from aqueous solutions by starch cryotextures. Russ. Chem. Bull. 1999, 48, 730–733. [Google Scholar] [CrossRef]
- Golovnya, R.V.; Terenina, M.B.; Krikunova, N.I.; Yuryev, V.P.; Misharina, T.A. Formation of supramolecular structures of aroma compounds with polysaccharides of corn starch cryotextures. Starch/Stärke 2001, 53, 269–277. [Google Scholar] [CrossRef]
- Terenina, M.B.; Misharina, T.A.; Krikunova, N.I.; Golovnya, R.V. Sorption of aliphatic ketones from aqueous solutions by starch cryotextures. Russ. Chem. Bull. 2001, 50, 1032–1036. [Google Scholar] [CrossRef]
- Terenina, M.B.; Krikunova, N.I.; Misharina, T.A. Binding of cyclic compounds by starch cryotextures from aqueous solutions. Russ. Chem. Bull. 2002, 51, 1689–1693. [Google Scholar] [CrossRef]
- Terenina, M.B.; Misharina, T.A. Sorption of components from a mixture of essential oils by cryotextured cornstarches. Appl. Biochem. Microbiol. 2002, 41, 407–412. [Google Scholar] [CrossRef]
- Damshkaln, L.G.; Simenel, I.A.; Lozinsky, V.I. Study of cryostructuration of polymer systems. 15. Freeze-thaw-induced formation of cryoprecipitate matter from the low-concentrated aqueous solutions of poly(vinyl alcohol). J. Appl. Polym. Sci. 1999, 74, 1978–1986. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Kurskaya, E.A.; Kulakova, V.K.; Lozinsky, V.I. Cryotropic structuring of aqueous dispersions of fibrous collagen: The influence of the initial pH values. Food Hydrocoll. 2000, 14, 111–120. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Zubov, A.L.; Savina, I.N.; Plieva, F.M. Study of cryostructuration of polymer systems. 14. Poly(vinyl alcohol) cryogels: Apparent yield of the freeze-thaw-induced gelation of concentrated aqueous solutions of the polymer. J. Appl. Polym. Sci. 2000, 77, 1822–1831. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, C.R.T.; Norton, I.T. Study of cryostructuration of polymer systems. 16. Freeze–thaw-induced effects in the low-concentration systems amylopectin–water. J. Appl. Polym. Sci. 2000, 75, 1740–1748. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G. Study of cryostructuration of polymer systems. 17. Poly(vinyl alcohol) cryogels: Dynamics of the cryotropic gel-formation. J. Appl. Polym. Sci. 2000, 77, 2017–2023. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, C.R.T.; Norton, I.T. Study of cryostructuration of polymer systems. 18. Freeze-thaw-influence on water-solubilized artificial mixtures of amylopectin and amylose. J. Appl. Polym. Sci. 2000, 78, 371–381. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, C.R.T.; Norton, I.T. Study of cryostructuring of polymer systems. 19. On the nature of intermolecular links in the cryogels of locust bean gum. Polym. Int. 2000, 49, 1434–1443. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G. Study of cryostructuration of polymer systems. 20. Foamed poly(vinyl alcohol) cryogels. J. Appl. Polym. Sci. 2001, 82, 1609–1619. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. The polymeric composition for the preparation of macroporous agarose gel and the method for the gel producing. Russian Patent 2,220,987, 10 January 2004. [Google Scholar]
- Mattiasson, B.; Galaev, I.; Lozinsky, V.; Plieva, F. Macroporous gel, its preparation and use. U.S. Patent 7,547,395 B2, 16 June 2009. [Google Scholar]
- Lozinsky, V.I.; Savina, I.N.; Davankov, V.A. The Composition for the Preparation of poly(vinyl alcohol) Cryogel and the Method for the Cryogel Producing. Russian Patent 2,190,644, 10 October 2002. [Google Scholar]
- Lozinsky, V.I.; Savina, I.N. Study of cryostructuration of polymer systems. 22. Poly (vinyl alcohol) composite cryogels filled with dispersed particles of various hydrophilicity/hydrophobicity. Colloid J. 2002, 64, 336–343. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Brown, C.R.T.; Norton, I.T. Study of cryostructuration of polymer systems. 21. Cryotropic gel-formation of the water-maltodextrin systems. J. Appl. Polym. Sci. 2002, 83, 1658–1667. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G. Polymeric Composition for the Preparation of poly(vinyl alcohol) Cryogel. Russian Patent 2,252,945, 27 May 2005. [Google Scholar]
- Savina, I.N.; Lozinsky, V.I. Study of cryostructuration of polymer systems. 23. Poly(vinyl alcohol) composite cryogels filled with dispersed particles that contain ionogenic groups. Colloid J. 2004, 66, 343–349. [Google Scholar] [CrossRef]
- Savina, E.N.; Hanora, A.; Plieva, F.M.; Galaev, I.Y.; Mattiasson, B.; Lozinsky, V.I. Study of cryostructuration of polymer systems. 24. Poly(vinyl alcohol) cryogels filled with particles of strong anion-exchanger: Properties of the composite materials and potential application. J. Appl. Polym. Sci. 2005, 95, 529–538. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuration of polymer systems. 25. Influence of surfactants on the properties and structure of gas-filled (foamed) poly(vinyl alcohol) cryogels. Colloid J. 2005, 67, 589–601. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Bakeeva, I.V.; Presnyak, E.P.; Damshkaln, L.G.; Zubov, V.P. Study of cryostructuring of polymer systems. 26. Heterophase organic-inorganic cryogels prepared via freezing-thawing of aqueous solutions of poly(vinyl alcohol) with added tetramethoxysilane. J. Appl. Polym. Sci. 2007, 105, 2689–2702. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Shaskol’skii, B.L.; Babushkina, T.A.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 27. Physicochemical properties of poly(vinyl alcohol) cryogels and features of their macroporous morphology. Colloid J. 2007, 69, 747–764. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 28. Physicochemical and morphological properties of poly(vinyl alcohol) cryogels formed via multiple freezing-thawing technique. Colloid J. 2008, 70, 189–198. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 33. Effect of rate of chilling aqueous poly(vinyl alcohol) solutions during their freezing on physicochemical properties and porous structure of resulting cryogels. Colloid J. 2012, 74, 319–327. [Google Scholar] [CrossRef]
- Ivanov, R.V.; Lozinsky, V.I.; Noh, S.K.; Han, S.S.; Lyoo, W.S. Preparation and characterization of polyacrylamide cryogels produced from a high-molecular weight precursor. I. Influence of the reaction temperature and concentration of the cross-linking agent. J. Appl. Polym. Sci. 2007, 106, 1470–1475. [Google Scholar] [CrossRef]
- Ivanov, R.V.; Lozinsky, V.I.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Lyoo, W.S. Preparation and characterization of polyacrylamide cryogels produced from a high-molecular-weight precursor. II. The influence of the molecular weight of the polymeric precursor. J. Appl. Polym. Sci. 2008, 107, 382–390. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Bloch, K.O.; Vardi, P.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y. Cryostructuring of polymer systems. 29. Preparation and characterization of supermacroporous (spongy) agarose-based cryogels used as three-dimensional scaffolds for culturing insulin-producing cell aggregates. J. Appl. Polym. Sci. 2008, 108, 3046–3062. [Google Scholar] [CrossRef]
- Bloch, K.; Lozinsky, V.I.; Galaev, I.Y.; Yavriyanz, K.; Vorobeychik, M.; Azarov, D.; Damshkaln, L.G.; Mattiasson, B.; Vardi, P. Functional activity of insulinoma cells (INS-1E) and pancreatic islets cultured in agarose cryogel sponges. J. Biomed. Mater. Res. A 2005, 75, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.A.; Petrenko, A.Y.; Lozinsky, V.I.; Gurin, I.V.; Gorokhova, N.A.; Volkova, N.A.; Sandomirskii, B.P. Culturing of stromal cells-precursors in the 3D carriers. Transplantologiya (Kyev.) 2007, 9, 221–223. (In Russian) [Google Scholar]
- Bloch, K.; Vanichkin, A.; Damshkaln, L.G.; Lozinsky, V.I.; Vardi, P. Vascularization of wide pore agarose-gelatin cryogel scaffolds implanted subcutaneously in diabetic and non diabetic mice. Acta Biomater. 2010, 6, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Komarova, G.A.; Starodubtsev, S.G.; Lozinsky, V.I.; Kalinina, E.V.; Landfester, K.; Khokhlov, A.R. Intelligent gels and cryogels with entrapped emulsions. Langmuir 2008, 24, 4467–4469. [Google Scholar] [CrossRef]
- Komarova, G.A.; Starodubtsev, S.G.; Lozinsky, V.I.; Nasimova, I.R.; Khokhlov, A.R. Intelligent gels and cryogels with embedded emulsions of various oils. J. Appl. Polym. Sci. 2013, 127, 2703–2709. [Google Scholar] [CrossRef]
- Wasserman, L.A.; Vasil’ev, V.G.; Motyakin, M.V.; Blaszchak, W.; Fornal, J.; Damshkaln, L.G.; Lozinsky, V.I.; Yuryev, V.P. Influence of gluten and gums additives, as well as of the cryogenic treatment, on physicomechanical properties, morphology and local mobility of water in complex gels of wheat starch. Starch/Stärke 2009, 61, 377–388. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Korlyukov, A.A.; Lozinsky, V.I. Cryostructuring of polymer systems. 30. Poly(vinyl alcohol)-based composite cryogels filled with small disperse oil droplets: A gel system capable of mechanically-induced releasing of the lipophilic constituents. J. Appl. Polym. Sci. 2010, 117, 1332–1349. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Vorontsova, T.V.; Lozinsky, V.I. Study of cryostructuring of polymer systems. 32. Morphology and physico-chemical properties of composite poly(vinyl alcohol) cryogels filled with microdroplets of hydrophobic liquid. Colloid J. 2012, 74, 110–120. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Sakhno, N.G.; Damshkaln, L.G.; Bakeeva, I.V.; Zubov, V.P.; Kurochkin, I.N.; Kurochkin, I.I. Study of cryostructuring of polymer systems. 31. Influence of the additives of alkaline metals chlorides on physicochemical properties and morphology of poly(vinyl alcohol) cryogels. Colloid J. 2011, 73, 234–243. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Zaborina, O.E. Process for the Preparation of Cross-Linked Hydrophilic Polymer Exhibiting Superabsorbent Properties. Russian Patent 2,467,017, 20 November 2012. [Google Scholar]
- Zaborina, O.E.; Gasanov, R.G.; Peregudov, A.S.; Lozinsky, V.I. Cryostructuring of polymeric systems. 38. The causes of the covalently-crosslinked cryogels formation upon the homopolymerization of N,N-dimethylacrylamide in moderately-frozen aqueous media. Eur. Polym. J. 2014, 61, 226–239. [Google Scholar] [CrossRef]
- Burova, T.V.; Grinberg, N.V.; Kalinina, E.V.; Ivanov, R.V.; Lozinsky, V.I.; Alvarez-Lorenzo, C.; Grinberg, V.Y. Thermoresponsive copolymer cryogel possessing molecular memory: Synthesis, energetics of collapse and interaction with ligands. Macromol. Chem. Phys. 2011, 212, 72–80. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; D’yakonova, E.A.; Kolosova, O.Y.; Klabukova, L.F.; Lozinsky, V.I. Study of cryostructuring of polymer systems. 34. Composite poly(vinyl alcohol) cryogels filled with microparticles of polymeric dispersion. Colloid J. 2012, 74, 711–719. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Ezernitskaya, M.G.; Glotova, Y.K.; Antonov, Y.A. Cryostructuring of polymer systems. 35. Wide pore poly(vinyl alcohol) cryogels prepared using a combination of liquid-liquid phase separation and cryotropic gel-formation processes. Soft Matter 2012, 8, 8493–8504. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Kurochkin, I.N.; Kurochkin, I.I. Cryostructuring of polymeric systems. 36. Poly(vinyl alcohol) cryogels prepared from solutions of the polymer in water/low-molecular alcohol mixtures. Eur. Polym. J. 2014, 53, 189–205. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Podorozhko, E.A. Method for Molding of the poly(vinyl alcohol) Cryogels. Russian Patent 2,561,120, 28 July 2015. [Google Scholar]
- Podorozhko, E.A.; D’yakonova, E.A.; Lozinsky, V.I. Cryostructuring of polymeric systems. 37. Composite cryogels from dispersions of poly(butadiene-co-styrene) latex in aqueous poly(vinyl alcohol) solution. Colloid J. 2015, 77, 46–57. [Google Scholar] [CrossRef]
- Oschepkova, M.V.; Oschepkov, A.S.; Zaborina, O.E.; Fedorova, O.A.; Fedorov, Y.V.; Lozinsky, V.I. Fluorescent cryogels based on copolymers of N,N-dimethylacrylamide and allyl derivatives of 1,8-naphtalimide. Polym. Sci. Ser. B 2015, 57, 631–637. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Lunev, I.A.; Ryabev, A.N.; Kil’deeva, N.R.; Lozinsky, V.I. Cryostructuring of polymeric systems. 39. Composite poly(vinyl alcohol) cryogels filled with chitosan microparticles. Colloid J. 2015, 77, 186–195. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Lozinsky, V.I. Cryostructuring of polymeric systems. 40. Proteinaceous wide-pore cryogels generated by the action of denaturant/reductant mixtures on bovine serum albumin in moderately-frozen aqueous media. Soft Matter 2015, 11, 4921–4931. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Ul’yabaeva, G.R.; Kil’deeva, N.R.; Tikhonov, V.E.; Antonov, Y.A.; Zhuravleva, I.L.; Lozinsky, V.I. A Study of cryostructuring of polymer systems. 41. Complex and composite poly(vinyl alcohol) cryogels containing soluble and insoluble forms of chitosan. Colloid J. 2016, 78, 90–101. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Ul’yabaeva, G.R.; Tikhonov, V.E.; Grachev, A.V.; Vladimirov, L.V.; Antonov, Y.A.; Kil’deeva, N.R.; Lozinsky, V.I. Cryostructuring of polymeric systems. 43. Microstructural features of chitosan-containing complex and composite poly(vinyl alcohol) cryogels. Colloid J. 2017, 79, 94–105. [Google Scholar] [CrossRef]
- Ul’yabaeva, G.R.; Podorozhko, E.A.; Kil’deeva, N.R.; Lozinsky, V.I. Study of the acidic textle dye sorption from aqueous solutions by the chitosan-containing composite cryogel of poly(vinyl alcohol). Fibre Chem. 2019, 51, 199–203. [Google Scholar] [CrossRef]
- Podorozhko, E.A.; Ul’yabaeva, G.R.; Kil’deeva, N.R.; Lozinsky, V.I. A study of cryostructuring of polymer systems. 53. “Anomalous” character of the properties variation for the chitosan-containing composite poly(vinyl alcohol) cryogels as a result of multiple freezing-thawing. Colloid J. 2020, 82, 36–48. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Lozinsky, V.I. Study of cryo-structuring of polymeric systems. 42. Physical-chemical properties and microstructure of wide-porous covalently cross-linked albumin cryogels. Colloid J. 2016, 78, 492–504. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Shabatina, T.I.; Lozinsky, V.I. Cryostructuring of polymer systems. 44. Freeze-dried and then chemically cross-linked wide porous cryostructurates based on serum albumin. e-Polymers 2017, 17, 263–274. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Leonova, I.M.; Ivanov, R.V.; Bakeeva, I.V. A study of cryostructuring of polymer systems. 46. Physicochemical properties and microstructure of poly(vinyl alcohol) cryogels formed from polymer solutions in mixtures of dimethyl sulfoxide with low-molecular-mass alcohols. Colloid J. 2017, 79, 788–796. [Google Scholar] [CrossRef]
- Kolosova, O.Y.; Kurochkin, I.N.; Kurochkin, I.I.; Lozinsky, V.I. Cryostructuring of polymeric systems. 48. Influence of organic non-ionic and ionic chaotropes or kosmotropes on the cryotropic gel-formation of aqueous poly(vinyl alcohol) solutions, as well as on the properties and microstructure of the resultant cryogels. Eur. Polym. J. 2018, 102, 169–177. [Google Scholar] [CrossRef]
- Kolosova, O.Y.; Lozinsky, V.I. Influence of trehalose additives on the properties of poly(vinyl alcohol) cryogels formed in aqueous, as well as in organic media. IOP Conf. Ser. Mater. Sci. Eng. 2019, 525, 012024. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kolosova, O.Y.; Michurov, D.A.; Dubovik, A.S.; Vasil’ev, V.G.; Grinberg, V.Y. Cryostructuring of polymeric systems. 49. Unexpected “kosmotropic-like” impact of organic chaotropes on the PVA freeze-thaw-induced gelation in DMSO. Gels 2018, 4, 81. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Michurov, D.A.; Kolosova, O.Y. Polymeric Composition for the Preparation of poly(vinyl alcohol) Cryogels and Method for Increasing Their Strength and Heat Endurance. Russian Patent 2,678,281, 24 January 2019. [Google Scholar]
- Podorozhko, E.A.; Vasil’ev, V.G.; Vasiliev, N.K.; Lozinsky, V.I. Study of cryostructuring of polymer systems. 51. The combined influence of porous cellulose-containing dispersed fillers and salting-out electrolytes on physicochemical properties of composite poly(vinyl alcohol) cryogels. Colloid J. 2019, 81, 261–271. [Google Scholar] [CrossRef]
- Zvukova, N.D.; Klimova, T.P.; Ivanov, R.V.; Ryabev, A.N.; Tsiskarashvili, A.V.; Lozinsky, V.I. Cryostructuring of polymeric systems. 52. Properties, microstructure and an example of a potential biomedical use of the wide-pore alginate cryostructurastes. Gels 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Bakeeva, I.V.; Orlova, M.A.; Lozinsky, V.I. Poly(vinyl alcohol) cryogels formed from polymer solutions in dimethyl sulfoxide with tetramethoxysilane additives. Fine Chem. Technol. 2019, 14, 41–50. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Shchekoltsova, A.O.; Sinitskaya, E.S.; Vernaya, O.I.; Nuzhdina, A.V.; Bakeeva, I.V.; Ezernitskaya, M.G.; Semenov, A.M.; Shabatina, T.I.; Melnikov, M.Y. Influence of succinylation of a wide-pore albumin cryogels on their properties, structure, biodegradability, and release dynamics of dioxidine loaded in such spongy carriers. Int. J. Biol. Macromol. 2020, 160, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, S.V.; Slonimskii, G.L.; Rogowina, L.Z.; Vainerman, E.S.; Pivovarov, P.P. Structuration of myofibril krill proteins. Food/Nahrung 1981, 25, 391–396. [Google Scholar] [CrossRef]
- Rogozhin, S.V.; Belavtseva, E.M.; Vernerman, E.S.; Radchenko, L.G.; Pivovarov, P.P.; Golovina, T.O.; Pertsevoy, F.V. Electron-microscopic studies of structure formation of isolated myofibrillar proteins during the freezing-thawing processes. Food/Nahrung 1984, 28, 165–171. [Google Scholar] [CrossRef]
- Vainerman, E.S.; Lozinsky, V.I.; Rogozhin, S.V.; Raskina, L.P.; Shapiro, L.A.; Yakubovich, V.S.; Bronshtein, V.Y. Method for the Preparation of Porous Alginate Material. SU Patent 1,171,474, 8 April 1985. [Google Scholar]
- Vainerman, E.S.; Lozinsky, V.I.; Rogozhin, S.V.; Raskina, L.P.; Shapiro, L.A.; Yakubovich, V.S.; Shenker, M.B.; Komissarova, A.L.; Potapov, V.D.; Gudochkova, V.M. Method for the Preparation of Porous Material Possessing the Wound-Healing Ability. SU Patent 1,171,476, 8 April 1985. [Google Scholar]
- Duda, V.I.; Krivenko, V.I.; Rogozhin, S.V.; Lozinsky, V.I.; Mamtsis, A.M.; Vainerman, E.S.; Domotenko, L.V.; Shtil’man, M.I.; Ivanova, S.A. Method for the Preparation of Solid Nutritional Medium for Culturing of Microorganisms. SU Patent 1,213,069, 22 October 1985. [Google Scholar]
- Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Method for the Preparation of Immobilized Cells. SU Patent 1,400,071, 1 February 1988. [Google Scholar]
- Faleev, N.G.; Lozinsky, V.I.; Sadovnikova, M.S.; Vainerman, E.S.; Ruvinov, S.B.; Safiullin, R.M.; Belikov, V.M.; Rogozhin, S.V. Method for the Preparation of the Fluorine-Derivatives of l-tyrosine. SU Patent 1,351,971, 15 July 1987. [Google Scholar]
- Lozinsky, V.I.; Faleev, N.G.; Zubov, A.L.; Ruvinov, S.B.; Antonova, T.A.; Vainerman, E.S.; Belikov, V.M.; Rogozhin, S.V. Use of PVA-cryogel entrapped Citrobacter intermedius cells for continuous production of 3-fluoro-L-tyrosine. Biotechnol. Lett. 1989, 11, 43–48. [Google Scholar] [CrossRef]
- Rainina, E.I.; Lozinsky, V.I.; Galkina, I.A.; Morozova, S.A.; Vainerman, E.S.; Sinitsyn, A.P.; Shtil’man, M.I.; Rogozhin, S.V. Method for the Preparation of Immobilized Yeast Cells Used for Ethanol Production. SU Patent 1,387,412, 8 December 1987. [Google Scholar]
- Tavobilov, I.M.; Lozinsky, V.I.; Manolov, R.G.; Vainerman, E.S.; Bezborodova, S.I.; Rogozhin, S.V.; Bezborodov, A.M. Method for the Cultivation of Mycelium Fungi Capable of Producing Ribonucleases. SU Patent 1,405,310, 22 February 1988. [Google Scholar]
- Alexandrova, I.V.; Vainerman, E.S.; Domotenko, L.V.; Epifanov, A.E.; Kruglova, N.P.; Lozinsky, V.I.; Mamtsis, A.M.; Morozova, O.M.; Nikolaeva, V.V.; Rogozhin, S.V.; et al. Method for Culturing of Isolated Plants Tissues and Cells Capable to Produce the Biologically Active Substances. SU Patent 1,438,236, 15 July 1988. [Google Scholar]
- Rogozhin, S.V.; Egorov, S.N.; Lozinsky, V.I.; Gavrilenko, L.V.; Vainerman, E.S.; Semenova, I.N. Method for the Production of Acid Phosphatase by the Yeast Saccharomyces cerevisiae. SU Patent 1,505,972, 08 May 1989. [Google Scholar]
- Rainina, V.I.; Lozinsky, V.I.; Bachurina, G.P.; Makhlis, T.A.; Sinitsyn, A.P.; Vainerman, E.S.; Morozov, A.M.; Badalov, A.B.; Kleysov, A.A.; Rogozhin, S.V. Method for the Production of Ethanol. SU Patent 1,589,627, 1 May 1990. [Google Scholar]
- Lozinsky, V.I.; Zubov, A.L.; Makhlis, T.A. Entrapment of Zymomonas mobilis cells into PVA-cryogel carrier in the presence of polyol cryoprotectants. Prog. Biotechnol. 1996, 11, 112–117. [Google Scholar]
- Shtil’man, M.I.; Denisova, L.A.; Ostaeva, G.Y.; Donetsky, I.A.; Kondaryuk, V.V.; Pchelintseva, O.A.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Polymeric Reagent for the Covalent Immobilization of Serum Albumin. SU Patent 1,531,440, 22 August 1989. [Google Scholar]
- Arzumanov, E.N.; Alebian, G.P.; Tozalakian, P.V.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V.; Shtil’man, M.I. Method for the Preparation of l-aspartic Acid. SU Patent 1,559,716, 22 December 1989. [Google Scholar]
- Arzumanov, E.N.; Alebian, G.P.; Tozalakian, P.V.; Mkrtchian, M.V.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Method for the preparation of l-alanine. SU Patent 1,559,719, 22 December 1989. [Google Scholar]
- Alebian, G.P.; Arzumanov, E.N.; Mkrtchian, M.V.; Tozalakian, P.V.; Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V. Kinetic aspects of l-aspartate-β-decarboxylase functioning in free and immobilized Alcaligenes faecalis cells in the course of l-aspartic acid transformation to l-alanine. Sov. Biotechnol. 1990, 6, 36–40. [Google Scholar]
- Rogozhin, S.V.; Vainerman, E.S.; Pichugin, N.M.; Kirilenko, Y.K.; Lozinsky, V.I.; Kosolapov, A.M.; Gnezdilova, T.V.; Chumakov, S.D.; Aboyants, R.K.; Istranov, L.P. Method for the preparation of collagen material. SU Patent 1,603,723, 1 July 1990. [Google Scholar]
- Lozinsky, V.I.; Gnezdilova, T.V. Method for the Preparation of Fungous Collagen-Containing Material. Russian Patent 2,053,796, 10 February 1996. [Google Scholar]
- Podorozhko, E.A.; Kulakova, V.K.; Kurskaya, E.I.; Lozinsky, V.I. Method for the Obtaining of Porous Collagen-Containing Material. Russian Patent 2,116,801, 10 August 1998. [Google Scholar]
- Rogozhin, S.V.; Pichugin, N.M.; Vainerman, E.S.; Gnezdilova, T.V.; Kirilenko, Y.K.; Lozinsky, V.I.; Chumakov, S.D. Method for the Preparation of Collagen Material. SU Patent 1,653,318, 1 February 1991. [Google Scholar]
- Rogozhin, S.V.; Pichugin, N.M.; Vainerman, E.S.; Gnezdilova, T.V.; Kirilenko, Y.K.; Lozinsky, V.I.; Chumakov, S.D. Method for the Preparation of Collagen-Containing Material. SU Patent 1,678,021, 15 May 1991. [Google Scholar]
- Lozinsky, V.I.; Gnezdilova, T.V.; Vainerman, E.S.; Rogozhin, S.V. Method for the Preparation of Collagen-Containing Material. SU Patent 1,769,539, 15 June 1992. [Google Scholar]
- Mikhalev, O.I.; Petrov, A.N.; Gnezdilova, T.V.; Lozinsky, V.I.; Alfimov, M.V. Method for the Preparation of Collagen-Containing Material. Russian Patent 2,008,362, 28 February 1994. [Google Scholar]
- Kalinina, E.V.; Semenova, N.N.; Rogozhin, S.V.; Lozinsky, V.I. Method for the Preparation of Porous-Fibrous Material. Russian Patent 2,116,350, 27 July 1998. [Google Scholar]
- Lozinsky, V.I.; Vainerman, E.S.; Rogozhin, S.V.; Barkovskaya, E.N.; Razbegin, B.N.; Chapaev, A.A.; Maksimyak, R.V. Method for the Fixation of a Thawed Ground. SU Patent 1,705,500, 15 September 1991. [Google Scholar]
- Lozinsky, V.I.; Zubov, A.L. Device for the Formation of Spherical Granules on the Basis of Aqueous Systems. Russian Patent 2,036,095, 27 May 1995. [Google Scholar]
- Lozinsky, V.I.; Zubov, A.L. Set-Up for the Formation of Granules. Russian Patent 2,104,866, 20 February 1998. [Google Scholar]
- Zubov, A.L.; Lozinsky, V.I. Method for the Preparation of Granulated Artificial Bait. Russian Patent 2,054,254, 20 February 1996. [Google Scholar]
- Simonian, A.L.; Rainina, E.I.; Lozinsky, V.I.; Badalian, I.E.; Khachatrian, G.A.; Tatikian, S.S.; Makhlis, T.A.; Varfolomeev, S.D. A biosensor for l-proline determination by use of immobilized microbial cells. Appl. Biochem. Biotechnol. 1992, 36, 199–210. [Google Scholar] [CrossRef]
- Velizarov, S.; Rainina, E.I.; Lozinsky, V.I.; Zubov, A.L.; Sinitsyn, A.P.; Varfolomeev, S.D. l-lysine production by Corynebacterium glutamicum cells entrapped in PVA-cryogel. Biotechnol. Lett. 1992, 14, 291–296. [Google Scholar] [CrossRef]
- Nikitina, O.A.; Zatsepin, S.S.; Kalyuzhnyi, S.V.; Rainina, E.I.; Varfolomeev, S.D.; Zubov, A.L.; Lozinsky, V.I. Production of hydrogen by thermophylic anaerobic bacterium Clostridium thermosaccharolyticum immobilized into polyvinyl alcohol cryogel. Microbiology 1993, 62, 296–301. [Google Scholar]
- Lozinsky, V.I.; Konstantinova, N.R.; Solov’eva, N.R. Method for the Preparation of Porous Protein Gel. Russian Patent 2,058,083, 20 April 1996. [Google Scholar]
- Korolenko, I.I.; Lozinsky, V.I.; Sobolev, A.V.; Fesenko, A.V.; Chebyshev, A.V.; Chuiko, K.K.; Shumilkin, A.V. Method for the Determination of Toxic Substances in Gaseous Mixtures. Russian Patent 2,066,449, 10 September 1996. [Google Scholar]
- Pusheva, M.A.; Ryabokon’, A.N.; Rainina, E.I.; Detkova, E.I.; Zubov, A.L.; Lozinsky, V.I.; Varfolomeev, S.D.; Zavarzin, G.A. Microbial Method for the Preparation of Acetate. Russian Patent 2,080,388, 25 May 1997. [Google Scholar]
- Rainina, E.I.; Pusheva, M.A.; Ryabokon’, A.M.; Bolotina, N.P.; Lozinsky, V.I.; Varfolomeev, S.D. Microbial cells immobilized in poly(vinyl alcohol) cryogels: Biocatalytic reduction of CO2 by the thermophilic homoacetogenic bacterium Acetogenium kivuii. Biotechnol. Appl. Biochem. 1994, 19, 321–329. [Google Scholar]
- Ryabokon’, A.M.; Kevbrina, M.V.; Pusheva, M.A.; Zubov, A.L.; Lozinsky, V.I.; Rainina, E.A. Ecologically pure process of acetate synthesis on diverse gaseous substrates by homoacetogenic bacteria entrapped in poly(vinyl alcohol) cryogel. Prog. Biotechnol. 1996, 11, 106–111. [Google Scholar]
- Fokina, V.V.; Arinbasarova, A.Y.; Zubov, A.L.; Lozinsky, V.I.; Koshcheenko, K.A. Dehydrogenation of sterol substrates by bacterial cells Arthrobacter globiformis 193 entrapped into poly(vinyl alcohol) cryogels. Appl. Biochem. Microbiol. 1995, 31, 184–189. [Google Scholar]
- Fokina, V.; Susina, N.; Arinbasarova, A.; Zubov, A.; Lozinsky, V.; Koshcheenko, K. Immobilization of Arthrobacter globiformis 193 cells into PVA cryogel. Dehydrogenation of steroid substrates. Prog. Biotechnol. 1996, 11, 90–97. [Google Scholar]
- Vainerman, E.S.; Podorozhko, E.A.; Portnaya, I.B. Method for the Preparation of Porous Wares from Polymeric Dispersions. Russian Patent 2,062,277, 15 January 1998. [Google Scholar]
- Vainerman, E.S.; Portnaya, I.B. Methods for the Articles Manufactoring from Polymeric Dispersions. Russian Patent 2,108,350, 10 April 1998. [Google Scholar]
- Vainerman, E.S.; Portnaya, I.B. Porous Polymer Material and the Method for the Preparation Thereof. Russian Patent 2,109,766, 27 April 1998. [Google Scholar]
- Podorozhko, E.A.; Portnaya, I.B.; Kulakova, V.K.; Kurskaya, E.A. Method for the Preparation of Porous Material. Russian Patent 2,115,668, 20 July 1998. [Google Scholar]
- Podorozhko, E.A.; Andreeva, L.M.; Kurskaya, E.A.; Lozinsky, V.I. Method for the Preparation of Porous Protein Texturate. Russian Patent 2,118,495, 10 September 1998. [Google Scholar]
- Lozinsky, V.I.; Plieva, F.M.; Isaeva, E.I.; Zubov, A.L. Method for the Concentrating of Viruses. Russian Patent 2,130,069, 10 May 1999. [Google Scholar]
- Plieva, F.M.; Isaeva, E.I.; Lozinsky, V.I. Application of poly(vinyl alcohol) cryogels in biotechnology. V. Bioaffinity sorbents on the basis of supermacroporous carrier for the manipulation with virus particles. Biotechnol. Russ. 1998, 10, 12–17. [Google Scholar]
- Gough, S.; Barron, N.; Zubov, A.L.; Lozinsky, V.I.; McHale, A.P. Production of ethanol from molasses at 45 °C using Kluyveromyces marxianus IMB3 immobilized in calcium alginate gels and poly(vinyl alcohol) cryogel. Bioprocess Eng. 1998, 19, 87–90. [Google Scholar] [CrossRef]
- Belokon, Y.N.; Kochetkov, K.A.; Plieva, F.M.; Ikonnikov, N.S.; Maleev, V.I.; Parmar, V.S.; Kumar, R.; Lozinsky, V.I. Enantioselective hydrolysis of a Schiff’s base of D,L-phenylalanine ethyl ester in water-poor media through the reaction catalyzed with α-chymotrypsin immobilyzed in hydrophilic macroporous gel support. Appl. Biochem. Biotechnol. 2000, 88, 97–106. [Google Scholar] [CrossRef]
- Markvicheva, E.A.; Lozinsky, V.I.; Plieva, F.M.; Kochetkov, K.A.; Rumsh, L.D.; Zubov, V.P.; Maity, J.; Kumar, R.; Parmar, V.; Belokon, Y.N. Gel-immobilized enzymes as promising biocatalysts: Results from Indo-Russian collaborative studies. Pure Appl. Chem. 2005, 77, 227–236. [Google Scholar] [CrossRef]
- Plieva, F.M.; Kochetkov, K.A.; Singh, I.; Parmar, V.S.; Belokon’, Y.N.; Lozinsky, V.I. Immobilization of hog pancreas lipase in macroporous PVA-cryogel carrier for the biocatalysis in water-poor media. Biotechnol. Lett. 2000, 22, 551–554. [Google Scholar] [CrossRef]
- Bacheva, A.V.; Plieva, F.M.; Lysogorskaya, E.N.; Filippova, I.Y.; Lozinsky, V.I. Peptide synthesis in organic media with subtilisin 72 immobilized on poly(vinyl alcohol)-cryogel carrier. Bioorg. Med. Chem. Lett. 2001, 11, 1005–1008. [Google Scholar] [CrossRef]
- Filippova, I.Y.; Bacheva, A.V.; Baibak, O.V.; Plieva, F.M.; Lysogorskaya, E.N.; Oksenoit, E.S.; Lozinsky, V.I. Biocatalysts for peptide synthesis in organic media—Proteinases immobilized on poly(vinyl alcohol)-cryogel. Russ. Chem. Bull. 2001, 50, 1896–1901. [Google Scholar] [CrossRef]
- Bacheva, A.V.; Baibak, O.V.; Belyaeva, A.V.; Lysogorskaya, E.N.; Oksenoit, E.S.; Lozinsky, V.I.; Filippova, I.Y. Native and modified subtilisin 72 as a catalyst for peptide synthesis in media with a low water content. Russ. J. Bioorgan. Chem. 2003, 29, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Bacheva, A.V.; Baibak, O.V.; Belyaeva, A.V.; Oksenoit, E.S.; Velichko, T.I.; Lysogorskaya, E.N.; Gladilin, A.K.; Lozinsky, V.I.; Filippova, I.Y. Activity and stability of native and modified subtilisin in different media. Biochemistry (Mosc.) 2003, 68, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Bacheva, A.V.; Belyaeva, A.V.; Lysogorskaya, E.N.; Oksenoit, E.S.; Lozinsky, V.I.; Filippova, I.Y. Biocatalytic properties of native and immobilized subtilisin 72 in aqueous-organic and low water media. J. Mol. Catal. B Enzym. 2005, 32, 253–260. [Google Scholar] [CrossRef]
- Belyaeva, A.V.; Bacheva, A.V.; Oksenoit, E.S.; Lysogorskaya, E.N.; Lozinsky, V.I.; Filippova, I.Y. Peptide synthesis in organic media with the use of subtilisin 72 immobilised on a poly(vinyl alcohol) cryogel. Russ. J. Bioorgan. Chem. 2005, 31, 529–534. [Google Scholar] [CrossRef]
- Belyaeva, A.V.; Smirnova, Y.A.; Lysogorskaya, E.N.; Oksenoit, E.S.; Timofeeva, A.V.; Lozinsky, V.I.; Filippova, I.Y. Biocatalytic properties of thermolysin immobilized on poly(vinyl alcohol) cryogel. Russ. J. Bioorgan. Chem. 2008, 34, 435–441. [Google Scholar] [CrossRef]
- Lysogorskaya, E.N.; Roslyakova, T.V.; Belyaeva, A.V.; Bacheva, A.V.; Lozinsky, V.I.; Filippova, I.Y. Preparation and catalytic properties of trypsin immobilized on cryogels of poly(vinyl alcohol). Appl. Biochem. Microbiol. 2008, 44, 241–246. [Google Scholar] [CrossRef]
- Anikina, O.M.; Lysogorskaya, E.N.; Oksenoit, E.S.; Lozinsky, V.I.; Filippova, I.Y. Subtilysin Carlsberg in complex with sodium dodecyl sulfate is an effective catalysts for the solid phase segment coupling of peptides on poly(vinyl alcohol) cryogel. Russ. J. Bioorgan. Chem. Russ. 2008, 34, 329–333. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Damshkaln, L.G.; Reznikova, N.V. Biocatalyst and Its Preparation Method. Russian Patent 2,233,327, 27 July 2004. [Google Scholar]
- Shaskol’skiy, B.L.; Fogorasi, M.S.; Stanescu, M.D.; Lozinsky, V.I. Implementation of poly(vinyl alcohol) cryogels in biotechnology. VII. Composite immobilized biocatalysts containing particles of enzyme preparation entrapped in the matrix of poly(vinyl alcohol) cryogel. Biotechnol. Russ. 2009, 1, 100–115. [Google Scholar]
- Efremenko, E.N.; Spiricheva, O.V.; Varfolomeev, S.D.; Sineoky, S.P.; Baibak, A.V.; Lozinsky, V.I. Immobilized Biocatalyst, Its Preparation Method and Method for Production of Lactic Acid with the Use of This Biocatalyst. Russian Patent 2,253,677, 10 June 2005. [Google Scholar]
- Efremenko, E.N.; Spiricheva, O.V.; Veremeenko, D.V.; Baibak, A.V.; Lozinsky, V.I. L(+)-Lactic acid production using PVA-cryogel-entrapped Rhizopus oryzae fungal cells. J. Chem. Technol. Biotechnol. 2006, 81, 519–522. [Google Scholar] [CrossRef]
- Efremenko, E.; Spiricheva, O.; Varfolomeyev, S.; Lozinsky, V. Rhizopus oryzae fungus cells producing L(+)-lactic acid: Kinetic and metabolic parameters of free and PVA-cryogel-entrapped mycelium. Appl. Microbiol. Biotechnol. 2006, 72, 480–485. [Google Scholar] [CrossRef]
- Efremenko, E.; Lozinsky, V.; Sergeeva, V.; Plieva, F.; Makhlis, T.; Kazankov, G.; Gladilin, A.; Varfolomeyev, S. Additives of polybrene improve the stability of organophosphate hydrolase immobilized in poly(vinyl) alcohol cryogel carrier. J. Biochem. Biophys. Methods 2002, 51, 195–201. [Google Scholar] [CrossRef]
- Arvidsson, P.; Plieva, F.M.; Savina, I.N.; Lozinsky, V.I.; Fexby, S.; Bulow, L.; Galaev, I.Y.; Mattiasson, B. Chromatography of microbial cells using continuous supermacroporous affinity and ion exchange columns. J. Chromatogr. A 2002, 977, 27–38. [Google Scholar] [CrossRef]
- Arvidsson, P.; Plieva, F.M.; Lozinsky, V.I.; Galaev, I.Y.; Mattiasson, B. Direct chromatographic capture of enzyme from crude homogenate using immobilized metal affinity chromatography on a continuous supermacroporous adsorbent. J. Chromatogr. A 2003, 986, 275–290. [Google Scholar] [CrossRef]
- Martynenko, N.N.; Gracheva, I.M.; El-Registan, G.I.; Zubov, A.L.; Lozinsky, V.I. Method of the Preparation of Biocatalyst for Production of Alcohol-Containing Sparking Drinks. Russian Patent 2,239,658, 10 November 2004. [Google Scholar]
- Martynenko, N.N.; Gracheva, I.M.; Sarishvili, N.G.; Zubov, A.L.; El-Registan, G.I.; Lozinsky, V.I. Immobilization of Champagne yeasts by the entrapment in cryogel of polyvinyl alcohol: Means of prevention of cell release from the carrier matrix. Appl. Biochem. Microbiol. 2004, 40, 158–164. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Spiricheva, O.V.; Veremeenko, D.V.; Lozinsky, V.I. New approaches to the production of lactic acid: Immobilized biocatalyst based on fungus cells Rhizopus oryzae entrapped in PVA cryogel. Chem. Ind. (Belgrade) 2004, 58, 116–117. [Google Scholar]
- Cunningham, C.J.; Ivshina, I.B.; Lozinsky, V.I.; Kuyukina, M.S.; Philp, J.C. Bioremediation of diesel-contaminated soil by microorganisms immobilised in polyvinyl alcohol. Int. Biodeterior. Biodegr. 2004, 54, 167–174. [Google Scholar] [CrossRef]
- Vanin, A.F.; Lozinsky, V.I.; Kapel’ko, V.I. Polymeric Composition for the Preparation of Stabilized Form of Dinitrozyl Iron Complex and Method for the Preparation of Aforementioned Form of the Complex. Russian Patent 2,291,880, 20 January 2007. [Google Scholar]
- Vanin, A.F.; Sanina, N.A.; Serezhenkov, V.A.; Burbaev, D.S.; Lozinsky, V.I.; Aldoshin, S.M. Dinitrosyl-iron complexes with thiol-containing ligands: Spatial and electronic structures. Nitric Oxide Biol. Chem. 2007, 16, 82–93. [Google Scholar] [CrossRef]
- Veliev, E.I.; Kotov, S.V.; Shishlo, V.K.; Serezhenkov, V.A.; Lozinsky, V.I.; Vanin, A.F. Beneficial effect of dinitrosyl iron complexes with thiol-containing ligands on the rat penile cavernous bodies. Biophysics 2008, 53, 153–157. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Podorozhko, E.A. Composition for the Preparation of Oil-Filled Cryogel of poly(vinyl alcohol), the Method for the Preparation of This Cryogel and the Oil-Filled Cryogel Itself. Russian Patent 2,326,908, 20 June 2008. [Google Scholar]
- Kuyukina, M.S.; Ivshina, I.B.; Gavrin, A.Y.; Podorozhko, E.A.; Lozinsky, V.I.; Jeffree, C.E.; Philp, J.C. Immobilization of hydrocarbon-oxidizing bacteria in poly(vinyl alcohol) cryogels hydrophobized using a biosurfactant. J. Microbiol. Methods 2006, 65, 596–603. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Serebrennikova, M.K.; Krivorutchko, A.V.; Podorozhko, E.A.; Ivanov, R.V.; Lozinsky, V.I. Petroleum-contaminated water treatment in a fluidized-bed bioreactor with immobilized Rhodococcus cells. Int. Biodeterior. Biodegr. 2009, 63, 427–432. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Sen’ko, O.V.; Spiricheva, O.V.; Varfolomeev, S.D.; Lozinsky, V.I. Immobilized Biocatalyst for the Bioremediation of Fat-Containing Waste Waters and Its Preparation Method. Russian Patent 2,315,102, 20 January 2008. [Google Scholar]
- Sen’ko, O.V.; Spiricheva, O.V.; Lozinsky, V.I.; Efremenko, E. Immobilized biocatalyst for the remediation of fat-containing waste waters of food industry plants. Catal. Ind. 2007, 1, 55–61. (In Russian) [Google Scholar]
- Efremenko, E.; Sen’ko, O.; Zubaerova, D.; Podorozhko, E.; Lozinsky, V. New biocatalyst with multiple enzymatic activities for treatment of complex food wastewaters. Food Technol. Biotechnol. 2008, 46, 208–212. [Google Scholar]
- Petrenko, Y.A.; Petrenko, A.Y.; Damshkaln, L.G.; Volkova, N.A.; Lozinsky, V.I. Growth and adipogenic differentiation of mesenchimal stromal bone marrow cells during culturing in 3D macroporous agarose cryogel sponges. Bull. Exp. Biol. Med. 2008, 146, 129–132. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Volkova, N.A.; Zhulikova, E.P.; Damshkaln, L.G.; Lozinsky, V.I.; Petrenko, A.Y. Choice of conditions for human bone marrow stromal cells seeding into polymer macroporous sponges. Biopolym. Cell 2008, 24, 399–405. [Google Scholar] [CrossRef]
- Shmarov, M.M.; Tutykhina, I.L.; Logunov, D.Y.; Tokarskaya, E.A.; Naroditsky, B.S.; Damshkaln, L.G.; Ivanov, R.V.; Lozinsky, V.I. Method of Virus Replication. Russian Patent 2,381,272, 10 February 2010. [Google Scholar]
- Efremenko, E.N.; Sen’ko, O.V.; Spiricheva, O.V.; Varfolomeev, S.D.; Shaskol’skiy, B.L.; Lozinsky, V.I. Immobilized Biocatalyst for Microbiological Production of Pectinases. Russian Patent 2,383,618, 10 March 2010. [Google Scholar]
- Stanescu, M.D.; Fogorasi, M.; Dochia, M.; Mihuta, S.; Lozinsky, V.I. Biotechnology for textile waste valorization. Rev. Chim. 2009, 60, 59–62. [Google Scholar]
- Stanescu, M.D.; Fogorasi, M.; Gavrilas, S.; Shaskolskiy, B.L.; Lozinsky, V.I. New potential biocatalysts by laccase immobilization in PVA cryogel type carrier. Appl. Biochem. Biotechnol. 2010, 160, 1947–1954. [Google Scholar] [CrossRef]
- Stanescu, M.D.; Stanislav, A.; Ivanov, R.V.; Hirtopeanu, A.; Lozinsky, V.I. Immobilized laccase on a new cryogel carrier and kinetics of two anthraquinone derivatives oxidation. Appl. Biochem. Biotechnol. 2011, 165, 1789–1798. [Google Scholar] [CrossRef]
- Stanescu, M.D.; Gavrilas, S.; Ludwig, R.; Haltrich, D.; Lozinsky, V.I. Preparation of immobilized Trametes pubensces laccase on a cryogel-type polymeric carrier and application of the biocatalyst to apple juice phenolic compounds oxidation. Eur. Food Res. Technol. 2012, 234, 655–662. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Rubtsova, E.V.; Ivshina, I.B.; Ivanov, R.V.; Lozinsky, V.I. Selective adsorption of hydrocarbon-oxidizing Rhodococcus cells in a column with hydrophobized poly(acrylamide) cryogel. J. Microbiol. Methods 2009, 79, 76–81. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Rubtsova, E.V.; Ivshina, I.B.; Ivanov, R.V.; Lozinsky, V.I. Adsorption immobilization of rhodococci cells in hydrophobized derivatives of wide-porous poly(acrylamide) cryogel. Appl. Biochem. Microbiol. 2011, 47, 158–164. [Google Scholar] [CrossRef]
- Evtyugin, V.G.; Margulis, A.B.; Damshkaln, L.G.; Lozinsky, V.I.; Kolpakov, A.I.; Il’inskaya, O.N. Sorption of microorganisms by wide-porous agarose cryogels containing grafted aliphatic chains of different lengths. Microbiology 2009, 78, 603–608. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Zav’yalova, N.V.; Lyagin, I.V.; Sen’ko, O.V.; Gudkov, D.A.; Aksenov, A.V.; Stepanov, N.A.; Sirotkina, M.S.; Spiricheva, O.V.; Ivanov, R.V.; et al. Method for the Biodestruction of Organophosphate Compounds in the Composition of Reaction Masses Formed as a Result of Chemical Extermination of the Vx-Type Substances. Russian Patent 2,408,724, 10 January 2011. [Google Scholar]
- Evtyugin, V.G.; Margulis, A.B.; Bushmanova, O.V.; Nikitina, E.V.; Kolpakov, A.I.; Damshkaln, L.G.; Lozinsky, V.I.; Ilinskaya, O.N. Hydrophobized derivatives of wide-porous poly(vinyl alcohol) cryogel: New possibilities of biotechnological implementation. Her. Kazan Technol. Univ. 2010, 9, 89–96. (In Russian) [Google Scholar]
- Veleshko, I.E.; Nikonorov, V.V.; Veleshko, A.N.; Rumyantseva, E.V.; Mikhailov, S.N.; Lozinsky, V.I.; Ivanov, R.V.; Gal’braikh, L.S.; Kil’deeva, N.R. Sorption of Eu(III) ions from their solutions by covalently cross-linked chitosan cryogels. Fibre Chem. 2011, 42, 364–369. [Google Scholar] [CrossRef]
- Kil’deeva, N.R.; Veleshko, I.E.; Vladimirov, L.V.; Nikonorov, V.V.; Lozinsky, V.I.; Ivanov, R.V.; Perminov, P.A.; Mikhailov, S.N. Modification of chitosan cryogels by pyridoxal phosphate to improve sorption capability. Fibre Chem. 2012, 43, 426–432. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Petrenko, A.Y. Comparison of the methods for seeding human bone marrow mesenchimal stem cells to macroporous alginate cryogel carriers. Bull. Exp. Biol. Med. 2011, 150, 543–546. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Ivanov, R.V.; Petrenko, A.Y.; Lozinsky, V.I. Coupling of gelatin to inner surfaces of pore walls in spongy alginate-based scaffolds facilitates the adhesion, growth and differentiation of human bone marrow mesenchymal stromal cells. J. Mater. Sci. Mater. Med. 2011, 22, 1529–1540. [Google Scholar] [CrossRef]
- Petrenko, Y.A.; Katsen-Globa, A.; Meiser, I.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A.Y. Cryopreservation of mesenchymal stromal cells within wide-porous three-dimensional alginate-gelatin scaffolds. Probl. Cryobiol. Cryomed 2013, 23, 351–354. [Google Scholar]
- Katsen-Globa, A.; Meiser, I.; Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A.Y. Towards a ready-to-use 3-D scaffolds for regenerative medicine: Adhesion-based cryopreservation of human mesenchymal stem cells attached and spread within alginate-gelatin cryogel scaffolds. J. Mater. Sci. Mater. Med. 2014, 25, 857–871. [Google Scholar] [CrossRef]
- Ivanov, R.V.; Lozinsky, V.I.; Balakin, K.V.; Bachurin, S.O. Pharmaceutical composition based on hydrogenated pyrido(4,3-b)indole for treatment of neurodegenerative diseases, method for preparation of the composition and pharmacological dosage form on the basis of this composition. Russian Patent 2,428,185, 10 September 2011. [Google Scholar]
- Yarullina, D.R.; Damshkaln, L.G.; Mikheeva, R.O.; Il’inskaya, O.N.; Lozinsky, V.I. Complex Probiotic Formulation and the Process of Its Preparation. Russian Patent 2,491,079, 27 August 2013. [Google Scholar]
- Yarullina, D.R.; Damshkaln, L.G.; Bruslik, N.L.; Konovalova, O.A.; Ilinskaya, O.N.; Lozinsky, V.I. Towards effective and stable probiotics. Int. J. Risk Saf. Med. 2015, 27, 65–66. [Google Scholar] [CrossRef] [Green Version]
- Efremenko, E.N.; Stepanov, N.A.; Gudkov, D.A.; Senko, O.V.; Lozinsky, V.I.; Varfolomeev, S.D. Immobilized fungal biocatalysts for the production of cellulase complex hydrolyzing renewable plant feedstock. Catal. Ind. 2013, 5, 190–198. [Google Scholar] [CrossRef]
- Carpova-Rodina, N.V.; Andryushina, V.A.; Yaderetz, V.V.; Druzhinina, A.V.; Stytsenko, T.S.; Shaskol’skiy, B.L.; Lozinsky, V.I.; Huy, L.D.; Voishvillo, N.E. Transformation of Δ4-3-ketosteroids by free and immobilized cells of Rhodococcus erythropolis actinobacterium. Appl. Biochem. Microbiol. 2011, 47, 386–392. [Google Scholar] [CrossRef]
- Andryushina, V.A.; Ryabev, A.N.; Druzhinina, A.V.; Podorozhko, E.A.; Karpova, N.V.; Stytsenko, T.S.; Yaderets, V.V.; Lozinsky, V.I. Immobilized biocatalyst for microbial biotransforming of steroid compounds. Russian Patent 2,524,434, 04 June 2014. [Google Scholar]
- Andryushina, V.A.; Karpova, N.V.; Druzhinina, A.V.; Stytsenko, T.S.; Podorozhko, E.A.; Ryabev, A.N.; Lozinsky, V.I. Novel immobilized biocatalyst for microbiological synthesis of pharmaceutical steroids. Appl. Biochem. Microbiol. 2015, 51, 530–538. [Google Scholar] [CrossRef]
- Gritsay, D.V.; Lebedinsky, A.S.; Ochenashko, O.V.; Rogul’skaya, E.Y.; Petrenko, Y.A.; Lozinsky, V.I.; Ivanov, R.V.; Petrenko, A.Y. Transplantation of cryopreserved fetal liver cells seeded into macroporous alginate-gelatin scaffolds in rats with liver failure. Her. Transpl. Artif. Organs 2015, 17, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Lozinsky, V.I.; Kulakova, V.K.; Petrenko, A.Y.; Petrenko, Y.A.; Ershov, A.G.; Sukhanov, Y.V. Composition for the preparation of macroporous carrier which is used upon 3D culturing of animal or human cells, and method for the fabrication of such carrier. Russian Patent 2,594,427, 22 July 2016. [Google Scholar]
- Mezhevikina, L.M.; Malenko, G.P.; Kornienko, E.V.; Lozinsky, V.I.; Kosovsky, G.Y. Liposomal EGFP-transfection of cattle mesenchymal cells. Russ. J. Biopharm. 2016, 8, 13–20. [Google Scholar]
- Lozinsky, V.I.; Kulakova, V.K.; Ivanov, R.V.; Petrenko, A.Y.; Rogulska, O.Y.; Petrenko, Y.A. Cryostructuring of polymer systems. 47. Preparation of wide porous gelatin-based cryostructurates in sterilizing organic media and assessment of the suitability of thus formed matrices as spongy scaffolds for 3D cell culturing. e-Polymers 2018, 18, 175–186. [Google Scholar] [CrossRef]
- Korovina, D.G.; Stafford, V.V.; Gulyukin, A.M.; Rodionov, I.A.; Kulakova, V.K.; Lozinsky, V.I.; Savchenkova, I.P. Maintenance of multipotent mesynchymal stem cells of farm animals in cryogels based on naturally-derived polymers. Agric. Biol. 2019, 54, 1214–1224. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Ryabev, A.N.; Pavlova, L.A.; Tsyurupa, M.P.; Blinnikova, Z.K.; Davankov, V.A. Macroporous polymeric material filled with sorbent particles, composition for its preparation and method for the material preparation. Russian Patent 2,601,605, 13 October 2016. [Google Scholar]
- Lozinsky, V.I.; Rodionov, I.A.; Tsiskarashvili, A.V.; Es’kin, N.A. Antibacterial Protein Sponge for Chemotherapy of Infected Wounds and Method of Its Preparation. Russian Patent 2,637,634, 05 December 2017. [Google Scholar]
- Kolesnikova, E.S.; Kolosova, O.Y.; Lozinsky, V.I. Poly(vinyl alcohol) cryogels containing additives of biologically active substances. Adv. Chem. Chem. Technol. 2017, 31, 21–23. (In Russian) [Google Scholar]
- Vernaya, O.V.; Shabatin, V.P.; Nuzhdina, A.V.; Zvukova, N.D.; Khvatov, D.I.; Semenov, A.M.; Lozinsky, V.I.; Shabatina, T.I.; Mel’nikov, M.Y. Cryochemical synthesis and antibacterial activity of hybrid nanocomposites of dioxydine with Ag and Cu nanoparticles entrapped in biopolymeric cryostructurates. Russ. Chem. Bull. 2017, 66, 2152–2156. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Nuzhdina, A.V.; Zvukova, N.D.; Shabatin, V.P.; Semenov, A.M.; Lozinsky, V.I.; Mel’nikov, M.Y. Hybrid nanosystems based on an antibacterial preparation of dioxydine and metal nanoparicles (Ag, Cu) included in biopolymer cryostructures. Nanotechnol. Russ. 2018, 13, 182–188. [Google Scholar] [CrossRef]
- Tykhvynska, O.A.; Rogulska, O.Y.; Volkova, N.O.; Grischuk, V.P.; Revenko, O.B.; Mazur, S.P.; Lozinsky, V.I.; Petrenko, Y.A.; Petrenko, A.Y. Blood plasma-based macroporous scaffolds as biocompatible coatings to restore full-thickness excision wounds. Probl. Cryobiol. Cryomed. 2018, 28, 44–48. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Karlova, D.L.; Nuzhdina, A.V.; Shabatin, V.P.; Semenov, A.M.; Lozinsky, V.I.; Mel’nikov, M.Y. Hybrid systems of drug delivery of long-acting drugs based on gentanicine sulfate, silver, and copper nanoparicples, and gelatin biopolymer matrices. Nanotechnol. Russ. 2018, 13, 546–550. [Google Scholar] [CrossRef]
- Sazhnev, N.A.; Drozdova, M.G.; Rodionov, I.A.; Kil’deeva, N.R.; Balabanova, T.V.; Markvicheva, E.A.; Lozinsky, V.I. Preparation of chitosan cryostructurates with controlled porous morphology and their use as 3D-scaffolds for the cultivation of animal cells. Appl. Biochem. Microbiol. 2018, 54, 459–467. [Google Scholar] [CrossRef]
- Krasnov, M.S.; Shaikhaliev, A.I.; Korshakov, E.V.; Efimenko, M.V.; Soloshenkov, P.P.; Davydova, T.R.; Zvukova, N.D.; Sinitskaya, E.S.; Yamskova, V.P.; Yamskov, I.A.; et al. Induction of the rat bone tissue osteogenesis using cryogenically-structured porous 3D-materials containing a bioregulator. Bull. Exp. Biol. Med. 2019, 168, 99–103. [Google Scholar] [CrossRef]
- Aslanli, A.; Stepanov, N.; Razheva, T.; Podorozhko, E.; Lozinsky, V.I.; Efremenko, E. Enzymatically functionalized composite materials based on nanocellulose and PVA cryogel and possessing antimicrobial activity. Materials 2019, 12, 3619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Semenov, A.M.; Melnikov, M.Y.; Lozinsky, V.I. Metal nanoparticle containing nanocomposites of drug substances and their potential biomedical applications. Appl. Sci. 2020, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Shaikhaliev, A.I.; Korshakov, E.V.; Kolosova, O.Y.; Krasnov, M.S.; Lozinsky, V.I. Temporary implant for the patients with infected defects in the maxillofacial region and a method for treating them using such an implant. Russian Patent 2,729,929, 13 August 2020. [Google Scholar]
- Sergeev, G.B.; Batyuk, V.A. Reactions in frozen multicomponent systems. Russ. Chem. Rev. 1976, 45, 391–408. [Google Scholar] [CrossRef]
- Rogozhin, S.V.; Lozinsky, V.I.; Vainerman, E.S.; Korshak, V.V. The influence of the freezing of polymerising monomer solutions on the molecular weights of the polymers obtained. Dokl. Akad. Nauk SSSR 1983, 273, 1140–1143. (In Russian) [Google Scholar]
- Lozinsky, V.I.; Ivanov, R.V.; Kalinina, E.V. Method for the preparation of polyacrylamide. Russian Patent 2,196,780, 20 January 2003. [Google Scholar]
- Lozinsky, V.I.; Ivanov, R.V.; Kalinina, E.V.; Timofeeva, G.I.; Khokhlov, A.R. Redox-initiated radical polymerization of acrylamide in moderately frozen water solutions. Macromol. Rapid Commun. 2001, 22, 1441–1446. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kalinina, E.V.; Putilina, O.I.; Kulakova, V.K.; Kurskaya, E.A.; Dubovik, A.S.; Grinberg, V.Y. The influence of phase state of the reacting system on the properties of poly(N-isopropylacrylamide) at polymer synthesis in aqueous medium. Polym. Sci. Ser. A 2002, 44, 1122–1128. [Google Scholar]
- Lozinsky, V.I.; Ivanov, R.V. Polymer synthesis in moderately frozen solutions of monomers. In Synthesis and Modification of Polymers; Monakov, Y.B., Ed.; Khimiya: Moscow, Russia, 2003; pp. 68–86. ISBN 5-7245-1229-7. (In Russian) [Google Scholar]
- Ivanov, R.V.; Babushkina, T.A.; Lozinsky, V.I. Specifics of acrylamide cryopolymerisation at temperatures above and below the eutectic point of the frozen reaction system. Polym. Sci. Ser. A 2005, 47, 791–799. [Google Scholar]
- Ivanov, R.V.; Lozinsky, V.I. Influence of thermal pre-history of moderately frozen reaction system on the results of acrylamide cryopolymerisation. Polym. Sci. Ser. A 2006, 48, 1232–1239. [Google Scholar] [CrossRef]
- Zaborina, O.E.; Buzin, M.I.; Lozinsky, V.I. Cryopolymerization of N,N-dimethylacrylamide in moderately frozen formamide. Polym. Sci. Ser. B 2012, 54, 354–361. [Google Scholar] [CrossRef]
- Lipatov, Y.S. Relaxation and viscoelastic properties of heterogeneous polymeric compositions. Adv. Polym. Sci. 1977, 22, 1–59. [Google Scholar] [CrossRef]
- Kudaibergenov, S.E.; Ibraeva, Z.E.; Yashkarova, M.G.; Bekturov, E.A. Hydrogel Composite Materials; Shakarim State University Publ.: Semei, Kazakhstan, 2011; 148p. (In Russian) [Google Scholar]
- Altunina, L.K.; Manzhai, V.N.; Fufaeva, M.S. Mechanical and thermal properties of cryogels and foamed cryogels produced from aqueous solutions of poly(vinyl alcohol). Russ. J. Appl. Chem. 2006, 79, 1669–1672. [Google Scholar] [CrossRef]
- Fellows, P. Freeze drying and freeze concentration. In Food Processing Technology: Principles and Practice; Woodhead Publishing/Elsevier Science: Kent, UK, 2017; pp. 929–940. ISBN 978-0081005231. [Google Scholar]
- Pivovarov, P.P.; Pertsevoy, F.V.; Vainerman, E.S.; Rogozhin, S.V. Method for the preparation of structured gel-like products. SU Patent 1,012,872, 03 December 1981. [Google Scholar]
- Lozinsky, V.I.; Plieva, F.M.; Zubov, A.L. Application of poly(vinyl alcohol) in biotechnology. V. Supermacroporous carriers for the immobilization of molecules. Biotechnol. Russ. 1995, 2, 1–9. [Google Scholar]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [Green Version]
- Cao, L. Carrier-Bound Immobilized Enzymes: Principles, Applications and Design; Wiley–VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; 578p, ISBN 978-3-527-31232-0. [Google Scholar]
- Ajdary, R.; Tardy, B.L.; Mattos, B.D.; Bai, L.; Rojas, O.J. Plant nanomaterials and inspiration from nature: Water interactions and hierarchically structured hydrogels. Adv. Mater. 2020, e2001085. [Google Scholar] [CrossRef]
- Liu, K.Q.; Wei, S.; Song, L.X.; Liu, H.L.; Wang, T.F. Conductive hydrogels—A novel material: Recent advances and future perspectives. J. Agric. Food. Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef]
- Shahbazi, M.A.; Ghalkhani, M.; Maleki, H. Directional freeze-casting: A bioinspired method to assemble multifunctional aligned porous structures for advanced applications. Adv. Eng. Mater. 2020, 22, 2000033. [Google Scholar] [CrossRef]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable cryogels for biomedical applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Gallette, A.; Francis, M.; Wilkowski, J.; Salas-de la Cruz, D.; Hu, X. Protein-polysaccharide composite materials: Fabrication and applications. Polymers 2020, 12, 464. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; Garcia-Gonzalez, C.A.; Landin, M.; Aquino, R.P. Technologies and formulation design of polysaccharide-based hydrogels for drug delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jimenez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-based scaffolds for soft-tissue engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Bacon, K.; Lavoie, A.; Rao, B.M.; Daniele, M.; Menegatti, S. Past, present, and future of affinity-based cell separation technologies. Acta Biomater. 2020, 112, 29–51. [Google Scholar] [CrossRef]
- Jyothilekshmi, I.; Jayaprakash, N.S.; Jayaprakash, N. S. Trends in monoclonal antibody production using various bioreactor systems. J. Microbiol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Altunina, L.K.; Burkov, V.P.; Burkov, P.V.; Dudnikov, V.Y.; Osadchaya, G.G.; Ovsyannikova, V.S.; Fufaeva, M.S. Cryogels as a solution to the issues of environmental management and trunk pipeline operation in Arctic. Sci. Technol. Oil Oil Prod. Pipeline Transp. 2020, 10, 173–185. (In Russian) [Google Scholar] [CrossRef]







| Example | Precursors | Dispersion Medium | Conditions of Cryogenic Processing | What was Studied and Found | References | Publication Year |
|---|---|---|---|---|---|---|
| 1 | Thiol-containing poly(acylamide) | Aqueous medium | –8 °C/18 h | Detection that water-dissolved oxygen causes the formation of disulfide-crosslinked macroporous cryogels | [166] | 1981 |
| 2 | The systems: polymer + cross-linker, vinyl and divinyl comonomers + initiator; polycondesation comonomers | Aqueous and organic solvents | 1–60 °C below the solvent crystallization point/1–72 h | Elaboration of the synthetic approaches to the preparation of the chemically cross-linked cryogels | [167] | 1982 |
| 3 | The systems: polymer + cross-linker | Water; DMSO | –8 °C/18 h; +2 °C/15 h | Demonstration of the possibility to prepare chemically crosslinked cryogels both in aqueous and organic non-deeply-frozen media | [168] | 1982 |
| 4a b c | Chitosan + glutar aldehyde | Weakly acidic aqueous medium | −8 to −30 °C/18–24 h | Detection of the effect of an apparent decrease in the critical concentration of the cryotropic gelation | [169] [170] [171] | 1982 2010 2011 |
| 5a b | AAM + MBAAM + TEMED + APS | Water FA | −8 °C/18 h −10 °C/24 h | Preparation of poly(acrylamide)-based polymerization-type cryogels in frozen aqueous or formamide media and the studies of the resultant cryogels properties | [172] [173,174] | 1983 1984 |
| 6a b c d e | Gelatine; Agar-agar; Agaroide; PVA | Water DMSO | −10 °C/24 h −10 to −20 °C/19–24 h −10 °C/24 h | Demonstration of general character of the phenomenon of the formation of non-covalent (physical) cryogels | [175] | 1984 |
| 7a b | Poly(NVP-co-MA) + DADPO; PS + PXDC + SnCl4 | DMSO NB | −8 °C/18 h −4.5 to −27 °C/1–24 h | Preparation of covalent cryogels in frozen organic media and studies of the resultant cryogels properties; the first use of the term “cryogels” | [176] | 1984 |
| 8 | AAM + MBAAM + TEMED + APS | Water | −10 to −30 °C/1–48 h | Detection of the gel-formation acceleration effects and of a bell-shaped dependence of the gel-formation efficiency on the cryogenic processing temperature | [177] | 1986 |
| 9 | PVA | Water | −10 to −20 °C/12–240 h | The evidence of the non-covalent nature of the intermolecular links in the PVA cryogels | [178] | 1986 |
| 10 | PVA | Water | −10 to −20 °C/1–240 h | Studies of the influence of PVA molecular weight and concentration; freezing temperature and duration on the physico-mechanical properties of the PVA cryogels and their microstructure | [179] | 1986 |
| 11 | PVA | Water | −10 to −30 °C/1–24 h | The first demonstration of the principal significance of the heating rate upon the frozen samples defrosting for the properties of the resultant PVA cryogels | [180] | 1988 |
| 12 | PVA + Aspergillus clavatus spores | Aqueous medium | −20 °C/20 h | Studies of the biological activity of immobilized biocatalyst capable of producing the guanyl-ribonuclease | [181] | 1988 |
| 13 | AAM + MBAAM + TEMED + APS + Escherichia coli cells | Aqueous medium | −10 to −30 °C/18–24 h | Studies of the influence of cryopolymerization conditions on the microstructure of cryogel-based carriers, as well as on the cells viability and activity | [182] | 1988 |
| 14 | Sodium silicate + HCl + Renobacter vacuolatum (or Blastobacter viscosus, or Methhylobacterium) cells | Aqueous media, pH ~6 | −10 °C/24 h | Preparation of immobilized biocatalysts (bacterial cells entrapped in cryo-silicagel) exhibiting the hydrogen-oxidizing activity and studies of the physico-chemical properties and activity of such biocatalytic systems | [183] | 1988 |
| 15 | AAM + MBAAM + TEMED + APS | Water | −10 to −30 °C/24 h or −196 °C/0.5 h followed by −10 to −30 °C/24 h (low-temperature quenching technique) | Studies of the dynamics of cryotropic gel-formation, evaluations of the properties and microstructure of the resultant gel matrices as dependent on the freezing techniques | [184] | 1989 |
| 16a b | Thiol-containing poly(acrylamide) derivative | Aqueous medium, pH 7.5 | −10 to −30 °C/1–24 h | Studies of the dynamics of cryotropic gel-formation and the measurements of the amount of reactive pendant SH-groups of the polymer during such process | [185] [186] | 1989 2000 |
| 17 | PVA | Water | −10 to −30 °C/24–240 h | Studies of the properties of the PVA cryogels as dependent on the frozen storing duration | [187,188] | 1989 |
| 18 | PVA | Water | −10 to −20 °C/1–24 h | Studies of the thermomechanical properties of the PVA cryogels formed under various thermal regimes | [189] | 1989 |
| 19 | PVA | D2 O | −20 to −66 °C/1–6 h | 2H and 13C NMR studies of the frozen PVA solutions | [190] | 1990 |
| 20 | PVA + yeast cells | Aqueous medium | −15 °C/19 h | Studies of the filler particles (yeasts) influence on the physico-mechanical properties of the resultant composite PVA cryogels | [191] | 1990 |
| 21 | AAM + MBAAM + TEMED + APS + cells (Renobacter vacuolatum, or Blastobacter viscusus, or Methhylobacterium sp.) | Aqueous medium | −10 °C/24 h | Preparation of immobilized biocatalysts; studies of their physico-chemical properties and hydrogen-oxidizing activity | [192] | 1991 |
| 22 | PVA + nitroxyl-type spin probe | Water | −5 to −20 °C during the time of measurements | The ESR spectroscopic measurements of the UFLMP volume at various minus temperatures | [193] | 1991 |
| 23a b | PVA + oligoethylene glycols | Water | −5 to −20 °C/1–240 h −20 °C/18 h | Preparation and studies of the complex PVA cryogels that contained the additives of various oligoethylene glycols | [194] [195] | 1992 1995 |
| 24 | PVA + particles of cross-linked dextran (series of Sephadexes) | Water | −15 °C/18 h | Detection of the key role of the filler porosity for its influence on the properties of the composite PVA cryogels | [196] | 1992 |
| 25 | AAM + MBAAM + TEMED + APS | D2O | −13 to −28 °C/1–60 min or −196 °C/0.5 h followed by −13 to −28 °C/1–60 min (low-temperature quenching technique) | 1H- and 2H NMR studies of the poly(acrylamide) cryogels formation in frozen aqueous systems | [197] | 1993 |
| 26 | Water-soluble salts of polyacids | Aqueous media | −15 to −78 °C/1–12 h | Approaches for the preparation of the polyacids-based spongy cryostructurates | [198] | 1994 |
| 27 | Water-soluble salts of polymeric bases | Aqueous media | −8 to −78 °C/2–24 h | Approaches for the preparation of spongy cryostructurates consisting of the polymeric bases | [199] | 1994 |
| 28 | PVA + low-molecular polyols | Water | −20 °C/18 h | Preparation and studies of the PVA cryogels that contained the additives of low-molecular polyols | [195] | 1995 |
| 29a b | HEMA + MBAAM + TEMED + SPS | Water Water + dioxane | −8 to −10 °C/120 h −8 to −18 °C/24–75 h | Synthesis and studies of the poly(hydroxyethyl methacrylate)-based cryogels prepared in frozen aqueous of mixed water-organic media | [200] [201] | 1996 1996 |
| 30 | PVA | Water | −20 °C/24 h | Studies of the PVA cryogels swelling behavior in the salt-containing aqueous solutions | [202] | 1996 |
| 31 | PVA + low-molecular electrolytes | Water | −20 °C/24 h | Studies of the low-molecular electrolytes influence on the formation and properties of the PVA cryogels | [203] | 1996 |
| 32 | DEAAM + MBAAM + TEMED + APS | Water | −24 °C/0.5 h and further −10 °C/18 h | Preparation and studies of the first example of the temperature-responsive polymeric cryogels | [204] | 1997 |
| 33a b | PVA + disperse mineral or organic fillers of various porosity | Water | −15 °C/18 h −10 to −30 °C/12 h | Studies of the influence of micro-, meso- and macroporous fillers on the properties of the composite PVA cryogels | [205] [206] | 1997 2017 |
| 34 | Ovalbumin + urea | Water | −8 to −32 °C/0.1–48 h | Studies of the denaturation-induced cryotropic gel-formation of ovalbumin | [207] | 1997 |
| 35a b c d | Gelatinized starch + aroma compounds | Aqueous media | −18 °C/16 h | Preparation of the starch-based cryogels that contained various aroma compounds and study of their adsorption and release | [208] [209] [210,211] [212,213] | 1998 1999 2001 2002 |
| 36 | PVA | Water | −10 to −30 °C/24 h | Studies of the cryostructuring processes in the low-concentrated PVA solutions | [214] | 1999 |
| 37 | Microparticulate dispersions of fibrous collagen | Aqueous media, pH 1.5–12.4 | −15 °C/16–18 h | Studies of the microfibrillar collagen based cryogels that were formed at various pH values of the feed systems | [215] | 2000 |
| 38 | PVA | Water | −18 °C/18 h | Evaluation of the apparent yield of the PVA cryotropic gel-formation | [216] | 2000 |
| 39 | Amylopectin | Water | −6 to −24 °C/18 h | Studies of the cryoprecipitation effects in the low-concentrated solutions of amylopectin | [217] | 2000 |
| 40 | PVA | Water | −20 °C/24 h | Studies of the PVA cryotropic gel-formation dynamics | [218] | 2000 |
| 41 | Amylopectin + amylose + NaCl | Water | −6 to −24 °C/18 h | Studies of the cryostructuring processes in frozen aqueous solutions of the amylopectin/amylose mixtures | [219] | 2000 |
| 42 | Locust bean gum | Water | −20 °C/18 h | Studies of the locust bean gum cryotropic gel-formation and the elucidation of the nature of intermolecular links in the resultant cryogels | [220] | 2000 |
| 43 | PVA + gas bubbles | Water | −10 to −30 °C/18 h | Preparation and studies of the gas-filled (foamed) PVA cryogels | [221] | 2001 |
| 44 | Agarose + gelation-slowing additives | Aqueous media | −5 to −50 °C/1–24 h | Preparation and studies of the agarose-based wide-pore cryogels | [222,223] | 2001 |
| 45a b | PVA + hydophobic disperse particles | DMSO | −15 to −30 °C/1–24 h | Development of the approach for the preparation of composite PVA cryogels with entrapped hydrophobic fillers; studies of the properties of such composites | [224] [225] | 2001 2002 |
| 46 | Maltodextrin | Water | −6 to −24 °C/18 h | Studies of cryostructuring of the maltodextrin-containing aqueous solutions | [226] | 2002 |
| 47 | PVA + surfactants | Water | −5 to −78 °C/5–48 h | Revelation of the possibility to affect the macroporous morphology of PVA cryogels with the aid of surfactant additives | [227] | 2003 |
| 48a b | PVA + particles of ion-exchange resins | Water | −20 °C/5.5 h | Studies of the charged fillers influence of the properties and microstructure of composite PVA cryogels | [228] [229] | 2004 2005 |
| 49 | PVA + gas bubbles + surfactants | Water | −20 to −30oC/24 h | Studies of the surfactants influence on the physico-chemical properties and porous structure of the gas-filled (foamed) PVA cryogels | [230] | 2005 |
| 50 | PVA + TMOS | Water | −15 to −30 °C/12 h | Preparation and studies of the hybrid organic–inorganic PVA cryogels formed with simultaneous sol–gel transformation of TMOS precursor | [231] | 2007 |
| 51a b c | PVA | Water | −10 to −40 °C/1–240 h | Systematic studies of the influence of all parameters of the cryostructuring processes on the properties and structure of PVA cryogels | [232] [233] [234] | 2007 2008 2012 |
| 52a b | Poly(acrylamide) + glutar aldehyde | Aqueous medium, pH 10 | −5 to −20 °C/24 h | Preparation and studies of the covalently-linked macroporous cryogels based on pre-synthesized poly(acrylamide) | [235] [236] | 2007 2008 |
| 53a b c d | Agarose + gelation-slowing additives | Aqueous media | −5 to −30 °C/24 h | Preparation of the wide-pore agarose cryogel with grafted gelatin and its use as a scaffold for culturing of insulin-producing cells | [237] [238] [239] [240] | 2008 2005 2007 2010 |
| 54a b | NIPAAM + MBAAM + TEMED + APS + oil microdroplets | Aqueous medium | −15 °C/24 h | Preparation and studies of composite thermoresponsive wide-pore cryogels filled with oil dispersion | [241] [242] | 2008 2013 |
| 55 | Gelatinized starch + gluten or gums additives | Aqueous medium | −9 to −40oC/23 h | Studies of the influence of gluten and gums (guar, xanthan) additives on the physico-chemical properties and macro-porous morphology of the complex starch-based cryogels | [243] | 2009 |
| 56a b | PVA + microdroplets of hydrophobic liquids | Aqueous media | −10 to −60 °C/1–24 h | Studies of composite PVA cryogels filled with microdroplets of hydrophobic liquids | [244] [245] | 2010 2012 |
| 57 | PVA + alkali metal chlorides | Water | −20 °C/12 h | Studies of the influence of alkali metal chloride additives on the formation, properties and macroporous morphology of the PVA cryogels | [246] | 2011 |
| 58 | DMAAM + ionic acrylates + TEMED + APS | Water | −5 to −40 °C/4–24 h | Method for the preparation of poly(N,N’-dimethylacrylamide)-based cryogels that possess the superabsorbent properties and study of the mechanisms for such materials formation | [247] [248] | 2011 2014 |
| 59 | NIPAAM + DMAPA + BAC + TEMED + APS + ibuprofen | Aqueous medium | −10 °C/20 h | Synthesis of the ibuprofen-templated wide-pore thermoresponsive cryogels and the studies of their specificity with respect of various ligands | [249] | 2011 |
| 60 | PVA + dispersed PVAc microparticles | Aqueous medium | −20 °C/12 h | Preparation of composite PVA cryogels and studies of the influence of particulate polymeric fillers on the properties and microstructure of the resultant gel materials | [250] | 2012 |
| 61 | PVA + guar gum | Aqueous media, pH 5–11 | −5 to −30 °C/12 h | Preparation and studies of the wide pore PVA cryogels using a combination of the liquid–liquid phase separation and the cryotropic gel-formation processes | [251] | 2012 |
| 62 | PVA + series of the low-molecular aliphatic alcohols | Aqueous medium | −15 to −35 °C/12 h | Studies of the influence which the additives of low-molecular aliphatic alcohols exert on the physico-chemical properties and macroporous morphology of the PVA cryogels | [252] | 2014 |
| 63 | PVA | Water | −10 to −50 °C/1–48 h | Elaboration of the approach for the secondary molding of the PVA cryogels | [253] | 2014 |
| 64 | PVA + dispersions of butadiene-co-styrene latex | Water | −20 °C/12 h | Preparation and studies of the latex-filled composite PVA cryogels | [254] | 2015 |
| 65 | Aqueous dispersions of butadiene-co-styrene latex | Water | −20 °C/12 h | Preparation of the latex-based cryostructurates and studies of their microstructure | [254] | 2015 |
| 66 | DMAAM + allyl derivatives of 1,8-naphthalimide + TEMED + APS | Water | −10 to −30 °C/24 h | Preparation and studies of the osmotic and optical properties of fluorescent copolymeric cryogels | [255] | 2015 |
| 67 | PVA + chitosan powder | Water | −20 °C/12 h | Preparation of chitosan-filled composite PVA cryogels, studies of their properties, microstructure and absorption capacity with respect of copper ions | [256] | 2015 |
| 68 | Serum albumin + urea + cystein | Aqueous medium | −15 to −25 °C/20 h | Preparation of the wide-pore serum-albumin-based cryogels; studies of their physico-chemical properties, porosity characteristics and the mechanisms of the 3D network formation | [257] | 2015 |
| 69a b c d | PVA + chitosan hydrochloride | Aqueous medium | −20 °C/12 h | Preparation of complex and composite chitosan-containing PVA cryogels with the use the in situ filler formation, studies of their properties, microstructure and absorption characteristics | [258] [259] [260] [261] | 2016 2017 2019 2020 |
| 70 | Serum albumin + EDC | Aqueous medium | −15 to −25 °C/20 h | Preparation and studies of EDC-assisted cross-linked albumin cryogels, studies of their properties and macroporous morphology | [262] | 2016 |
| 71 | Serum albumin | Water | −15 to −25 °C/18 h | Preparation of the wide-pore albumin-based cryostructurates followed by their chemical tanning and studies of the properties | [263] | 2017 |
| 72 | PVA + low-molecular aliphatic alcohols | DMSO | −11.6 to −31.6 °C/12 h | Studies of the influence of low-molecular aliphatic alcohol additive on the properties and microstructure of the PVA cryogels formed in a frozen DMSO medium | [264] | 2017 |
| 73a b | PVA + organic chaotropes and kosmotropes | Aqueous media | −20 °C/12 h | Studies of the influence of chaotropic and kosmotropic additives on the physico-chemical properties and microstructure of PVA cryogels | [265] [266] | 2018 2019 |
| 74 | PVA + organic chaotropes and kosmotropes | DMSO | −11.6 to −31.6 °C/12 h | Studies of the influence of chaotropic and kosmotropic additives on the physico-chemical properties and microstructure of the PVA cryogels formed in a frozen DMSO medium; revealing of the unexpected “kosmotropic-like” effects of the organic chaotropes | [267,268] | 2018 |
| 75 | PVA + cellulose-based fillers + salting-out electrolyte | Aqueous medium | −20 °C/12 h | Studies of the combined fillers and electrolytes influence on the properties and structure of composite PVA cryogels | [269] | 2019 |
| 76 | Sodium alginate | Water | −10 to −30 °C/1 h | Preparation of Ca-alginate-based wide-pore cryostructurates, studies of their properties and porous morphology, as well as the evaluation of their suitability as drug delivery carriers | [270] | 2019 |
| 77 | PVA + TMOS + aqueous HCl as a catalyst | DMSO | −21.6 °C/18 h | Preparation and studies of the hybrid organic–inorganic PVA cryogels formed in the frozen DMSO medium | [271] | 2019 |
| 78a b | BSA + urea + cysteinBSA + GHC + cystein | Aqueous media, pH ~8 | −15 to −25 °C/18 h | Preparation of the wide-pore albumin-based cryogels followed by their chemical modification via the succinylation, studies of the properties of the resultant spongy matrices and evaluation of their potential as drug delivery materials | [272] | 2020 |
| Example | Precursors | Dispersion Medium | Conditions of Cryogenic Processing | Elaboration Targets and Related Studies | References | Publication Year |
|---|---|---|---|---|---|---|
| 1 | Coagulates of the plant proteins isolates | Water | −10 to −60 °C/1–48 h | Method for the preparation of the porous gels based on plant proteins | [163] | 1972 |
| 2a b c | Paste-like dispersion of fish or crustacean proteins isolates | Aqueous media, pH 5–7 | −13 to −15 °C/1.5–48 h | Method for the preparation of the cryogels based on myofibrillar proteins; studies of the cryogels properties | [164] [273] [274] | 1977 1981 1984 |
| 3 | Sodium alginate | Water | −20 to −40 °C/0.5–24 h | Elaboration of the Ca-alginate cryostructurates that are of interest as the biomedical materials | [275,276] | 1985 |
| 4 | PVA + nutrients | Water | −5 to −196 °C/0.5–24 h | Elaboration of the PVA-cryogel-based microbiological solid nutritional media | [277] | 1985 |
| 5 | PVA + microbial cells | Aqueous media | −10 to −70 °C/1–240 h | Method for the entrapment of microbial cells in the PVA cryogel carriers | [278] | 1986 |
| 6 | PVA + Citrobacter intermedius cells | Aqueous medium | −10 to −30 °C/12–24 h | Development and studies of the immobilized biocatalysts for the production of 3-fluoro-L-tyrosine | [279] [280] | 1986 1989 |
| 7 | AAM + MBAAM + TEMED + APS + Saccharomyces cerevisiae cells | Aqueous medium | −10 to −30 °C/18–36 h | Immobilized biocatalysts capable of ethanol producing | [281] | 1986 |
| 8 | PVA + spores of filamentous fungi | Aqueous medium | −10 to −40 °C/8–24 h | Immobilized biocatalysts capable of ribonucleases producing | [282] | 1986 |
| 9 | PVA + nutrients | Aqueous media | −18 °C/3 h | Solid nutritional media for the cultivation of plant tissues and cells | [283] | 1986 |
| 10 | PVA + Saccharomyces cerevisiae cells | Aqueous medium | −70 °C/5–10 min | Immobilized biocatalysts capable of acidic phosphatase producing | [284] | 1986 |
| 11 | PVA + Zymomonas mobilis cells | Aqueous medium | −10 to −60 °C/12–24 h | Immobilized biocatalyst for the production of ethanol | [285] [286] | 1986 1996 |
| 12 | AAM + MBAAM + AGE + TEMED + APS | Water | −20 °C/24 h | Elaboration of the cryogel-based carriers for the covalent immobilization of serum albumin | [287] | 1987 |
| 13 | PVA + Erwinia aroidea cells | Aqueous medium | −6 to −30 °C/12–48 h | Immobilized biocatalysts for the biotransformation of fumaric acid to L-aspartic acid | [288] | 1988 |
| 14 | PVA + Alcaligenes faecalis cells | Aqueous medium | −6 to −30 °C/12–48 h | Immobilized biocatalysts for the decarboxylation of L-aspartic acid to L- alanine | [289] [290] | 1988 1990 |
| 15a b c | Dispersion of collagen coagulate + gluar aldehyde | Aqueous medium | −5 to −20 °C/10–15 h | Methods for the preparation of collagen sponges | [291] [292] [293] | 1988 1992 1995 |
| 16a b c d | Dispersion of tanned leather particles + gluar aldehyde | Aqueous media, pH 3.5–5.5 | −5 to −60 °C/1–24 h | Methods for the preparation of leather-like materials based on the tanned leather wastes | [294,295] [296] [297] [298] | 1988 1989 1992 1995 |
| 17 | PVA + dispersed ground particles | Aqueous media | −2 to −60 °C/3–24 h | Method for the fixation of thawed grounds | [299] | 1990 |
| 18a b | PVA | Water | −5 to −40 °C/1–24 h | The variants of setup for the preparation of the beaded PVA cryogels | [300] [301] | 1992 1996 |
| 19 | PVA | Water | −1 to −60 °C/1–12 h | Method for the preparation of the PVA-cryogel-based beaded artificial baits for sporting and amateur fishing. | [302] | 1992 |
| 20 | PVA + Presudomonas sp. Cells | Aqueous medium | −20 °C/24 h | Preparation and study of immobilized biocatalyst for the use in a biosensor for L-proline detection and quantification | [303] | 1992 |
| 21 | PVA + Corynebacterium glutamicum cells | Aqueous medium | −20 °C/15 h | Immobilized biocatalysts for the biosynthesis of L-lysine | [304] | 1992 |
| 22 | PVA + Clostridium thermosaccharolyticum cells | Aqueous medium | −20 °C/24 h | Thermotolerant immobilized biocatalyst capable of hydrogen producing | [305] | 1993 |
| 23 | Water-soluble proteins + denaturants | Aqueous media | −3 to −196 °C/0.2–48 h | Methods for the preparation of macroporous proteinaceous cryogels | [306] | 1994 |
| 24 | Water-soluble salts of polyacids | Aqueous media | −15 to −78 °C/1–12 h | Application of the polyacids-based spongy cryostructurates in analytical systems | [307] | 1994 |
| 25a b | PVA + thermotolerant Acetogenium kivuii cells | Aqueous medium | −20 °C/24 h | Preparation and study of the immobilized biocatalyst for the production of acetate via the CO2 reduction | [308,309] [310] | 1994 1996 |
| 26a b | PVA + Arthrobacter globiformis cells | Aqueous media | −20 °C/24 h | Preparation and study of the immobilized biocatalysts for the biotransformation of sterol compounds | [311] [312] | 1995 1996 |
| 27a b | Latex-containing dispersions | Aqueous media | −4 to −100 °C/0.5–12 h | Method for the preparation of the latex-based porous cryogenically structured materials | [313] [314,315,316] | 1996 1998 |
| 28 | Dispersed particles of fibrillar proteins | Aqueous media | −5 to −40 °C/6–12 h | Method for the preparation of macroporous cryogels based on fibrillar proteins | [317] | 1997 |
| 29a b | PVA | Water | −20 °C/24 h | Covalent immobilization of specific antibodies in the beaded PVA cryogels and the use of such macroporous immunosorbents for the isolation of viruses | [318] [319] | 1997 1998 |
| 30 | PVA + Kluyveromyces marxianus cells | Aqueous medium | −20 °C/18 h | Preparation and study of the immobilized biocatalyst with the PVA-cryogel-entrapped thermotolerant yeasts for the production of ethanol at elevated temperatures | [320] | 1998 |
| 31 a b c d e f g h | PVA | Water | −20 °C/24 h | Preparation of the beaded PVA cryogel, subsequent covalent immobilization of enzymes and the use of the resultant biocatalysts for the operation in organic media. The enzymes: -α-chymotrypsin -hog pancreas lipase -subtilisin 72 -thermolysin | [321] [322] [323] [322] [324,325] [326,327] [328,329] [325] | 2000 2005 2000 2005 2005 2001 2008 2008 |
| 31i j k | -thermolysin -trypsin -subtilisin Carlsberg | [330] [331] [332] | 2008 2001 2003 | |||
| 32a b | PVA + dispersions of the cross-linked enzyme aggregates | Aqueous medium | −5 to −40 °C/4–48 h | Method for the preparation of the composite immobilized biocatalysts filled with disperse particles of the cross-linked enzyme aggregates | [333] [334] | 2002 2009 |
| 33a b | PVA + cells of lactic-acid-producing microorganisms | Aqueous media | −15 to −30 °C/6–30 h | Methods for the preparation of the immobilized biocatalysts capable of producing lactic acid and the studies of such biocatalysts | [335] [336,337] | 2002 2006 |
| 34 | PVA | Water | −20 °C/24 h | Preparation of immobilized OPH using additives of Polybrene as a protective agent for the enzyme activity | [338] | 2002 |
| 35 | AAM + AGE + MBAAM + TEMED + APS | Aqueous medium | −12 to −15 °C/5–15 h | Synthesis and study of the supermacroporous affinity and ion exchange columns for the isolation of microbial cells | [339] | 2002 |
| 36 | AAM + AGE + MBAAM + TEMED + APS | Aqueous medium | −10 °C/24 h | Synthesis and study of the supermacroporous metal-chelate affinity cryogels for the isolation and purification of enzymes | [340] | 2003 |
| 37a b | PVA + Champagne yeast cells | Aqueous medium | −20 °C/24 h | Preparation and study of the immobilized biocatalysts for the fermentation of sparkling wines | [341] [342] | 2003 2004 |
| 38 | PVA + Rhizopus oryzae spores | Aqueous medium | −10 to −30 °C/12–24 h | Preparation and studies of the immobilized biocatalysts capable of producing L(+)-lactic acid | [343] | 2004 |
| 39 | PVA + suspension of soil microorganisms | Aqueous medium | −20 °C/18 h | Preparation and study of immobilized biocatalysts for the bioremediation of diesel-contaminated soils | [344] | 2004 |
| 40a b c | Water-soluble neutral polysaccharides + dinitrosyl iron complexes | Aqueous media | −10 to −196 °C/0.1–24 h | Method for the preparation of the cryostructurates serving as the carries for the dinitrosyl iron complexes and the studies of these drug-release systems as the NO-donors | [345] [346] [347] | 2005 2007 2008 |
| 41 | PVA + microdroplets of hydrophobic liquids | Aqueous media | −10 to −60 °C/1–24 h | Method for the preparation of the composite PVA cryogels filled with microdroplets of hydrophobic liquids | [348] | 2006 |
| 42 | PVA + Rhodococcus ruber cells | Aqueous media | −18 °C/12 h | Preparation and studies of the immobilized biocatalysts for the oxidation of hydrocarbon pollutants | [349] [350] | 2006 2009 |
| 43a c c | PVA + Rhizopus oryzae spores | Aqueous medium, pH 6.0 | −16 to −22 °C/16–48 h | Preparation and studies of the immobilized biocatalysts for the bioremediation of industrial waters contaminated with food wastes | [351] [352] [96,353] | 2006 2007 2008 |
| 44 | Agarose + gelation-slowing additives | Aqueous medium | −5 to −20 °C/24 h | Preparation of the wide-pore agarose cryogel with grafted gelatin and its use as a scaffold for culturing of stem cells | [354,355] | 2008 |
| 45 | Series of synthetic and natural polymers | Aqueous media | −10 to −60 °C/1–48 h | Methods for the preparation of the cryogel-based carriers, their use as the wide-pore scaffolds for culturing of permissive cells followed by virus infection and propagation | [356] | 2008 |
| 46 | PVA + spores or cells of the pectinase-producing strains | Aqueous media | −13 to −20 °C/16–24 h | Preparation of the immobilized biocatalysts for the biosynthesis of pectinases | [357] | 2008 |
| 47a b c d | PVA | Water | −20 °C/24 h | Preparation of the beaded PVA cryogel, subsequent covalent immobilization of laccase and the use of the resultant biocatalysts for the remediation of dyed textile waste waters | [358] [359] [360] [361] | 2009 2010 2011 2012 |
| 48 | AAM + MBAAM + TEMED + APS | Water | −12 °C/18 h | Preparation of the wide-pore poly(acrylamide) cryogels, their hydrophobization and the use for the adsorption immobilization of the Rhodococcus cells | [362] [363] | 2009 2011 |
| 49 | Agarose + gelation-slowing additives | Aqueous medium | −5 to −20 °C/24 h | Preparation of the wide-pore agarose cryogels, their hydrophobization and the use as the bioaffinity adsorbents for the separation of microbial cells | [364] | 2009 |
| 50 | PVA + OPH-bearing microbial cells | Aqueous medium | −20 °C/24 h | Preparation of the immobilized biocatalyst for the decomposition of the organophosphate toxins | [365] | 2009 |
| 51 | PVA + guar gum | Aqueous medium | −5 to −30 °C/12 h | Preparation of the wide-pore PVA cryogels, their hydrophobization and use as the bioaffinity adsorbents for the selective attachment of microbial cells | [366] | 2010 |
| 52 | Chitosan + glutar aldehyde | Aqueous medium, pH 5 | −20 °C/24 h | Preparation of the wide-pore chitosan-based cryogel and its use for the sorption of the heavy metal ions | [367,368] | 2011 |
| 53 | Sodium alginate | Aqueous medium | −20 °C/12 h | Preparation of the Ca-alginate-based wide-pore cryostructurate, its chemical modification followed by the gelatin grafting and the subsequent use as scaffold for culturing and cryopreservation of stem cells | [369,370] [371] [372] | 2011 2013 2014 |
| 54 | Water-soluble biomedical polymers + hydrogenated pyrido(4,3-b)indole | Aqueous medium | −40 °C/1–24 h | Method for the preparation of the drug-containing cryostructurates for the treatment of neurodegenerative diseases | [373] | 2011 |
| 55a b | Starch + lacto- and bifidobacterial cells | Aqueous media | −10 to −196 °C/0.1–6 h | Method for the preparation of the starch cryostructurates that contain probiotic bacteria; the studies of the formulation’s biological activity | [374] [375] | 2012 2015 |
| 56 | PVA + spores of various filamentous fungi | Aqueous medium | −20 °C/24 h | Preparation and studies of the immobilized biocatalysts capable of producing cellulases | [376] | 2013 |
| 57a b | PVA + Pimelobacter simplex cells | Aqueous medium | −15 °C/18 h | Immobilized biocatalyst for the microbial biotransformation of sterols | [377] [378] [379] | 2011 2013 2015 |
| 58 | Sodium alginate | Aqueous medium | −20 °C/12 h | Preparation of Ca-alginate-based wide-pore cryostructurate, its chemical modification followed by the gelatin grafting and the use as scaffold for culturing, cryopreservation and in vivo transplantation of fetal liver cells | [380] | 2015 |
| 59a b c d | Gelatin | DMSO | +3 to −22 °C/1–24 h | Elaboration of the method for the preparation of the gelatin-based cryostructurates under sterilizing conditions and the use of the resultant materials as wide-pore 3D scaffolds for culturing of human or animal cells | [381] [382] [383] [384] | 2015 2016 2018 2019 |
| 60 | Water-soluble polyelectrolyte + particles of the hypercrosslinked PS | Aqueous media | −10 to −40 °C/1–8 h | Method for the preparation of the composite wide-pore cryostructurates and their use as the sorbents for toxicants and pollutants | [385] | 2015 |
| 61 | Proteins of blood serum + urea + cystein | Aqueous media | −10 to −30 °C/6–36 h | Method for the preparation of the sponges based on proteins of blood serum, their loading with antibiotics and the use as antimicrobial biodegradable materials for the treatment of the infected wounds and burns | [386] | 2016 |
| 62 | PVA + series of amino acids | Aqueous media | −20 °C/24 h | Preparation of the PVA-cryogel-based carriers of biologically active substances | [387] | 2017 |
| 63a b | Sodium alginate | Water | −20 °C/12 h | Preparation of the Ca-alginate-based wide-pore cryostructurates, their loading with nanocomposites dioxidine-metal (Ar, Cu) and the use as potential antibacterial materials | [388] [389] | 2017 2018 |
| 64 | Proteins of blood serum + urea + cystein | Aqueous medium | −20 °C/24 h | Preparation and in vivo studies of the proteinaceous sponges for the use as biocompatible coatings to restore the full-thickness excision wounds | [390] | 2018 |
| 65 | Gelatin | DMSO | −12 °C/24 h | Preparation of the gelatin-based wide-pore cryostructurates, their loading with nanocomposites dioxidine/metal (Ar, Cu) and the use as potential antibacterial materials | [389,391] | 2018 |
| 66 | Chitosan acetate | Aqueous media | −10 °C/15 h | Preparation of the chitosan-based cryostructurates followed by their tanning with genipin and the use as the wide-pore 3D-scaffolds for the culture of fibroblasts | [392] | 2018 |
| 67 | Chitosan; alginate; gelatin | Aqueous media | −15 to −30 °C/12–24 h | Preparation of the wide-pore biopolymeric cryostructurates and their use as the carriers of the osteogenic bioregulator | [393] | 2019 |
| 68 | PVA + bacterial nanocellulose + OPH | Aqueous medium | −20 °C/12 h | Preparation of the composite PVA cryogels possessing enzymatic activity and their use as potential antimicrobial biomaterials | [394] | 2019 |
| 69 | Chitosan; alginate; gelatin | Aqueous media | −15 to −30 °C/12–24 h | Preparation of biopolymeric wide-pore cryostruucturates, their loading with nanocomposites dioxidine/metal (Ar, Cu) or gentamicin/metal (Ar, Cu) and the use as potential antibacterial materials | [395] | 2020 |
| 70 | PVA + antibiotics | Aqueous media | Temporary implants for treating of infected bone defects | [396] | 2020 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. https://doi.org/10.3390/gels6030029
Lozinsky VI. Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels. 2020; 6(3):29. https://doi.org/10.3390/gels6030029
Chicago/Turabian StyleLozinsky, Vladimir I. 2020. "Cryostructuring of Polymeric Systems. 55. Retrospective View on the More than 40 Years of Studies Performed in the A.N.Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems" Gels 6, no. 3: 29. https://doi.org/10.3390/gels6030029