Alkyl Chain Length Effects of Imidazolium Ionic Liquids on Electrical and Mechanical Performances of Polyacrylamide/Alginate-Based Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design Strategy and Characterization of the pAMAL-IMCx-Ca Hydrogels
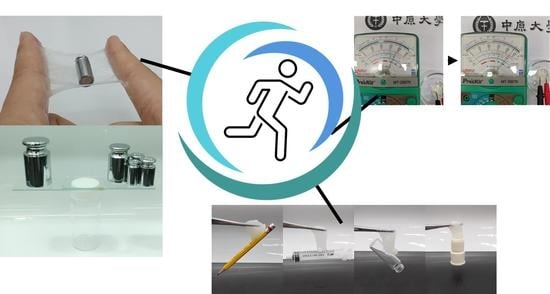
2.2. Mechanical Properties
2.3. Electrical Properties
2.4. Adhesive Capacity and Strain Sensor Testing
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Monomers
4.3. Preparation of pAMAL-Based Hydrogels
4.4. Measurements
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, B.; Huang, X. Seeking advanced thermal management for stretchable electronics. NPJ Flex. Electron. 2021, 5, 12. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, T.; Chen, H.; Wang, F.; Pu, Y.; Gao, C.; Li, S. Mini Review on Flexible and Wearable Electronics for Monitoring Human Health Information. Nanoscale Res. Lett. 2019, 14, 263. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, K.; Iniewski, K. Flexible, Wearable, and Stretchable Electronics, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Bao, Z.; Chen, X. Flexible and Stretchable Devices. Adv. Mater. 2016, 28, 4177–4179. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Wang, H.; Xia, Y.; Cui, D.; Shi, Y.; Dong, M.; Liu, C.; Ding, T.; Zhang, J.; Ma, Y.; et al. An overview of lead-free piezoelectric materials and devices. J. Mater. Chem. C 2018, 6, 12446–12467. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef]
- Lipomi, D.J. Stretchable Figures of Merit in Deformable Electronics. Adv. Mater. 2016, 28, 4180–4183. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.A.; Yeo, W.H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal Design Concepts for Stretchable Electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Shin, S.; Lee, S.; Song, J.; Kang, S.; Han, H.; Kim, S.; Kim, S.; Seo, J.; Kim, D.; et al. Highly Sensitive Multifilament Fiber Strain Sensors with Ultrabroad Sensing Range for Textile Electronics. ACS Nano 2018, 12, 4259–4268. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Ota, H.; Kiriya, D.; Takei, K.; Javey, A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Hwang, B.-U.; Kim, D.; Kim, B.-Y.; Lee, N.-E. Stretchable, Transparent, Ultrasensitive, and Patchable Strain Sensor for Human–Machine Interfaces Comprising a Nanohybrid of Carbon Nanotubes and Conductive Elastomers. ACS Nano 2015, 9, 6252–6261. [Google Scholar] [CrossRef]
- Shi, G.; Zhao, Z.; Pai, J.-H.; Lee, I.; Zhang, L.; Stevenson, C.; Ishara, K.; Zhang, R.; Zhu, H.; Ma, J. Highly Sensitive, Wearable, Durable Strain Sensors and Stretchable Conductors Using Graphene/Silicon Rubber Composites. Adv. Funct. Mater. 2016, 26, 7614–7625. [Google Scholar] [CrossRef]
- Liao, X.; Song, W.; Zhang, X.; Huang, H.; Wang, Y.; Zheng, Y. Directly printed wearable electronic sensing textiles towards human–machine interfaces. J. Mater. Chem. C 2018, 6, 12841–12848. [Google Scholar] [CrossRef]
- Xu, H.; Lv, Y.; Qiu, D.; Zhou, Y.; Zeng, H.; Chu, Y. An ultra-stretchable, highly sensitive and biocompatible capacitive strain sensor from an ionic nanocomposite for on-skin monitoring. Nanoscale 2019, 11, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, C.; Chen, X.; Zhao, Y.; Zhang, H.; Wen, N.; Fan, Z.; Pan, L. Facile Fabrication of High-Performance Pen Ink-Decorated Textile Strain Sensors for Human Motion Detection. ACS Appl. Mater. Interfaces 2020, 12, 19874–19881. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, R.; Sun, J.; Gao, L. A Stretchable and Highly Sensitive Graphene-Based Fiber for Sensing Tensile Strain, Bending, and Torsion. Adv. Mater. 2015, 27, 7365–7371. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Turan, M.; Clementson, C.P.; Sitti, M. Parallel Microcracks-based Ultrasensitive and Highly Stretchable Strain Sensors. ACS Appl. Mater. Interfaces 2016, 8, 5618–5626. [Google Scholar] [CrossRef]
- Zhai, W.; Xia, Q.; Zhou, K.; Yue, X.; Ren, M.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Multifunctional flexible carbon black/polydimethylsiloxane piezoresistive sensor with ultrahigh linear range, excellent durability and oil/water separation capability. Chem. Eng. J. 2019, 372, 373–382. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, Y.; Pan, J.; Chen, T.; Hu, Y.; Zheng, G.; Dai, K.; Liu, C.; Shen, C.; Sun, X.; et al. Design of Helically Double-Leveled Gaps for Stretchable Fiber Strain Sensor with Ultralow Detection Limit, Broad Sensing Range, and High Repeatability. ACS Appl. Mater. Interfaces 2019, 11, 4345–4352. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Gao, E.; Jian, M.; Xia, K.; Wang, Q.; Xu, Z.; Ren, T.; Zhang, Y. Carbonized Silk Fabric for Ultrastretchable, Highly Sensitive, and Wearable Strain Sensors. Adv. Mater. 2016, 28, 6640–6648. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhan, P.; Ren, M.; Zheng, G.; Dai, K.; Mi, L.; Liu, C.; Shen, C. Significant Stretchability Enhancement of a Crack-Based Strain Sensor Combined with High Sensitivity and Superior Durability for Motion Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 7405–7414. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Y.; Qiu, Y. Advanced Flexible Skin-Like Pressure and Strain Sensors for Human Health Monitoring. Micromachines 2021, 12, 695. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, F.; Di, C.-A.; Zhu, D. Advances of flexible pressure sensors toward artificial intelligence and health care applications. Mater. Horizons 2015, 2, 140–156. [Google Scholar] [CrossRef]
- Barlian, A.A.; Park, W.T.; Mallon, J.R.; Rastegar, A.J.; Pruitt, B.L. Review: Semiconductor Piezoresistance for Microsystems. Proc. IEEE 2009, 97, 513–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Chen, J.; Wang, L.; Gao, G.; Zhou, Y.; Wang, R.; Xu, T.; Yin, J.; Fu, J. Flexible and wearable strain sensors based on tough and self-adhesive ion conducting hydrogels. J. Mater. Chem. B 2019, 7, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Lv, X.; Sun, S. A highly sensitive strain sensor based on a silica@ polyaniline core–shell particle reinforced hydrogel with excellent flexibility, stretchability, toughness and conductivity. Soft Matter 2021, 17, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lin, N.; He, Y.; Zuo, B. Self-Healing, Self-Adhesive Silk Fibroin Conductive Hydrogel as a Flexible Strain Sensor. ACS Appl. Mater. Interfaces 2021, 13, 40013–40031. [Google Scholar] [CrossRef]
- Darnell, M.C.; Sun, J.-Y.; Mehta, M.; Johnson, C.; Arany, P.R.; Suo, Z.; Mooney, D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J. Designed fabrication of super-stiff, anisotropic hybrid hydrogels via linear remodeling of polymer networks and subsequent crosslinking. J. Mater. Chem. B 2015, 3, 1479–1483. [Google Scholar] [CrossRef]
- Li, J.; Illeperuma, W.R.K.; Suo, Z.; Vlassak, J.J. Hybrid Hydrogels with Extremely High Stiffness and Toughness. ACS Macro Lett. 2014, 3, 520–523. [Google Scholar] [CrossRef]
- Wang, J.; Wei, J.; Su, S.; Qiu, J.; Wang, S. Ion-linked double-network hydrogel with high toughness and stiffness. J. Mater. Sci. 2015, 50, 5458–5465. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Liu, X.; Qin, Z.; Yang, Y.; Zang, J.; Zhao, X. Fatigue-resistant adhesion of hydrogels. Nat. Commun. 2020, 11, 1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Zhang, J.; Chen, X.; Zhang, J.; Chen, G.; Zhang, K.; Lin, J.; Guo, C.; Liu, J. Trigger-Detachable Hydrogel Adhesives for Bioelectronic Interfaces. Adv. Funct. Mater. 2021, 2106446. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, F.; Gao, G. Transparent and conductive amino acid-tackified hydrogels as wearable strain sensors. Chem. Eng. J. 2019, 375, 121915. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Camp, J.E. Recent advances in the synthesis of imidazoles. Org. Biomol. Chem. 2020, 18, 3950–3964. [Google Scholar] [CrossRef]
- Green, M.D.; Long, T.E. Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies. Polym. Rev. 2009, 49, 291–314. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, X.; Tian, J.; Xu, L.; Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid Interface Sci. 2022, 606, 192–203. [Google Scholar] [CrossRef]
- Yan, K.; Sun, Y.; Huang, X. Effect of the alkyl chain length of a hydrophobic ionic liquid (IL) as an oil phase on the phase behavior and the microstructure of H2O/IL/nonionic polyoxyethylene surfactant ternary systems. RSC Adv. 2014, 4, 32363–32370. [Google Scholar] [CrossRef]
- Cai, Y.; Qin, J.; Li, W.; Tyagi, A.; Liu, Z.; Hossain, M.D.; Chen, H.; Kim, J.-K.; Liu, H.; Zhuang, M.; et al. A stretchable, conformable, and biocompatible graphene strain sensor based on a structured hydrogel for clinical application. J. Mater. Chem. A 2019, 7, 27099–27109. [Google Scholar] [CrossRef]
- Yu, F.; Cui, T.; Yang, C.; Dai, X.; Ma, J. κ-Carrageenan/Sodium alginate double-network hydrogel with enhanced mechanical properties, anti-swelling, and adsorption capacity. Chemosphere 2019, 237, 124417. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Ma, F.-X.; Wang, K.; Wang, F.-B.; Xia, X.-H. Synthesis of a hydrophilic poly-L-lysine/graphene hybrid through multiple non-covalent interactions for biosensors. J. Mater. Chem. B 2013, 1, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kuang, N.; Chen, L.; Fan, Y.; Fu, F.; Li, J.; Zhang, J. Synthesis and property of alkyl dioxyethyl α-D-xyloside. J. Mol. Liq. 2020, 315, 113770. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.-N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Zhang, Y.; Tao, L.; Li, S.; Wei, Y. Synthesis of Multiresponsive and Dynamic Chitosan-Based Hydrogels for Controlled Release of Bioactive Molecules. Biomacromolecules 2011, 12, 2894–2901. [Google Scholar] [CrossRef]
- Matsuda, Y.; Okuda, K.; Tasaka, S. Interfacial phase of nylon 6 strongly adsorbed on alumina particles. Polym. J. 2020, 52, 1121–1127. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Chaturvedi, G.C.; Gosavi, R.K. Infrared spectra and configurations of alkylurea derivatives: Normal vibrations on N, N’-dimethyl- and tetramethylurea. J. Mol. Spectrosc. 1968, 28, 526–535. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Double Reversible Networks: Improvement of Self-Healing in Hybrid Materials via Combination of Diels–Alder Cross-Linking and Hydrogen Bonds. Macromolecules 2018, 51, 6099–6110. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, J.; Yang, X.; Fan, D.; Cao, J.; Luo, Y.; Zhang, X. Naturally Derived Wearable Strain Sensors with Enhanced Mechanical Properties and High Sensitivity. ACS Appl. Mater. Interfaces 2020, 12, 22163–22169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Y.; Mohamed, A.; Ji, Q.; Jia, H. High strength and flexible aramid nanofiber conductive hydrogels for wearable strain sensors. J. Mater. Chem. C 2021, 9, 575–583. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Schubert, D.W. Highly Sensitive Ultrathin Flexible Thermoplastic Polyurethane/Carbon Black Fibrous Film Strain Sensor with Adjustable Scaffold Networks. Nano-Micro Lett. 2021, 13, 64. [Google Scholar] [CrossRef]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: Applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Duan, L.; Guan, S.; Gao, G.; Cheng, Y.; Ren, X.; Wang, Y. The effect of hydrophobic alkyl chain length on the mechanical properties of latex particle hydrogels. RSC Adv. 2017, 7, 44673–44679. [Google Scholar] [CrossRef] [Green Version]
- Hajighasem, A.; Kabiri, K. Cationic highly alcohol-swellable gels: Synthesis and characterization. J. Polym. Res. 2013, 20, 218. [Google Scholar] [CrossRef]
- Vats, K.; Benoit, D.S. Dynamic manipulation of hydrogels to control cell behavior: A review. Tissue Eng. Part B Rev. 2013, 19, 455–469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, M.; Han, X.; Fan, Z.; Zhang, H.; Li, Q. High-strength and highly electrically conductive hydrogels for wearable strain sensor. Chem. Phys. Lett. 2021, 769, 138437. [Google Scholar] [CrossRef]
- Souri, H.; Bhattacharyya, D. Wearable strain sensors based on electrically conductive natural fiber yarns. Mater. Des. 2018, 154, 217–227. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Yu, Z.; Chou, K.-C. Electrical conductivity of CaCl2–KCl–NaCl system at 1080 K. Thermochim. Acta 2012, 543, 107–112. [Google Scholar] [CrossRef]
- Heaney, M.B. Electrical Conductivity and Resistivity. In Electrical Measurement, Signal Processing, and Displays; Webster, J.G., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 7–11. [Google Scholar]
- Zhang, X.; Chen, J.; He, J.; Bai, Y.; Zeng, H. Mussel-inspired adhesive and conductive hydrogel with tunable mechanical properties for wearable strain sensors. J. Colloid Interface Sci. 2021, 585, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Pan, S.; Wu, L.; Tan, L.; Chen, D.; Huang, S.; Zhang, Y.; He, P. A self-adhesive wearable strain sensor based on a highly stretchable, tough, self-healing and ultra-sensitive ionic hydrogel. J. Mater. Chem. C 2020, 8, 17349–17364. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Duan, L.; Gao, G. Ultra-stretchable wearable strain sensors based on skin-inspired adhesive, tough and conductive hydrogels. Chem. Eng. J. 2019, 365, 10–19. [Google Scholar] [CrossRef]
- Bon, S.B.; Chiesa, I.; Esposti, M.D.; Morselli, D.; Fabbri, P.; De Maria, C.; Morabito, A.; Coletta, R.; Calamai, M.; Pavone, F.S.; et al. Carbon Nanotubes/Regenerated Silk Composite as a Three-Dimensional Printable Bio-Adhesive Ink with Self-Powering Properties. ACS Appl. Mater. Interfaces 2021, 13, 21007–21017. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Xu, Y.-J.; Rao, W.-H.; Luo, X.; Chen, L.; Wang, Y.-Z. Epoxidized soybean oil cured with tannic acid for fully bio-based epoxy resin. RSC Adv. 2018, 8, 26948–26958. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Lucero, D.; Castillo-Acosta, S.; Martínez-Palou, R. Glycerol Carbonate Synthesis Using Poly(1-alkyl-3-vinylimidazolium) Imidazolates as Catalysts. ChemistrySelect 2020, 5, 13694–13702. [Google Scholar] [CrossRef]
- Rahman, M.S.; Islam, M.M.; Islam, M.S.; Zaman, A.; Ahmed, T.; Biswas, S.; Sharmeen, S.; Rashid, T.U.; Rahman, M.M. Morphological Characterization of Hydrogels. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 819–863. [Google Scholar]
- Wang, H.-J.; Chu, Y.-Z.; Chen, C.-K.; Liao, Y.-S.; Yeh, M.-Y. Preparation of conductive self-healing hydrogels via an interpenetrating polymer network method. RSC Adv. 2021, 11, 6620–6627. [Google Scholar] [CrossRef]








| Hydrogels | Tensile Strength (kPa) | Elongation at Break (%) | Young’s Modulus (kPa) | Rs (Ω) | Resistivity (kΩ·cm) | Conductivity (μS/cm) |
|---|---|---|---|---|---|---|
| pAMAL | 0.64 | 871 | 0.17 | 42.0 | 5.3 | 190.5 |
| pAMAL-Ca | 1.28 | 491 | 0.24 | 24.1 | 3.0 | 332.0 |
| pAMAL-IMC2-Ca | 0.13 | 537 | 0.09 | 120.2 | 15.0 | 66.6 |
| pAMAL-IMC4-Ca | 0.39 | 1201 | 0.04 | 8.3 | 1.0 | 969.7 |
| pAMAL-IMC6-Ca | 8.25 | 868 | 1.48 | 11.2 | 1.4 | 714.3 |
| pAMAL-IMC8-Ca | 0.57 | 665 | 0.11 | 97.5 | 12.2 | 82.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-K.; Chen, P.-W.; Wang, H.-J.; Yeh, M.-Y. Alkyl Chain Length Effects of Imidazolium Ionic Liquids on Electrical and Mechanical Performances of Polyacrylamide/Alginate-Based Hydrogels. Gels 2021, 7, 164. https://doi.org/10.3390/gels7040164
Chen C-K, Chen P-W, Wang H-J, Yeh M-Y. Alkyl Chain Length Effects of Imidazolium Ionic Liquids on Electrical and Mechanical Performances of Polyacrylamide/Alginate-Based Hydrogels. Gels. 2021; 7(4):164. https://doi.org/10.3390/gels7040164
Chicago/Turabian StyleChen, Chen-Kang, Po-Wen Chen, Huan-Jung Wang, and Mei-Yu Yeh. 2021. "Alkyl Chain Length Effects of Imidazolium Ionic Liquids on Electrical and Mechanical Performances of Polyacrylamide/Alginate-Based Hydrogels" Gels 7, no. 4: 164. https://doi.org/10.3390/gels7040164