Ecological, Social, and Other Environmental Determinants of Dengue Vector Abundance in Urban and Rural Areas of Northeastern Thailand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Study Design and Sample Size
2.3. Entomological Survey
2.4. Mosquito Handling
2.5. Household and Demographic Survey
2.6. Knowledge, Attitudes and Practices Survey
2.7. Climate Data
2.8. Data Management and Statistical Analysis
3. Results
3.1. Entomological Collections and Indices
3.2. Container Characteristics and Breeding of Dengue Vectors
3.3. Knowledge, Attitudes, and Practices on Climate Change
3.4. Knowledge, Attitudes, and Practices on Dengue
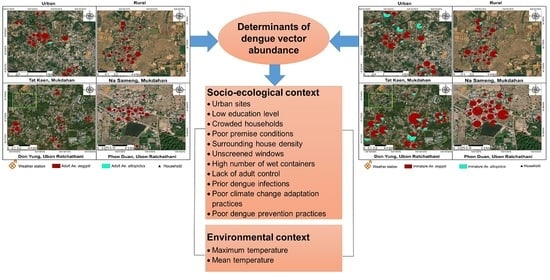
3.5. Ecological and Social Determinants of the Abundance of Adult Female and Immature Ae. aegypti
3.6. Climatic Determinants of the Abundance of Adult Females and Immature Ae. aegypti
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J. The economic burden of dengue. Am. J. Trop. Med. Hyg. 2012, 86, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhimal, M.; Gautam, I.; Joshi, H.D.; O′Hara, R.B.; Ahrens, B.; Kuch, U. Risk Factors for the Presence of Chikungunya and Dengue Vectors (Aedes aegypti and Aedes albopictus), Their Altitudinal Distribution and Climatic Determinants of Their Abundance in Central Nepal. PLoS Negl. Trop. Dis. 2015, 9, e0003545. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.K.; Dhar-Chowdhury, P.; Haque, C.E.; Al-Amin, H.M.; Goswami, D.R.; Kafi, M.A.H.; Drebot, M.A.; Lindsay, L.R.; Ahsan, G.U.; Brooks, W.A. Risk factors for the presence of dengue vector mosquitoes, and determinants of their prevalence and larval site selection in Dhaka, Bangladesh. PLoS ONE 2018, 13, e0199457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Murray, N.E.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Chareonsook, O.; Foy, H.M.; Teeraratkul, A.; Silarug, N. Changing epidemiology of dengue hemorrhagic fever in Thailand. Epidemiol. Infect. 1999, 122, 161–166. [Google Scholar] [CrossRef]
- Waewwab, P.; Sungvornyothin, S.; Potiwat, R.; Okanurak, K. Impact of dengue-preventive behaviors on Aedes immature production in Bang Kachao, Samut Prakan Province, Thailand: A cross-sectional study. BMC Public Health 2020, 20, 905. [Google Scholar] [CrossRef]
- Polwiang, S. The time series seasonal patterns of dengue fever and associated weather variables in Bangkok (2003–2017). BMC Infect. Dis. 2020, 20, 208. [Google Scholar] [CrossRef] [Green Version]
- Kosoltanapiwat, N.; Tongshoob, J.; Singkhaimuk, P.; Nitatsukprasert, C.; Davidson, S.A.; Ponlawat, A.J.P. Entomological Surveillance for Zika and Dengue Virus in Aedes Mosquitoes: Implications for Vector Control in Thailand. Pathogens 2020, 9, 442. [Google Scholar] [CrossRef]
- Department of Disease Control, Thailand. Weekly Disease Forecast No. 231_Dengue. Available online: https://ddc.moph.go.th/uploads/files_en/28120191219084236.pdf (accessed on 1 January 2021).
- Messina, J.P.; Brady, O.; Scott, T.W.; Zou, C.; Pigott, D.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef] [Green Version]
- WHO. Global Strategy for Dengue Prevention and Control 2012–2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/75303/9789241504034_eng.pdf;jsessionid=8BD7C5C13E1CC23A760ADD649728C11C?sequence=1 (accessed on 16 July 2020).
- MOPH Thailand. Weekly Disease Forecast No. 263_Dengue. Available online: https://ddc.moph.go.th/uploads/files_en/31120200619074616.pdf (accessed on 27 August 2020).
- Chareonviriyaphap, T.; Akratanakul, P.; Nettanomsak, S.; Huntamai, S. Larval habitats and distribution patterns of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Thailand. Southeast Asian J. Trop. Med. Public Health 2003, 34, 529–535. [Google Scholar]
- Kittayapong, P.; Strickman, D. Distribution of container-inhabiting Aedes larvae (Diptera: Culicidae) at a dengue focus in Thailand. J. Med. Entomol. 1993, 30, 601–606. [Google Scholar] [CrossRef]
- Koyadun, S.; Butraporn, P.; Kittayapong, P. Ecologic and Sociodemographic Risk Determinants for Dengue Transmission in Urban Areas in Thailand. Interdiscip. Perspect. Infect. Dis. 2012. [Google Scholar] [CrossRef]
- Vannavong, N.; Seidu, R.; Stenström, T.-A.; Dada, N.; Overgaard, H. Effects of socio-demographic characteristics and household water management on Aedes aegypti production in suburban and rural villages in Laos and Thailand. Parasites Vectors 2017, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Ndenga, B.A.; Mutuku, F.M.; Ngugi, H.N.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Vulule, J.; Mukoko, D.; Kitron, U.; LaBeaud, A.D. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE 2017, 12, e0189971. [Google Scholar] [CrossRef] [Green Version]
- Kuno, G.J. Review of the factors modulating dengue transmission. Epidemiol. Rev. 1995, 17, 321–335. [Google Scholar] [CrossRef]
- Descloux, E.; Mangeas, M.; Menkes, C.E.; Lengaigne, M.; Leroy, A.; Tehei, T.; Guillaumot, L.; Teurlai, M.; Gourinat, A.-C.; Benzler, J.; et al. Climate-Based Models for Understanding and Forecasting Dengue Epidemics. PLoS Negl. Trop. Dis. 2012, 6, e1470. [Google Scholar] [CrossRef] [Green Version]
- Regis, L.N.; Acioli, R.V.; Silveira, J.C., Jr.; de Melo-Santos, M.A.V.; da Cunha, M.C.S.; Souza, F.; Batista, C.A.V.; Barbosa, R.M.R.; de Oliveira, C.M.F.; Ayres, C.F. Characterization of the spatial and temporal dynamics of the dengue vector population established in urban areas of Fernando de Noronha, a Brazilian oceanic island. Acta Trop. 2014, 137, 80–87. [Google Scholar] [CrossRef]
- Misslin, R.; Vaguet, Y.; Vaguet, A.; Daudé, E. Estimating air temperature using MODIS surface temperature images for assessing Aedes aegypti thermal niche in Bangkok, Thailand. Environ. Monit. Assess. 2018, 190, 537. [Google Scholar] [CrossRef]
- Phanitchat, T.; Apiwathnasorn, C.; Sumroiphon, S.; Samung, Y.; Naksathit, A.; Thawornkuno, C.; Sungvornyothin, S. The influence of temperature on the developmental rate and survival of Aedes albopictus in Thailand. Southeast Asian J. Trop. Med. Public Health 2017, 48, 799–808. [Google Scholar]
- Brady, O.J.; Johansson, M.A.; Guerra, C.A.; Bhatt, S.; Golding, N.; Pigott, D.M.; Delatte, H.; Grech, M.G.; Leisnham, P.T.; Maciel-De-Freitas, R.; et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasites Vectors 2013, 6, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.M.; Lin, C.D.; Iamsirithaworn, S.; Scott, T.W. The Complex Relationship between Weather and Dengue Virus Transmission in Thailand. Am. J. Trop. Med. Hyg. 2013, 89, 1066–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, R.E.M.; Ya-Umphan, P.; Phumala-Morales, N.; Komalamisra, N.; Dujardin, J.-P. Climate associated size and shape changes in Aedes aegypti (Diptera: Culicidae) populations from Thailand. Infect. Genet. Evol. 2010, 10, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Nagao, Y.; Thavara, U.; Chitnumsup, P.; Tawatsin, A.; Chansang, C.; Campbell-Lendrum, D. Climatic and social risk factors for Aedes infestation in rural Thailand. Trop. Med. Int. Health 2003, 8, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Strickman, D.; Kittayapong, P.J. Dengue and its vectors in Thailand: Introduction to the study and seasonal distribution of Aedes larvae. Am. J. Trop. Med. Hyg. 2002, 67, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Arunachalam, N.; Tana, S.; Espino, F.; Kittayapong, P.; Abeyewickreme, W.; Wai, K.T.; Tyagi, B.K.; Kroeger, A.; Sommerfeld, J.; Petzold, M. Eco-bio-social determinants of dengue vector breeding: A multicountry study in urban and periurban Asia. Bull. World Health Organ. 2010, 88, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Da Silveira, L.T.C.; Tura, B.; Santos, M.J. Systematic review of dengue vaccine efficacy. BMC Infect. Dis. 2019, 19, 750. [Google Scholar] [CrossRef]
- Rosa, B.R.; da Cunha, A.J.L.A.; de Andrade Medronho, R.J.B. Efficacy, immunogenicity and safety of a recombinant tetravalent dengue vaccine (CYD-TDV) in children aged 2–17 years: Systematic review and meta-analysis. BMJ Open 2019, 9, e019368. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Karamehic-Muratovic, A.; Baghbanzadeh, M.; Amrin, M.; Zafar, S.; Rahman, N.N.; Shirina, S.U.; Haque, U. Climate change and dengue fever knowledge, attitudes and practices in Bangladesh: A social media–based cross-sectional survey. Trans. R. Soc. Trop. Med. Hyg. 2020, 115, 85–93. [Google Scholar] [CrossRef]
- Rahman, S.; Overgaard, H.J.; Pientong, C.; Mayxay, M.; Ekalaksananan, T.; Aromseree, S.; Phanthanawiboon, S.; Zafar, S.; Shipin, O.; Paul, R.E.; et al. Knowledge, attitudes, and practices on climate change and dengue in Lao People′s Democratic Republic and Thailand. Environ. Res. 2021, 193, 110509. [Google Scholar] [CrossRef]
- Rueda, L.M. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue Virus Transmission. Zootaxa 2004, 589, 1–60. [Google Scholar] [CrossRef]
- Knox, T.B.; Yen, N.T.; Nam, V.S.; Gatton, M.L.; Kay, B.H.; Ryan, P.A. Critical evaluation of quantitative sampling methods for Aedes aegypti (Diptera: Culicidae) immatures in water storage containers in Vietnam. J. Med. Entomol. 2007, 44, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Focks, D.A. A Review of Entomological Sampling Methods and Indicators for Dengue Vectors; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Bangs, M.J.; Focks, D.A. Abridged Pupa Identification Key to the Common Container-Breeding Mosquitoes in Urban Southeast Asia. J. Am. Mosq. Control Assoc. 2006, 22, 565–572. [Google Scholar] [CrossRef]
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Available online: http://www.ncbi.nlm.nih.gov/books/NBK143157 (accessed on 2 January 2021).
- Melki, I.S.; Beydoun, H.; Khogali, M.; Tamim, H.; Yunis, K. Household crowding index: A correlate of socioeconomic status and inter-pregnancy spacing in an urban setting. J. Epidemiol. Community Health 2004, 58, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.; Tipple, A. Economics of multihabitation: Housing conditions, household occupancy and household structure under rent control, inflation, and nonmarketability of ownership rights. World Dev. 1991, 19, 1705–1720. [Google Scholar] [CrossRef]
- Hustedt, J.; Doum, D.; Keo, V.; Ly, S.; Sam, B.; Chan, V.; Boyer, S.; Liverani, M.; Alexander, N.; Bradley, J.; et al. Ability of the Premise Condition Index to Identify Premises with Adult and Immature Aedes Mosquitoes in Kampong Cham, Cambodia. Am. J. Trop. Med. Hyg. 2020, 102, 1432–1439. [Google Scholar] [CrossRef]
- Andrighetti, M.T.M.; Galvani, K.C.; da Graça Macoris, M.d.L. Evaluation of premise condition index in the context of Aedes aegypti control in Marília, São Paulo, Brazil. Dengue Bull. 2009, 33, 167–175. [Google Scholar]
- Gardner, W.; Mulvey, E.P.; Shaw, E. Regression analyses of counts and rates: Poisson, overdispersed Poisson, and negative binomial models. Psychol. Bull. 1995, 118, 392. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M.B. Package ‘mass′. Cran r 2013, 538, 113–120. [Google Scholar]
- Wickham, H. ggplot2: Elegant graPhics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Fustec, B.; Phanitchat, T.; Hoq, M.I.; Aromseree, S.; Pientong, C.; Thaewnongiew, K.; Ekalaksananan, T.; Bangs, M.J.; Corbel, V.; Alexander, N.; et al. Complex relationships between Aedes vectors, socio-economics and dengue transmission—Lessons learned from a case-control study in northeastern Thailand. PLoS Negl. Trop. Dis. 2020, 14, e0008703. [Google Scholar] [CrossRef]
- Overgaard, H.J.; Olano, V.A.; Jaramillo, J.F.; Matiz, M.I.; Sarmiento, D.; Stenström, T.A.; Alexander, N. A cross-sectional survey of Aedes aegypti immature abundance in urban and rural household containers in central Colombia. Parasites Vectors 2017, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Wilke, A.B.B.; Chase, C.; Vasquez, C.; Carvajal, A.; Medina, J.; Petrie, W.D.; Beier, J.C. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci. Rep. 2019, 9, 15335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdousi, F.; Yoshimatsu, S.; Ma, E.; Sohel, N.; Wagatsuma, Y. Identification of Essential Containers for Aedes Larval Breeding to Control Dengue in Dhaka, Bangladesh. Trop. Med. Health 2015, 43, 253–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrera, R.; Amador, M.; Clark, G.G. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am. J. Trop. Med. Hyg. 2006, 74, 290–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seng, C.M.; Setha, T.; Nealon, J.; Socheat, D. Pupal sampling for Aedes aegypti (L.) surveillance and potential stratification of dengue high-risk areas in Cambodia. Trop. Med. Int. Health 2009, 14, 1233–1240. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, A.M.S.; Ryan, S.; Beltrán, E.; Mejía, R.; Silva, M.; Muñoz, Á. Dengue Vector Dynamics (Aedes aegypti) Influenced by Climate and Social Factors in Ecuador: Implications for Targeted Control. PLoS ONE 2013, 8, e78263. [Google Scholar] [CrossRef]
- Saifur, R.G.M.; Dieng, H.; Abu Hassan, A.; Salmah, R.C.; Satho, T.; Miake, F.; Hamdan, A. Changing Domesticity of Aedes aegypti in Northern Peninsular Malaysia: Reproductive Consequences and Potential Epidemiological Implications. PLoS ONE 2012, 7, e30919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadee, D.D.; Huntley, S.; Focks, D.A.; Chen, A.A. Aedes aegypti in Jamaica, West Indies: Container productivity profiles to inform control strategies. Trop. Med. Int. Health 2009, 14, 220–227. [Google Scholar] [CrossRef]
- Bureau, P.A.S. Dengue and Dengue Hemorrhagic Fever in the Americas: Guidelines for Prevention and Control; Pan American Health Organization: Washington, DC, USA, 1994. [Google Scholar]
- Sanchez, L.; Vanlerberghe, V.; Alfonso, L.; Marquetti, M.D.C.; Guzman, M.G.; Bisset, J.; Van Der Stuyft, P. Aedes aegypti Larval Indices and Risk for Dengue Epidemics. Emerg. Infect. Dis. 2006, 12, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Wijayanti, S.P.M.; Porphyre, T.; Chase-Topping, M.; Rainey, S.M.; McFarlane, M.; Schnettler, E.; Biek, R.; Kohl, A. The Importance of Socio-Economic Versus Environmental Risk Factors for Reported Dengue Cases in Java, Indonesia. PLoS Negl. Trop. Dis. 2016, 10, e0004964. [Google Scholar] [CrossRef]
- Udayanga, L.; Gunathilaka, N.; Iqbal, M.C.M.; Lakmal, K.; Amarasinghe, U.S.; Abeyewickreme, W. Comprehensive evaluation of demographic, socio-economic and other associated risk factors affecting the occurrence of dengue incidence among Colombo and Kandy Districts of Sri Lanka: A cross-sectional study. Parasites Vectors 2018, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Telle, O.; Vaguet, A.; Yadav, N.; Lefebvre, B.; Daudé, E.; Paul, R.E.; Cebeillac, A.; Nagpal, B.J. The spread of dengue in an endemic urban milieu—The case of Delhi, India. PloS ONE 2016, 11, e0146539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the Relationship between Vector Indices and Dengue Transmission: A Systematic Review of the Evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef] [PubMed]
- Juarez, J.; Garcia-Luna, S.; Medeiros, M.; Dickinson, K.; Borucki, M.; Frank, M.; Badillo-Vargas, I.; Chaves, L.; Hamer, G. The Eco-Bio-Social Factors That Modulate Aedes aegypti Abundance in South Texas Border Communities. Insects 2021, 12, 183. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Dye, C.; Etard, J.-F.; Smith, T.; Charlwood, J.D.; Garnett, G.P.; Hagan, P.; Hii, J.L.K.; Ndhlovu, P.D.; Quinnell, R.J.; et al. Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proc. Natl. Acad. Sci. USA 1997, 94, 338–342. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, Y.; Suwonkerd, W.; Chawprom, S.; Prajakwong, S.; Takagi, M. Different Spatial Distribution of Aedes Aegypti and Aedes Albopictus along an Urban–Rural Gradient and the Relating Environmental Factors Examined in Three Villages in Northern Thailand. J. Am. Mosq. Control Assoc. 2006, 22, 222–228. [Google Scholar] [CrossRef]
- Francisco, E.; Carlosmoisa, H.; Rafael, C.J. Factors that modify the larval indices of Aedes aegypti in Colima, Mexico. Pan Am. J. Public Health 2001, 10, 6–12. [Google Scholar]
- Nogueira, L.A.; Madeira, N.G.; Gushi, L.T.; Ribolla, P.E.M.; Miranda, J.E. Application of an Alternative Aedes Species (Diptera: Culicidae) Surveillance Method in Botucatu City, São Paulo, Brazil. Am. J. Trop. Med. Hyg. 2005, 73, 309–311. [Google Scholar] [CrossRef] [Green Version]
- Ohtomo, H.; Akao, N. Effect of global warming on infectious diseases. Nihon Rinsho. Jpn. J. Clin. Med. 2007, 65 (Suppl. 3), 653–658. [Google Scholar]
- Watts, N.; Amann, M.; Ayeb-Karlsson, S.; Belesova, K.; Bouley, T.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; Chambers, J.; et al. The Lancet Countdown on health and climate change: From 25 years of inaction to a global transformation for public health. Lancet 2018, 391, 581–630. [Google Scholar] [CrossRef]
- Kovats, R.S.; Campbell-Lendrum, D.H.; McMichel, A.J.; Woodward, A.; Cox, J.S.H. Early effects of climate change: Do they include changes in vector-borne disease? Philos. Trans. R. Soc. B Biol. Sci. 2001, 356, 1057–1068. [Google Scholar] [CrossRef]
- Confalonieri, U.; Menne, B.; Akhtar, R.; Ebi, K.L.; Hauengue, M.; Kovats, R.S.; Revich, B.; Woodward, A. Human Health Climate Change 2007: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Woodward, A.; Smith, K.R.; Campbell-Lendrum, D.; Chadee, D.D.; Honda, Y.; Liu, Q.; Olwoch, J.; Revich, B.; Sauerborn, R.; Chafe, Z.; et al. Climate change and health: On the latest IPCC report. Lancet 2014, 383, 1185–1189. [Google Scholar] [CrossRef]
- Fan, J.; Lin, H.; Wang, C.; Bai, L.; Yang, S.; Chu, C.; Yang, W.; Liu, Q. Identifying the high-risk areas and associated meteorological factors of dengue transmission in Guangdong Province, China from 2005 to 2011. Epidemiol. Infect. 2014, 142, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Phanitchat, T.; Zhao, B.; Haque, U.; Pientong, C.; Ekalaksananan, T.; Aromseree, S.; Thaewnongiew, K.; Fustec, B.; Bangs, M.J.; Alexander, N. Spatial and temporal patterns of dengue incidence in northeastern Thailand 2006–2016. BMC Infect. Dis. 2019, 19, 743. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.H.; Doan, H.T.M.; Phan, T.T.; Minh, N.N.T. Ecological factors associated with dengue fever in a central highlands Province, Vietnam. BMC Infect. Dis. 2011, 11, 172. [Google Scholar] [CrossRef]
- Siraj, A.S.; Oidtman, R.J.; Huber, J.; Kraemer, M.U.G.; Brady, O.J.; Johansson, M.A.; Perkins, T.A. Temperature modulates dengue virus epidemic growth rates through its effects on reproduction numbers and generation intervals. PLoS Negl. Trop. Dis. 2017, 11, e0005797. [Google Scholar] [CrossRef] [Green Version]
- Alto, B.W.; Juliano, S.A. Temperature effects on the dynamics of Aedes albopictus (Diptera: Culicidae) populations in the laboratory. J. Med. Entomol. 2001, 38, 548–556. [Google Scholar] [CrossRef] [Green Version]
- Morin, C.W.; Comrie, A.C.; Ernst, K. Climate and Dengue Transmission: Evidence and Implications. Environ. Health Perspect. 2013, 121, 1264–1272. [Google Scholar] [CrossRef]
- Rohani, A.; Wong, Y.C.; Zamre, I.; Lee, H.L.; Zurainee, M.N. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.). Southeast Asian J. Trop. Med. Public Health 2009, 40, 942–950. [Google Scholar]
- Halstead, S.B. Dengue virus–mosquito interactions. Annu. Rev. Entomol. 2008, 53, 273–291. [Google Scholar] [CrossRef] [Green Version]
- Rueda, L.; Patel, K.; Axtell, R.; Stinner, R. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 1990, 27, 892–898. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Canziani, O.F.; Leary, N.A.; Dokken, D.J.; White, K.S. Climate Change 2001: Impacts, Adaptation, and Vulnerability: Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001; Volume 2. [Google Scholar]
- Lambrechts, L.; Paaijmans, K.P.; Fansiri, T.; Carrington, L.B.; Kramer, L.D.; Thomas, M.B.; Scott, T.W. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl. Acad. Sci. USA 2011, 108, 7460–7465. [Google Scholar] [CrossRef] [Green Version]
- Hales, S.; de Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef]
- Halide, H.; Ridd, P. A predictive model for Dengue Hemorrhagic Fever epidemics. Int. J. Environ. Health Res. 2008, 18, 253–265. [Google Scholar] [CrossRef]
- Campbell, K.M.; Haldeman, K.; Lehnig, C.; Munayco, C.V.; Halsey, E.S.; Laguna-Torres, V.A.; Yagui, M.; Morrison, A.C.; Lin, C.-D.; Scott, T.W. Weather Regulates Location, Timing, and Intensity of Dengue Virus Transmission between Humans and Mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003957. [Google Scholar] [CrossRef]
- Mitovski, T.; Folkins, I.; Von Salzen, K.; Sigmond, M. Temperature, relative humidity, and divergence response to high rainfall events in the tropics: Observations and models. J. Clim. 2010, 23, 3613–3625. [Google Scholar] [CrossRef]
- Tun-Lin, W.; Lenhart, A.; Nam, V.S.; Rebollar-Téllez, E.; Morrison, A.; Barbazan, P.; Cote, M.; Midega, J.; Sanchez, F.; Manrique-Saide, P. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: A multi-country non-inferiority cluster randomized trial. Trop. Med. Int. Health 2009, 14, 1143–1153. [Google Scholar] [CrossRef] [Green Version]
- Kearney, M.; Porter, W.P.; Williams, C.; Ritchie, S.; Hoffmann, A.A. Integrating biophysical models and evolutionary theory to predict climatic impacts on species′ ranges: The dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 2009, 23, 528–538. [Google Scholar] [CrossRef]
- Gautam, I.; Aradhana, K.; Tuladhar, R.; Pandey, B.D.; Tamrakar, A.S.; Byanju, R.; Dhimal, M.; Aryal, K.; Kuch, U. Container preference of the Asian tiger mosquito (Aedes albopictus) in Kathmandu and Lalitpur districts of Nepal. J. Nat. Hist. Mus. 2012, 26, 181–193. [Google Scholar] [CrossRef]
- Patz, J.A.; Martens, W.J.; Focks, D.A.; Jetten, T.H. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ. Health Perspect. 1998, 106, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Banu, S.; Hu, W.; Hurst, C.; Tong, S. Dengue transmission in the Asia-Pacific region: Impact of climate change and socio-environmental factors. Trop. Med. Int. Health 2011, 16, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.C.; Currie, B.J.; Lindsay, M.D.; MacKenzie, J.S.; Ritchie, S.A.; Whelan, P.I. Dengue and climate change in Australia: Predictions for the future should incorporate knowledge from the past. Med. J. Aust. 2009, 190, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Huang, F.; Wang, J.J.; Zhang, S.S.; Su, Y.P.; Tang, L.H. Geographical, meteorological and vectorial factors related to malaria re-emergence in Huang-Huai River of central China. Malar. J. 2010, 9, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Index | Variables | Description | Classification Score |
|---|---|---|---|
| Premise condition index (PCI) | House condition | Good (well-maintained, e.g., newly painted or new house) | 1 |
| Intermediate (moderately well-maintained house) | 2 | ||
| Bad (not well-maintained house, e.g., paint peeling, broken items visible, and dilapidated old house) | 3 | ||
| Yard condition | Good (tidy yard) | 1 | |
| Intermediate (moderately tidy yard) | 2 | ||
| Bad (untidy yard) | 3 | ||
| Shade condition | Not shaded (very little or no shade) | 1 | |
| Intermediate (some shade: >25% but <50%) | 2 | ||
| Shady (plenty of shade: >50%) | 3 | ||
| Water supply and storage | Piped water | 1 | |
| Ground water/well water supply | 2 | ||
| Rainwater and/or open water source: river/stream/lake/mountain water/river water | 3 | ||
| Household crowding index (HCI) | Co-residents | Monthly number of co-residents per household | - |
| Number of rooms | Number of rooms per household | - | |
| Socio-economic status (SES) | House roof material | Ceramic/Wood/Metal | - |
| House walls material | Plastered/cement/bricks/wood | - | |
| Ownership of durable assets | television/VCD/refrigerator/washing machine/mobile/smartphone/computer/oven/microwave/airconditioner/car/pickup/motorcycle | - | |
| Ownership of toilet facility | Yes/no | - | |
| Toilet/bathroom floor material | Tiles/cement/earth | - | |
| Ownership of flush toilet/squat toilet | Yes/no | - |
| Species/Stage | Total Number (%) | Monthly Range, n | Monthly Mean ± SD | |||
|---|---|---|---|---|---|---|
| Urban | Rural | Urban | Rural | Urban | Rural | |
| Adult Ae. aegypti | ||||||
| Female | 551 (18.8) | 515 (21.8) | 6–110 | 23–81 | 45.9 ± 28.2 | 42.9 ± 19.5 |
| Male | 823 (28.2) | 769 (32.7) | 11–166 | 34–153 | 64.0 ± 47.3 | 68.5 ± 36.4 |
| Adult Ae. albopictus | ||||||
| Female | 36 (1.3) | 11 (0.4) | 0–17 | 0–3 | 3.0 ± 4.6 | 0.9 ± 0.9 |
| Male | 16 (0.6) | 12 (0.5) | 0–5 | 0–4 | 1.3 ± 1.6 | 1.0 ± 1.1 |
| Culex spp. | 1302 (44.6) | 677 (28.8) | 63–149 | 40–83 | 108.5 ± 26.5 | 56.4 ± 13.6 |
| Other species | 191 (6.5) | 370 (15.8) | 6–27 | 6–54 | 15.9 ± 5.9 | 30.8 ± 19 |
| Total adult mosquitoes | 2919 (100) | 2354 (100) | 0–166 | 0–153 | 39.8 ± 45.8 | 31.6 ± 27.9 |
| Immature Ae. aegypti | ||||||
| Larvae | 647 (42.7) | 525 (48.8) | 33–75 | 23–65 | 53.9 ± 14.4 | 43.7 ± 11.3 |
| Pupae | 543 (35.8) | 495 (46.0) | 16–112 | 10–148 | 16.4 ± 17.1 | 3.0 ± 3.8 |
| Immature Ae. Albopictus | ||||||
| Larvae | 197 (12.9) | 37 (3.4) | 0–50 | 0–14 | 45.2 ± 31.7 | 41.2 ± 39.3 |
| Pupae | 131 (8.6) | 19 (1.8) | 0–57 | 0–10 | 10.9 ± 16.3 | 1.5 ± 2.7 |
| Total immature mosquitoes | 1518 (100) | 1076 (100) | 0–112 | 0–148 | 33.4 ± 32.2 | 22.4 ± 28.8 |
| Larvae | Pupae | Total | ||||
|---|---|---|---|---|---|---|
| Container Characteristics | Description | Urban | Rural | Urban | Rural | |
| Shape of container | Square (Cemented tank, flower vase/pots) | 27 (7) | 66 (24) | 7 (6) | 21 (26) | 121 (14) |
| Round (jar, bucket, etc.) | 292 (79) | 198 (71) | 99 (79) | 54 (66) | 643 (75) | |
| Other (tree holes, bamboo, ant traps, solid waste, etc.) | 51 (14) | 15 (5) | 19 (15) | 7 (8) | 92 (11) | |
| Size of container | Small (<50 cm) | 161 (44) | 97 (35) | 56 (45) | 31 (38) | 345 (40) |
| Medium (50–100 cm) | 200 (54) | 172 (61) | 66 (53) | 46 (56) | 484 (57) | |
| Large (>150 cm) | 9 (2) | 10 (4) | 3 (2) | 5 (6) | 27 (3) | |
| Container Cover | Good | 12 (3) | 3 (1) | 2 (2) | 2 (2) | 19 (2) |
| Poorly fitted | 34 (9) | 15 (5) | 14 (11) | 7 (9) | 70 (8) | |
| None | 324 (88) | 261 (94) | 109 (87) | 73 (89) | 767 (90) | |
| Location | Indoor | 120 (32) | 129 (46) | 37 (30) | 42 (51) | 328 (38) |
| Outdoor | 250 (68) | 150 (54) | 88 (70) | 40 (49) | 528 (62) | |
| In toilet or not | In toilet | 108 (29) | 165 (59) | 35 (28) | 42 (51) | 350 (41) |
| Not in toilet | 262 (71) | 114 (41) | 90 (72) | 40 (49) | 506 (59) | |
| Larval control types | Abate | 20 (5) | 12 (4) | 5 (4) | 4 (5) | 41 (5) |
| Larval control washed in last week | 28 (8) | 42 (15) | 8 (6) | 7 (9) | 85 (10) | |
| No larvae control | 322 (87) | 225 (81) | 112 (90) | 71 (86) | 730 (85) | |
| Female Adults | Immatures | ||||
|---|---|---|---|---|---|
| Variables | n (%) | IRR (95% CI) | p-Value | IRR (95% CI) | p-Value |
| Sites types | |||||
| Urban | 64 (50) | 1.55 (1.21–1.98) | 0.000 | 1.47 (1.01–2.17) | 0.042 |
| Rural | 64 (50) | 1 | 1 | ||
| Education level | |||||
| <=Primary | 89 (69.5) | 1.39 (1.07–1.79) | 0.011 | 1.49 (1.02–2.18) | 0.032 |
| >Primary | 39 (30.5) | 1 | 1 | ||
| Socio-economic status | |||||
| Poor | 36 (28.1) | 0.94 (0.72–1.23) | 0.693 | 2.15 (1.39–3.32) | 0.001 |
| Intermediate | 52 (40.6) | 1.03 (0.81–1.3) | 0.801 | 1.63 (1.1–2.43) | 0.015 |
| Wealthy | 40 (31.3) | 1 | 1 | ||
| Household crowding index (HCI) | |||||
| 3 (Crowded) | 31 (24.2) | 1.76 (1.27–2.43) | 0.001 | 0.75 (0.46–1.23) | 0.263 |
| 2 (Medium crowded) | 65 (50.8) | 1.58 (1.22–2.05) | 0.000 | 0.50 (0.34–0.74) | 0.001 |
| 1 (Not crowded) | 32 (25.0) | 1 | 1 | ||
| Premise condition index (PCI) | |||||
| 9–10 (High) | 41 (32.0) | 1.97 (1.49–2.61) | 0.000 | 1.54 (1–2.37) | 0.043 |
| 7–8 (Medium) | 46 (36.0) | 1.20 (0.91–1.59) | 0.179 | 1.07 (0.72–1.59) | 0.730 |
| 5–6 (Low) | 41 (32.0) | 1 | 1 | ||
| House density (houses per km2) | |||||
| 100–200 | 8 (6.3) | 1.29 (0.83–1.99) | 0.246 | 0.67 (0.34–1.34) | 0.267 |
| 201–500 | 38 (29.7) | 1.37 (1.05–1.8) | 0.021 | 0.84 (0.55–1.29) | 0.433 |
| 501–1000 | 47 (36.7) | 1.19 (0.92–1.55) | 0.167 | 1.49 (1.02–2.18) | 0.038 |
| >1000 | 35 (27.3) | 1 | 1 | ||
| House type | |||||
| Single house, one family, two floors | 64 (50) | 1.009 (0.82–1.22) | 0.931 | 1.27 (0.91–1.77) | 0.146 |
| Single house, one family, one floor | 64 (50) | 1 | 1 | ||
| Roof materials type | |||||
| Metal (e.g., corrugated iron) | 97 (75.8) | 1.14 (0.83–1.58) | 0.400 | 0.89 (0.54–1.46) | 0.659 |
| Wood | 17 (13.3) | 1.03 (0.65–1.61) | 0.889 | 0.57 (0.28–1.16) | 0.125 |
| Ceramic | 14 (10.9) | 1 | 1 | ||
| Wall type | |||||
| Wood | 12 (9.4) | 1.11 (0.79–1.57) | 0.530 | 1.68 (1.01–2.81) | 0.045 |
| Cement/bricks | 33 (25.8) | 0.95 (0.76–1.18) | 0.652 | 1.48 (1.05–2.09) | 0.022 |
| Plastered | 83 (64.8) | 1 | 1 | ||
| Location of bathroom/toilet | |||||
| Indoors | 91 (71.1) | 1.46 (1.14–1.89) | 0.003 | 1.01 (0.68–1.5) | 0.925 |
| Outdoors | 37 (28.9) | 1 | 1 | ||
| Bathroom floor type | |||||
| Cement | 54 (42.2) | 1.15 (0.91–1.47) | 0.221 | 0.73 (0.5–1.07) | 0.109 |
| Tiles | 74 (57.8) | 1 | 1 | ||
| Eaves status | |||||
| Closed | 79 (61.7) | 1.14 (0.92–1.42) | 0.201 | 0.94 (0.67–1.3) | 0.715 |
| Opened | 49 (38.3) | 1 | 1 | ||
| Windows | |||||
| Unscreened | 107 (83.6) | 1.41 (1.04–1.92) | 0.025 | 1.65 (1.05–2.6) | 0.029 |
| Screened | 21 (16.4) | 1 | 1 | ||
| Number of wet container | |||||
| >50 | 109 (85.2) | 1.33 (1.01–1.75) | 0.037 | 1.41 (0.92–2.16) | 0.112 |
| <50 | 19 (14.8) | 1 | 1 | ||
| Use any kind of larvae control | |||||
| No | 93 (72.7) | 0.93 (0.75–1.14) | 0.503 | 1.20 (0.84–1.7) | 0.300 |
| Yes | 35 (27.3) | 1 | 1 | ||
| Use any kind of adult control | |||||
| No | 72 (56.2) | 1.24 (1.01–1.55) | 0.045 | 1.13 (0.79–1.61) | 0.496 |
| Yes | 56 (43.8) | 1 | 1 | ||
| Self-reported dengue infections | |||||
| Yes | 12 (9.4) | 1.68 (1.22–2.32) | 0.001 | 0.79 (0.46–1.35) | 0.398 |
| No | 116 (90.6) | 1 | 1 | ||
| Climate change knowledge | |||||
| Poor | 97 (75.8) | 0.87 (0.61–1.24) | 0.451 | 1.97 (1.19–3.25) | 0.008 |
| Good | 31 (24.2) | 1 | 1 | ||
| Climate change attitude | |||||
| Poor | 49 (38.3) | 0.97 (0.79–1.19) | 0.801 | 0.71 (0.52–0.99) | 0.043 |
| Good | 79 (61.7) | 1 | 1 | ||
| Climate change practice | |||||
| Poor | 105 (82.0) | 1.52 (1.07–2.16) | 0.017 | 1.84 (1.13–2.99) | 0.014 |
| Good | 23 (18.0) | 1 | 1 | ||
| Dengue knowledge | |||||
| Poor | 73 (57.0) | 0.91 (0.7–1.17) | 0.491 | 1.18 (0.8–1.75) | 0.391 |
| Good | 55 (43.0) | 1 | 1 | ||
| Dengue attitude | |||||
| Poor | 40 (31.3) | 1.24 (1.01–1.53) | 0.035 | 0.75 (0.54–1.04) | 0.092 |
| Good | 88 (68.8) | 1 | 1 | ||
| Dengue practice | |||||
| Poor | 94 (73.4) | 1.43 (1.03–1.99) | 0.029 | 1.93 (1.17–3.18) | 0.009 |
| Good | 34 (26.6) | 1 | 1 | ||
| Model fit | |||||
| Omnibus test | 131.2 | 0.000 | 957.2 | 0.000 | |
| AIC | 719.2 | 935.3 | |||
| BIC | 810.5 | 1026.6 |
| Meteorological Variables | Range (n) | Mean ± SD | ||
|---|---|---|---|---|
| Urban | Rural | Urban | Rural | |
| Mean temperature (°C) | 23.2–32.6 | 22.7–31.0 | 28.0 ± 2.3 | 27.2 ± 2.2 |
| Minimum temperature (°C) | 13.2–26.7 | 9.6–23.7 | 21.6 ± 3.7 | 19.1 ± 3.9 |
| Maximum temperature (°C) | 31.0–41.8 | 33.0–41.8 | 35.5 ± 3.0 | 37.0 ± 2.6 |
| Relative humidity (%) | 58.0–88.1 | 60.0–88.7 | 73.5 ± 8.1 | 76.3 ± 8.2 |
| Total rainfall (mm) | 0–803.3 | 0–1229.4 | 102.9 ± 173.3 | 166.5 ± 254.7 |
Publisher′s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.S.; Ekalaksananan, T.; Zafar, S.; Poolphol, P.; Shipin, O.; Haque, U.; Paul, R.; Rocklöv, J.; Pientong, C.; Overgaard, H.J. Ecological, Social, and Other Environmental Determinants of Dengue Vector Abundance in Urban and Rural Areas of Northeastern Thailand. Int. J. Environ. Res. Public Health 2021, 18, 5971. https://doi.org/10.3390/ijerph18115971
Rahman MS, Ekalaksananan T, Zafar S, Poolphol P, Shipin O, Haque U, Paul R, Rocklöv J, Pientong C, Overgaard HJ. Ecological, Social, and Other Environmental Determinants of Dengue Vector Abundance in Urban and Rural Areas of Northeastern Thailand. International Journal of Environmental Research and Public Health. 2021; 18(11):5971. https://doi.org/10.3390/ijerph18115971
Chicago/Turabian StyleRahman, Md. Siddikur, Tipaya Ekalaksananan, Sumaira Zafar, Petchaboon Poolphol, Oleg Shipin, Ubydul Haque, Richard Paul, Joacim Rocklöv, Chamsai Pientong, and Hans J. Overgaard. 2021. "Ecological, Social, and Other Environmental Determinants of Dengue Vector Abundance in Urban and Rural Areas of Northeastern Thailand" International Journal of Environmental Research and Public Health 18, no. 11: 5971. https://doi.org/10.3390/ijerph18115971