Mechanisms of ATP Release by Inflammatory Cells
Abstract
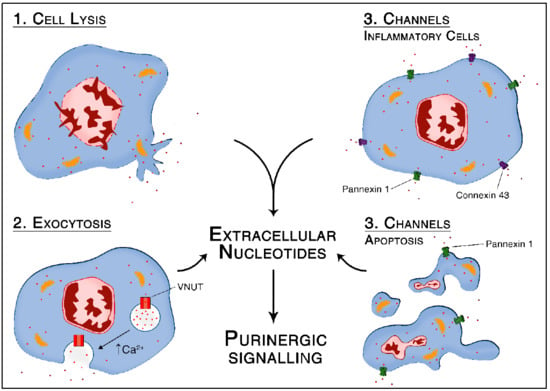
:1. Introduction
2. Non-Specific ATP Release from Necrotic Cells
3. Active ATP Release via Vesicular Exocytosis
3.1. Vesicular ATP Release in Response to Inflammation Mediates Chronic Inflammatory Pain
3.2. Vesicular Exocytosis-Mediated ATP Release in Response to Infection
3.3. Vesicular Exocytosis Mediated ATP Release in Response to Hypoxia
4. Active ATP Release via Pore-Forming Channels
4.1. Connexins and Pannexins—Structural and Functional Differences
4.2. Structure and General Functions of Connexin Hemichannels
4.3. Connexin-Mediated ATP Release in Response to Pathogen Associated Molecular Patterns
4.4. Connexin-Mediated ATP Release in Atherosclerosis
4.5. Hypoxia Regulates Connexin-Dependent ATP Release
4.6. Regulation of Pannexin Channels
4.7. Pannexin-1 Mediated ATP Release from Apoptotic Cells
4.8. Functional Consequences of Pannexin-1 Mediated ATP Release during Inflammation
4.8.1. Neutrophils
4.8.2. Monocytes/Macrophages
4.8.3. Dendritic Cells
4.8.4. Pannexin-Mediated Intravascular Crosstalk
5. Conclusions
Conflicts of Interest
Abbreviations
| ATP | Adenosine 5′-triphosphate |
| BMMф | Bone marrow macrophages |
| cGMP-PKG | Cyclic guanosine monophosphate-protein kinase G |
| DCs | Dendritic cells |
| fMLP | N-formyl Met-Leu-Phe |
| LPS | Lipopolysaccharides |
| MCP-1 | Macrophages chemoattractant protein 1 |
| Nlrp3 | NOD-like receptor family, pyrin domain containing 3 |
| NEM | N-ethylmaleimide |
| NFκB | Nuclear factor κ B |
| PAR-1 | protease activated receptor 1 |
| PMф | Peritoneal macrophages |
| PMN | Polymorphonuclear neutrophil |
| SNARE | Soluble N-Ethylmaleimide-sensitive factor attachment protein receptor |
| TLR | Toll-like receptor |
| UTP | Uridine 5′-triphosphate |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VNUT | Vesicular nucleotide transporter |
References
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A. Extracellular ATP in the immune system: More than just a “danger signal”. Sci. Signal 2009, 2, pe6. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Pulskens, W.P.; Sadler, J.J.; Butter, L.M.; Teske, G.J.; Ulland, T.K.; Eisenbarth, S.C.; Florquin, S.; Flavell, R.A.; Leemans, J.C.; et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 2009, 106, 20388–20393. [Google Scholar] [CrossRef] [PubMed]
- Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Vuerich, M. Purinergic signaling in the immune system. Auton. Neurosci. 2015, 191, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Sumi, Y.; Woehrle, T.; Chen, Y.; Bao, Y.; Li, X.; Yao, Y.; Inoue, Y.; Tanaka, H.; Junger, W.G. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock 2014, 42, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kondo, Y.; Bao, Y.; Staudenmaier, L.; Lee, A.; Zhang, J.; Ledderose, C.; Junger, W.G. Systemic Adenosine Triphosphate Impairs Neutrophil Chemotaxis and Host Defense in Sepsis. Crit. Care Med. 2017, 45, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Cauwels, A.; Rogge, E.; Vandendriessche, B.; Shiva, S.; Brouckaert, P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014, 5, e1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandran, K.S. Beginnings of a good apoptotic meal: The find-me and eat-me signaling pathways. Immunity 2011, 35, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; McMahon, H.T. Mechanisms of membrane fusion: Disparate players and common principles. Nat. Rev. Mol. Cell Biol. 2008, 9, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, Y.; Hiasa, M.; Sakamoto, S.; Omote, H.; Nomura, M. Vesicular nucleotide transporter (VNUT): Appearance of an actress on the stage of purinergic signaling. Purinergic Signal 2017, 13, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Echigo, N.; Juge, N.; Miyaji, T.; Otsuka, M.; Omote, H.; Yamamoto, A.; Moriyama, Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 5683–5686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaji, T.; Sawada, K.; Omote, H.; Moriyama, Y. Divalent cation transport by vesicular nucleotide transporter. J. Biol. Chem. 2011, 286, 42881–42887. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Morizawa, Y.; Komatsu, R.; Shibata, K.; Shinozaki, Y.; Kasai, H.; Moriishi, K.; Moriyama, Y.; Koizumi, S. Microglia release ATP by exocytosis. Glia 2013, 61, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C.; Rothman, J.E. Membrane fusion: Grappling with SNARE and SM proteins. Science 2009, 323, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hiasa, M.; Ichikawa, R.; Hasuzawa, N.; Kadowaki, A.; Iwatsuki, K.; Shima, K.; Endo, Y.; Kitahara, Y.; Inoue, T.; et al. Identification of a vesicular ATP release inhibitor for the treatment of neuropathic and inflammatory pain. Proc. Natl. Acad. Sci. USA 2017, 201704847. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.; Zetterqvist, A.V.; Wright, W.R.; Kirkby, N.S.; Mitchell, J.A.; Paul-Clark, M.J. Differential role of pannexin-1/ATP/P2X7 axis in IL-1β release by human monocytes. FASEB J. 2017, 31, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Sáez, P.J.; Vargas, P.; Shoji, K.F.; Harcha, P.A.; Lennon-Duménil, A.M.; Sáez, J.C. ATP promotes the fast migration of dendritic cells through the activity of pannexin 1 channels and P2X7 receptors. Sci. Signal 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, W.; Xu, X.; Xiong, Y.; Zhang, Y.; Zhang, H.; Sun, B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc. Natl. Acad. Sci. USA 2017, 114, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, H.; Wang, L.; Zhang, N.; Huang, H.; Xiong, Q.; Yan, Y.; Wu, N.; Ren, H.; Han, H.; et al. Virus-Triggered ATP Release Limits Viral Replication through Facilitating IFN-β Production in a P2X7-Dependent Manner. J. Immunol. 2017, 199, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.A.; McClain, J.L.; Watson, R.E.; Patel, B.A.; Gulbransen, B.D. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhang, G.; Zhang, X.; Tan, B.; Lv, Z.; Liu, M.; Ren, H.; Qian, M.; Du, B. TLR-Activated Gap Junction Channels Protect Mice against Bacterial Infection through Extracellular UDP Release. J. Immunol. 2016, 196, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Lim To, W.K.; Kumar, P.; Marshall, J.M. Hypoxia is an effective stimulus for vesicular release of ATP from human umbilical vein endothelial cells. Placenta 2015, 36, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Lohman, A.W.; Leskov, I.L.; Butcher, J.T.; Johnstone, S.R.; Stokes, T.A.; Begandt, D.; DeLalio, L.J.; Best, A.K.; Penuela, S.; Leitinger, N.; et al. Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation. Nat. Commun. 2015, 6, 7965. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bao, Y.; Zhang, J.; Woehrle, T.; Sumi, Y.; Ledderose, S.; Li, X.; Ledderose, C.; Junger, W.G. Inhibition of Neutrophils by Hypertonic Saline Involves Pannexin-1, CD39, CD73, and Other Ectonucleotidases. Shock 2015, 44, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Molica, F.; Morel, S.; Meens, M.J.; Denis, J.F.; Bradfield, P.F.; Penuela, S.; Zufferey, A.; Monyer, H.; Imhof, B.A.; Chanson, M.; et al. Functional role of a polymorphism in the Pannexin1 gene in collagen-induced platelet aggregation. Thromb. Haemost. 2015, 114, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Calder, B.W.; Matthew Rhett, J.; Bainbridge, H.; Fann, S.A.; Gourdie, R.G.; Yost, M.J. Inhibition of connexin 43 hemichannel-mediated ATP release attenuates early inflammation during the foreign body response. Tissue Eng. Part A 2015, 21, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Németh, Z.H.; Törő, G.; Idzko, M.; Zech, A.; Koscsó, B.; Spolarics, Z.; Antonioli, L.; Cseri, K.; Erdélyi, K.; et al. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015, 29, 3626–3637. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Teng, Y.; Tan, B.; Zhang, X.; Jiang, W.; Liu, M.; Du, B.; Qian, M. Toll-like receptor-triggered calcium mobilization protects mice against bacterial infection through extracellular ATP release. Infect. Immun. 2014, 82, 5076–5085. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Wright, J.R.; Vial, C.; Evans, R.J.; Mahaut-Smith, M.P. Amplification of human platelet activation by surface pannexin-1 channels. J. Thromb. Haemost. 2014, 12, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, H.; Tsukimoto, M.; Harada, H.; Moriyama, Y.; Kojima, S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS ONE 2013, 8, e59778. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling and disorders of the central nervous system. Nat. Rev. Drug Discov. 2008, 7, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Perretti, M.; McMahon, S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005, 6, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.P.; Vulchanova, L.; Hargreaves, K.M.; Elde, R.; McCleskey, E.W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature 1997, 387, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P.; Herrstedt, J.; Group, E.G.W. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2014, 25 (Suppl. 3), iii124–iii137. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, V.; Bidula, S.; Campwala, H.; Katikaneni, D.; Fountain, S.J. Constitutive lysosome exocytosis releases ATP and engages P2Y receptors in human monocytes. J. Cell Sci. 2012, 125 Pt 19, 4567–4575. [Google Scholar] [CrossRef] [PubMed]
- McLeish, K.R.; Dean, W.L.; Wellhausen, S.R.; Stelzer, G.T. Role of intracellular calcium in priming of human peripheral blood monocytes by bacterial lipopolysaccharide. Inflammation 1989, 13, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Gouwy, M.; Struyf, S.; Noppen, S.; Schutyser, E.; Springael, J.Y.; Parmentier, M.; Proost, P.; Van Damme, J. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol. Pharmacol. 2008, 74, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Badolato, R.; Johnston, J.A.; Wang, J.M.; McVicar, D.; Xu, L.L.; Oppenheim, J.J.; Kelvin, D.J. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J. Immunol. 1995, 155, 4004–4010. [Google Scholar] [PubMed]
- Hishikawa, T.; Cheung, J.Y.; Yelamarty, R.V.; Knutson, D.W. Calcium transients during Fc receptor-mediated and nonspecific phagocytosis by murine peritoneal macrophages. J. Cell Biol. 1991, 115, 59–66. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, J.P.; Pope, B.L. The involvement of protein kinase C, calcium, and 5-lipoxygenase in the production of tumor necrosis factor by a cloned interleukin-3 dependent cell line with natural cytotoxic activity. Int. J. Immunopharmacol. 1991, 13, 175–184. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Kuhlicke, J.; Frick, J.S.; Morote-Garcia, J.C.; Rosenberger, P.; Eltzschig, H.K. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE 2007, 2, e1364. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Ibla, J.C.; Furuta, G.T.; Leonard, M.O.; Jacobson, K.A.; Enjyoji, K.; Robson, S.C.; Colgan, S.P. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 2003, 198, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.F.; Eltzschig, H.K.; Ibla, J.C.; Van De Wiele, C.J.; Resta, R.; Morote-Garcia, J.C.; Colgan, S.P. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J. Exp. Med. 2004, 200, 1395–13405. [Google Scholar] [CrossRef] [PubMed]
- Spaans, F.; de Vos, P.; Bakker, W.W.; van Goor, H.; Faas, M.M. Danger signals from ATP and adenosine in pregnancy and preeclampsia. Hypertension 2014, 63, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Lohman, A.W.; Isakson, B.E. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett. 2014, 588, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin channels are not gap junction hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Scemes, E.; Spray, D.C.; Meda, P. Connexins, pannexins, innexins: Novel roles of “hemi-channels”. Pflugers Arch. 2009, 457, 1207–1226. [Google Scholar] [CrossRef] [PubMed]
- D’hondt, C.; Ponsaerts, R.; De Smedt, H.; Bultynck, G.; Himpens, B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays 2009, 31, 953–974. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Gehi, R.; Laird, D.W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta 2013, 1828, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Mazo, A.; Barberà-Cremades, M.; Pelegrín, P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim. Biophys. Acta 2013, 1828, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008, 28, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; De Bock, M.; Decrock, E.; Bol, M.; Gadicherla, A.; Vinken, M.; Rogiers, V.; Bukauskas, F.F.; Bultynck, G.; Leybaert, L. Paracrine signaling through plasma membrane hemichannels. Biochim. Biophys. Acta 2013, 1828, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Tran Van Nhieu, G.; Clair, C.; Bruzzone, R.; Mesnil, M.; Sansonetti, P.; Combettes, L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat. Cell Biol. 2003, 5, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Schock, S.C.; Leblanc, D.; Hakim, A.M.; Thompson, C.S. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem. Biophys. Res. Commun. 2008, 368, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Schalper, K.A.; Shoji, K.F.; Orellana, J.A.; Bennett, M.V.; Sáez, J.C. Possible involvement of different connexin43 domains in plasma membrane permeabilization induced by ischemia-reperfusion. J. Membr. Biol. 2007, 218, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Lopez, W.; Ramachandran, J.; Alsamarah, A.; Luo, Y.; Harris, A.L.; Contreras, J.E. Mechanism of gating by calcium in connexin hemichannels. Proc. Natl. Acad. Sci. USA 2016, 113, E7986–E7995. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Wang, J.; Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006, 580, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Batra, N.; Riquelme, M.A.; Jiang, J.X. Biological role of connexin intercellular channels and hemichannels. Arch. Biochem. Biophys. 2012, 524, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.; Wong, E.; Hackos, D.H. Pannexin-1 is blocked by its C-terminus through a delocalized non-specific interaction surface. PLoS ONE 2014, 9, e99596. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; De Bock, M.; Decrock, E.; Bol, M.; Gadicherla, A.; Bultynck, G.; Leybaert, L. Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening. Neuropharmacology 2013, 75, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Iyyathurai, J.; Wang, N.; D’hondt, C.; Jiang, J.X.; Leybaert, L.; Bultynck, G. The SH3-binding domain of Cx43 participates in loop/tail interactions critical for Cx43-hemichannel activity. Cell. Mol. Life Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Leybaert, L.; Lampe, P.D.; Dhein, S.; Kwak, B.R.; Ferdinandy, P.; Beyer, E.C.; Laird, D.W.; Naus, C.C.; Green, C.R.; Schulz, R. Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications. Pharmacol. Rev. 2017, 69, 396–478. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 2007, 217, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Connexin43 phosphorylation: Structural changes and biological effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Reuss, L.; Altenberg, G.A. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J. Biol. Chem. 2004, 279, 20058–20066. [Google Scholar] [CrossRef] [PubMed]
- García, I.E.; Sánchez, H.A.; Martínez, A.D.; Retamal, M.A. Redox-mediated regulation of connexin proteins; focus on nitric oxide. Biochim. Biophys. Acta 2018, 1860, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Cortés, C.J.; Reuss, L.; Bennett, M.V.; Sáez, J.C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: Induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 4475–4480. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; De Vuyst, E.; Ponsaerts, R.; Boengler, K.; Palacios-Prado, N.; Wauman, J.; Lai, C.P.; De Bock, M.; Decrock, E.; Bol, M.; Vinken, M.; et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2013, 108, 309. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T.; Mager, A.; Küper, N.; Karcher, C.; Weissmüller, T.; Boengler, K.; Schulz, R.; Robson, S.C.; Colgan, S.P. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ. Res. 2006, 99, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Lang, S.; Lambert, P.A.; Martin, P.E. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. Biochem. J. 2010, 432, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Rhett, J.M.; Fann, S.A.; Yost, M.J. Purinergic signaling in early inflammatory events of the foreign body response: Modulating extracellular ATP as an enabling technology for engineered implants and tissues. Tissue Eng. Part B Rev. 2014, 20, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol. Rep. 2008, 60, 12–20. [Google Scholar] [PubMed]
- Ferrari, D.; Vitiello, L.; Idzko, M.; la Sala, A. Purinergic signaling in atherosclerosis. Trends Mol. Med. 2015, 21, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Wang, Q.; Wu, D.; Yu, M.; Zhang, S.; Li, L.; Tao, L.; Harris, A.L. Monocyte-endothelial adhesion is modulated by Cx43-stimulated ATP release from monocytes. Biochem. Biophys. Res. Commun. 2012, 420, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Lichtman, A.H. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Christen, T.; Roth, I.; Chadjichristos, C.E.; Derouette, J.P.; Foglia, B.F.; Chanson, M.; Goodenough, D.A.; Kwak, B.R. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat. Med. 2006, 12, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Faigle, M.; Seessle, J.; Zug, S.; El Kasmi, K.C.; Eltzschig, H.K. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE 2008, 3, e2801. [Google Scholar] [CrossRef] [PubMed]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Sandilos, J.K.; Chiu, Y.H.; Chekeni, F.B.; Armstrong, A.J.; Walk, S.F.; Ravichandran, K.S.; Bayliss, D.A. Pannexin 1, an ATP release channel, is activated by caspase cleavage of its pore-associated C-terminal autoinhibitory region. J. Biol. Chem. 2012, 287, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Jin, X.; Medina, C.B.; Leonhardt, S.A.; Kiessling, V.; Bennett, B.C.; Shu, S.; Tamm, L.K.; Yeager, M.; Ravichandran, K.S.; et al. A quantized mechanism for activation of pannexin channels. Nat. Commun. 2017, 8, 14324. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.R.; de Rivero Vaccari, J.P.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef] [PubMed]
- Gulbransen, B.D.; Bashashati, M.; Hirota, S.A.; Gui, X.; Roberts, J.A.; MacDonald, J.A.; Muruve, D.A.; McKay, D.M.; Beck, P.L.; Mawe, G.M.; et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 2012, 18, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Surprenant, A. The P2X(7) receptor-pannexin connection to dye uptake and IL-1β release. Purinergic Signal 2009, 5, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Locovei, S.; Roque, A.; Alberto, A.P.; Dahl, G.; Spray, D.C.; Scemes, E. P2X7 receptor-Pannexin1 complex: Pharmacology and signaling. Am. J. Physiol. Cell Physiol. 2008, 295, C752–C760. [Google Scholar] [CrossRef] [PubMed]
- Gödecke, S.; Roderigo, C.; Rose, C.R.; Rauch, B.H.; Gödecke, A.; Schrader, J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am. J. Physiol. Cell Physiol. 2012, 302, C915–C923. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.R.; Paramos-de-Carvalho, D.; Certal, M.; Costa, M.A.; Costa, C.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Sévigny, J.; Correia-de-Sá, P. Histamine induces ATP release from human subcutaneous fibroblasts, via pannexin-1 hemichannels, leading to Ca2+ mobilization and cell proliferation. J. Biol. Chem. 2013, 288, 27571–27583. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.R.; Paramos-de-Carvalho, D.; Certal, M.; Costa, C.; Magalhães-Cardoso, M.T.; Ferreirinha, F.; Costa, M.A.; Correia-de-Sá, P. Bradykinin-induced Ca2+ signaling in human subcutaneous fibroblasts involves ATP release via hemichannels leading to P2Y12 receptors activation. Cell Commun. Signal 2013, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A. Connexin and Pannexin hemichannels are regulated by redox potential. Front. Physiol. 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.K.J.; Swayne, L.A. P2X7 receptor cross-talk regulates ATP-induced pannexin 1 internalization. Biochem. J. 2017, 474, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Dahl, G. A permeant regulating its permeation pore: Inhibition of pannexin 1 channels by ATP. Am. J. Physiol. Cell Physiol. 2009, 296, C250–255. [Google Scholar] [CrossRef] [PubMed]
- Whyte-Fagundes, P.; Zoidl, G. Mechanisms of pannexin1 channel gating and regulation. Biochim. Biophys. Acta 2018, 1860, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Poornima, V.; Vallabhaneni, S.; Mukhopadhyay, M.; Bera, A.K. Nitric oxide inhibits the pannexin 1 channel through a cGMP-PKG dependent pathway. Nitric Oxide 2015, 47, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Ayna, G.; Krysko, D.V.; Kaczmarek, A.; Petrovski, G.; Vandenabeele, P.; Fésüs, L. ATP release from dying autophagic cells and their phagocytosis are crucial for inflammasome activation in macrophages. PLoS ONE 2012, 7, e40069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauber, K.; Blumenthal, S.G.; Waibel, M.; Wesselborg, S. Clearance of apoptotic cells: Getting rid of the corpses. Mol Cell 2004, 14, 277–287. [Google Scholar] [CrossRef]
- Qu, Y.; Misaghi, S.; Newton, K.; Gilmour, L.L.; Louie, S.; Cupp, J.E.; Dubyak, G.R.; Hackos, D.; Dixit, V.M. Pannexin-1 is required for ATP release during apoptosis but not for inflammasome activation. J. Immunol. 2011, 186, 6553–6561. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, Y.; Ledderose, C.; Li, L.; Junger, W.G. Pannexin 1 channels link chemoattractant receptor signaling to local excitation and global inhibition responses at the front and back of polarized neutrophils. J. Biol. Chem. 2013, 288, 22650–22657. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yao, Y.; Sumi, Y.; Li, A.; To, U.K.; Elkhal, A.; Inoue, Y.; Woehrle, T.; Zhang, Q.; Hauser, C.; et al. Purinergic signaling: A fundamental mechanism in neutrophil activation. Sci. Signal 2010, 3, ra45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Corriden, R.; Inoue, Y.; Yip, L.; Hashiguchi, N.; Zinkernagel, A.; Nizet, V.; Insel, P.A.; Junger, W.G. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006, 314, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Riteau, N.; Baron, L.; Villeret, B.; Guillou, N.; Savigny, F.; Ryffel, B.; Rassendren, F.; Le Bert, M.; Gombault, A.; Couillin, I. ATP release and purinergic signaling: A common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012, 3, e403. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1β release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Gombault, A.; Baron, L.; Couillin, I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front. Immunol. 2012, 3, 414. [Google Scholar] [CrossRef] [PubMed]
- Connors, B.W. Tales of a dirty drug: Carbenoxolone, gap junctions, and seizures. Epilepsy Curr. 2012, 12, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 2014, 33, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; de Andrade Mello, P.; Figliuolo, V.R.; de Avelar Almeida, T.F.; Santana, P.T.; Oliveira, S.D.S.; Silva, C.L.M.; Feldbrügge, L.; Csizmadia, E.; Minshall, R.D.; et al. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J. Hepatol. 2017, 67, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Tschopp, J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007, 14, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Zerr, M.; Hechler, B.; Freund, M.; Magnenat, S.; Lanois, I.; Cazenave, J.P.; Léon, C.; Gachet, C. Major contribution of the P2Y₁receptor in purinergic regulation of TNFα-induced vascular inflammation. Circulation 2011, 123, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Riegel, A.K.; Faigle, M.; Zug, S.; Rosenberger, P.; Robaye, B.; Boeynaems, J.M.; Idzko, M.; Eltzschig, H.K. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood 2011, 117, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Kronlage, M.; Song, J.; Sorokin, L.; Isfort, K.; Schwerdtle, T.; Leipziger, J.; Robaye, B.; Conley, P.B.; Kim, H.C.; Sargin, S.; et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal 2010, 3, ra55. [Google Scholar] [CrossRef] [PubMed]



| Selected Reading | Type | Cells | Mechanisms | |||
|---|---|---|---|---|---|---|
| First Author | Last Author | Journal | Year | |||
| Kato, Y. | Miyaji, T. | PNAS | 2017 | Exocytosis | Neurons, microglia, immune cells | Reduction of neuropathic and inflammatory pain by clodronate (inhibitor of exocytosis) in mice [21] |
| Parzych, K. | Paul-Clark, M.J. | FASEB | 2017 | Pannexin-1 | THP-1 cells | IL1 β secretion from monocytes upon TLR2 stimulation is dependent on pannexin-1/ATP/P2X7 axis [22] |
| Saez, P.J. | Saez, J.C. | SCI SIGNAL | 2017 | Pannexin-1 | Dendritic cells | Dendritic cells release ATP via PANX1 hemichannels in response to ATP-dependent P2X7 activation. Released ATP amplifies DCs activation in autocrine manner [23] |
| Wang, X. | Sun, B. | PNAS | 2017 | Connexin 43 | dHL-60 | Neutrophils release ATP via CX43 in response to LPS stimulation. MLCK is activated and phosphorylates MLC, leading to chemotaxis stoppage [24] |
| Zhang, C. | Du, B. | J IMMUNOL | 2017 | Exocytosis + pannexin-1 | RAW 264.7 cells/293 T cells | ATP is released by virus infected macrophages and protects cells-limiting virus replication-via P2X7 and increased IFNgamma production [25] |
| Brown, I.A. | Gulbransen, B.D. | CELL MOL GASTROENTEROL HEPATOL | 2016 | Connexin 43 | Enteric glia | Upon oxidative stress, enteric glia release ATP via CX43. This mechanism is potentiated by NO. ATP further activates P2X7 leading to neuron death [26] |
| Qin, J. | Du, B. | J IMMUNOL | 2016 | Connexin 43 | RAW 264.7 | TLRs induce increased CX43 expression in macrophages and UDP release. UDP interacts with P2Y6 receptor and induces MCP-1 release [27] |
| Lim To, W.K. | Marshall, J.M. | PLACENTA | 2015 | Exocytosis | Endothelial cells (HUVEC) | Hypoxia induces ATP release which leads to vasodilation via an increased synthesis of PGs and NO [28] |
| Lohman, A.W. | Isakson, B.E. | NAT COMMUN | 2015 | Pannexin-1 | Endothelial cells (HUVEC) | TNF α released upon inflammation induces ATP release from vascular endothelial cells via PANX1 [29] |
| Chen, Y. | Junger, W.G. | SHOCK | 2015 | Pannexin-1 | PMNs | Hypertonic saline reduces PMNs overactivation by inducing ATP release via PANX1 channels. ATP is degraded to adenosine that interacts with A2a receptors on PMNs [30] |
| Yang, D. | Núñez, G. | IMMUNITY | 2015 | Pannexin-1 | BMMφ | Intracellular LPS activated caspase-11 cleaves PANX1 which releases ATP. ATP further activates P2X7 receptors ending with pyroptosis [31] |
| Molica, F. | Kwak, B.R. | J THROMB HAEMOST | 2015 | Pannexin-1 | Platelets | Collagen induces ATP release from blood platelets and leads to platelet aggregation [32] |
| Calder, B.W. | Yost, M.J. | TISSUE ENG | 2015 | Connexin 43 | HMVEC | CX43 mediated ATP release in HMVEC was decreased upon treatment with a CX43 mimetic peptide (JM2) and FFAs [33] |
| Csóka, B. | Haskó, G. | FASEB | 2015 | Connexin 43 | - | ATP is released during sepsis and CX43 blocking leads to increased inflammatory cytokines and bacterial load [34] |
| Ren, H. | Qian, M. | INFECT IMMUN | 2014 | Exocytosis | Macrophages | ATP is released from macrophages through TLR activation upon stimulation with LPS and Pam3CSK4 [35] |
| Taylor, K.A. | Mahaut-Smith, M.P. | J THROMB HAEMOST | 2014 | Pannexin-1 | Platelets | Arterial shear rates induce ATP release via PANX1 in vitro, which ATP interacts with P2X1 and leads to platelet aggregation [36] |
| Imura, Y. | Koizumi, S. | GLIA | 2013 | Exocytosis | Microglia | Stimulation with ionomycin or LPS induces release of ATP from microglia by increasing VNUT-dependent exocytotic mechanisms [19] |
| Sakaki, H. | Kojima, S. | PLOS ONE | 2013 | Exocytosis | THP-1 monocytes | LPS induced ATP release leads to autocrine P2Y11 activation, M1 polarization and cytokines secretion [37] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. https://doi.org/10.3390/ijms19041222
Dosch M, Gerber J, Jebbawi F, Beldi G. Mechanisms of ATP Release by Inflammatory Cells. International Journal of Molecular Sciences. 2018; 19(4):1222. https://doi.org/10.3390/ijms19041222
Chicago/Turabian StyleDosch, Michel, Joël Gerber, Fadi Jebbawi, and Guido Beldi. 2018. "Mechanisms of ATP Release by Inflammatory Cells" International Journal of Molecular Sciences 19, no. 4: 1222. https://doi.org/10.3390/ijms19041222