Design of Antibacterial Agents: Alkyl Dihydroxybenzoates against Xanthomonas citri subsp. citri
Abstract
:1. Introduction
2. Results
2.1. Synthesis, Purification and Identification of Alkyl Dihydroxybenzoates
2.2. Partition Coefficient Study
2.3. Determination of MIC and MBC against Xanthomonas citri subsp. citri Assay
2.4. Cytotoxicity Assay
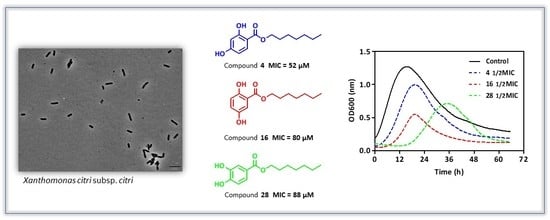
2.5. Growth Curve
2.6. Cellular Membrane Permeability of Xcc Using the Live/Dead Kit
2.7. Inhibition Assays of GTPase Activity
3. Discussion
4. Methods
4.1. Synthesis of Alkyl Dihydroxybenzoates
4.2. Partition Coefficient Determination Log Po/w by HPLC
4.3. Determination of MIC and MBC against Xanthomonas citri subsp. citri Assay
4.4. Determination of Cytotoxicity
4.5. Growth Curve
4.6. Cellular Membrane Permeability of Xcc Using the Live/Dead Kit
4.7. Inhibition Assays of GTPase Activity
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schaad, N.W.; Postnikova, E.; Lacy, G.H.; Sechler, A.; Agarkova, I.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.K. Reclassification of Xanthomonas campestris pv. citri (ex Hasse 1915) dye 1978 forms, A.; B/C/D, and E as, X. smithii subsp. citri (ex Hasse) sp. nov. nom. rev. comb. nov., X. fuscans subsp. aurantifolii (ex Gabriel 1989) sp. nov. nom. rev. comb. nov., and, X. alfalfae subsp. citrumelo (ex Riker and Jones) Gabriel et al., 1989 sp. nov.nom. rev. comb. nov.; X. campestris pv. malvacearum (ex smith 1901) dye1978 as, X. smithii subsp. smithii nov. comb. nov. nom. nov.; X. campestris pv. alfalfae (ex Riker and Jones, 1935) dye 1978 as, X. alfalfae subsp. alfalfa ex Riker et al., 1935) sp. nov. nom. rev.; and “var. fuscans” of, X. campestris pv. phaseoli (ex Smith, 1987) dye 1978 as, X. fuscans subsp. fuscans sp2005. nov. Syst. Appl. Microbiol. 2005, 28, 494–518. [Google Scholar] [PubMed]
- Schaad, N.W.; Postnikova, E.; Lacy, G.; Sechler, A.; Agarkova, I.V.; Stromberg, P.E.; Stromberg, V.K.; Vidaver, A.M. Emended classification of xanthomonad pathogens on citrus. Syst. Appl. Microbiol. 2006, 29, 690–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottwald, T.R.; Graham, J.H.; Schubert, T.S. Citrus canker: The pathogen and its impact. Plant Health Prog. 2002, 10, 32. [Google Scholar] [CrossRef]
- Dalla Pria, M.; Christiano, R.C.; Furtado, E.L.; Amorim, L.; Bergamin Filho, A. Effect of temperature and leaf wetness duration on infection of sweet oranges by Asiatic citrus canker. Plant Pathol. 2006, 55, 657–663. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.H.; Gottwald, T.R.; Cubero, J.; Achor, D.S. Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brunings, A.M.; Gabriel, D.W. Xanthomonas citri: Breaking the surface. Mol. Plant Pathol. 2003, 4, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.H.; Parker, P.E.; Gottwald, T.R. Effect of simulated winddriven rain on duration and distance of dispersal of Xanthomonas axonopodis pv. citri from canker-infected citrus trees. Plant Dis. 2005, 89, 71–80. [Google Scholar] [CrossRef]
- São Paulo (Estado). Resolução da Secretaria de Agricultura e Abastecimento (SAA) No 10; Diário Oficial do Estado de São Paulo: São Paulo, Brazil, 2017. [Google Scholar]
- Kuhara, S. Present epidemic status and control of the citrus canker disease (Xanthomonas citri (Hasse) Dowson) in Japan. Rev. Plant Prot. Res. 1978, 11, 132–142. [Google Scholar]
- Stall, R.E.; Miller, J.W.; Marco, G.M.; Canteros, B.I. Timing of sprays to control cancrosis of grapefruit in Argentina. Proc. Int. Soc. Citric. 1982, 1, 414–417. [Google Scholar]
- Leite, R.P., Jr.; Mohan, S.K. Integrated management of the citrus bacterial canker disease caused by Xanthomonas campestris pv. citri in the State of Paraná, Brazil. Crop Protect. 1990, 9, 3–7. [Google Scholar] [CrossRef]
- Behlau, F.; Amorim, L.; Belasque, J., Jr.; Bergamin Filho, A.; Leite, R.P., Jr.; Graham, J.H.; Gottwald, T.R. Annual and polyetic progression of citrus canker on trees protected with copper sprays. Plant Pathol. 2010, 59, 1031–1036. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.H. Varietal susceptibility to citrus canker: Observations from southern Brazil. Citrus Ind. 2001, 82, 15–17. [Google Scholar]
- Schubert, T.S.; Rizvi, S.A.; Sun, X.; Gottwald, T.R.; Graham, J.H.; Dixon, W.N. Meeting the challenge of eradication citrus canker in Florida—Again. Plant Dis. 2001, 85, 340–356. [Google Scholar] [CrossRef]
- Leite, R.P., Jr.; Mohan, S.K.; Pereira, A.L.; Campacci, C.A. Controle integrado de cancro cítrico—Efeito da resistência genética e da aplicação de bactericidas. Fitopatol. Brás. 1987, 12, 257–263. [Google Scholar]
- Stall, R.E.; Miller, J.W.; Marco, G.M.; Canteros, B.I. Population dynamics of Xanthomonas citri causing cancrosis of citrus in Argentina. Proc. Fla. State Hort. Soc. 1980, 93, 10–14. [Google Scholar]
- Behlau, F.; Scandelai, L.H.M.; da Silva, G.J., Jr.; Lanza, F.E. Soluble and insoluble copper formulations and metallic copper rate for control of citrus canker on sweet orange trees. Crop Prot. 2017, 94, 185–191. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Huguenot, D.; Jézéquel, K.; Lollier, M.; Lebeau, T. Bioremediation of copper-contaminated soils by bacteria. World J. Microbiol. Biotechnol. 2017, 33, 26. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, G.; de Melo, G.W.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Pii, Y.; Mimmo, T.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.; Preston, G.M. The impact of transition metals on bacterial plant disease. FEMS Microbiol. Rev. 2013, 37, 495–519. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; He, Z.; Ma, L.Q.; Stoffella, P.J. Accumulation and availability of copper in citrus grove soils as affected by fungicide application. J. Soil. Sediment. 2011, 11, 639–648. [Google Scholar] [CrossRef]
- Alva, A.K.; Graham, J.H.; Anderson, C.A. Soil pH and copper effects on young ‘Hamlin’ orange trees. Soil Sci. Soc. Am. J. 1995, 59, 481–487. [Google Scholar] [CrossRef]
- Behlau, F.; Canteros, B.I.; Minsavage, G.V.; Jones, J.B.; Graham, J.H. Molecular characterization of copper resistance genes from Xanthomonas citri subsp. citri and Xanthomonas alfafae subsp. citrumelonis. Appl. Environ. Microbiol. 2011, 77, 4089–4096. [Google Scholar] [CrossRef] [PubMed]
- Canteros, B.I. Copper resistance in Xanthomonas campestris pv. citri. In Proceedings of the 9th International Conference Centre for Advanced Study in Botany Plant Pathogenic Bacteria, Chennai, India, 21–23 December 1999; pp. 455–459. [Google Scholar]
- Silva, I.C.; Regasini, L.O.; Petronio, M.S.; Silva, D.H.; Bolzani, V.D.; Belasque, J.; Sacramento, L.V.; Ferreira, H. Antibacterial activity of alkyl gallates against Xanthomonas citri subsp. citri. J. Bacteriol. 2013, 195, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Król, E.; de Sousa Borges, A.; da Silva, I.; Polaquini, C.R.; Regasini, L.O.; Ferreira, H.; Scheffers, D.J. Antibacterial activity of alkyl gallates is a combination of direct targeting of FtsZ and permeabilization of bacterial membranes. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Savietto, A.; Polaquini, C.R.; Kopacz, M.; Scheffers, D.J.; Marques, B.C.; Regasini, L.O.; Ferreira, H. Antibacterial activity of monoacetylated alkyl gallates against Xanthomonas citri subsp. citri. Arch. Microbiol. 2018, 200, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Merkl, R.; Hradkova, I.; Filip, V.; Smi Drkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food 2010, 28, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Nihei, K.; Nihei, A.; Kubo, I. Rational Design of Antimicrobial Agents: Antifungal Activity of Alk(en)yl Dihydroxybenzoates and Dihydroxyphenyl Alkanoates. Biol. Med. Chem. Lett. 2003, 13, 3993–3996. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Antibacterial activities of phenolic benzaldehydes and benzoic acids against campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2003, 10, 1811–1821. [Google Scholar] [CrossRef]
- OECD. Test No. 117: Partition Coefficient (N-Octanol/Water), HPLC Method; OECD Publishing: Paris, France, 2004; ISBN 9789264069824. [Google Scholar]
- Chatterjee, T.; Chatterjee, B.K.; Majumdar, D.; Chakrabarti, P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim. Biophys. Acta 2015, 2, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Erickson, H.P. Rapid in vitro assmbly dynamics and subunit turnover of ftsz dementrated by fluorescence resonance energy tranfer. J. Biol. Chem. 2005, 280, 22549–22554. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Lutkenhaus, J. Guanine nucleotide-dependent assembly of FtsZ INTO filaments. J. Bacteriol. 1994, 176, 2754–2758. [Google Scholar] [CrossRef] [PubMed]
- de Boer, P.A.; Crossley, R.E.; Rothfield, L.I. Roles of minC and minD in the site-specific septation block mediated by the minCDE System of Escherichia coli. J. Bacteriol. 1992, 174, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, M.M.; Lorenzoni, A.S.; Polaquini, C.R.; Regasini, L.O.; Scheffers, D.J. Purification and characterization of FtsZ from the citrus canker pathogen Xanthomonas citri subsp. citri. Microbiol. Open 2018, 5, e00706. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.M.; Merfa, M.V.; Takita, M.A.; de Souza, A.A. Persistence in phytogenic bacteria: Do know enough? Front. Microniol. 2018, 9, 1–14. [Google Scholar]
- Gochez, A.M.; Huguet-Tapia, J.C.; Minsavage, G.V.; Shantaraj, D.; Jalan, N.; Strauß, A.; Lahaye, T.; Wang, N.; Canteros, B.I.; Jones, J.B.; Potnis, N. Pacbio sequencing of copper-tolerant Xanthomonas citri reveals presence of a chimeric plasmid structure and provides insights into reassortment and shuffling of transcription activator-like effectors among, X. citri strains. BMC Genom. 2018, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Tribot, N.; Boyer, C.; Terville, M.; Boyer, K.; Javegny, S.; Roux-Cuvelier, M.; Pruvost, O.; Moreau, A.; Chabirand, A.; et al. First report of copper-resistant Xanthomonas citri pv. citri pathotype a causing Asiatic citrus canker in Réunion, France. Plant Dis. 2017, 101, 503. [Google Scholar] [CrossRef]
- Yu, X.; Armstrong, C.M.; Zhou, M.; Duan, Y. Bismerthiazol Inhibits Xanthomonas citri subsp. citri Growth and Induces Differential Expression of Citrus Defense-Related Genes. Phytopathology 2016, 7, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, N. Foliar application of biofilm formation-inhibiting compounds enhances control of citrus canker caused by Xanthomonas citri subsp. citri. Phytopathology 2014, 104, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Filocamo, A.; La Camera, E.; Zummo, S.; Fera, M.T.; Mandalari, G. Antibacterial activities of almond skins on cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC Microbiol. 2013, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bisignano, C.; D’Arrigo, M.; Ginestra, G.; Arena, A.; Tomaino, A.; Wickham, M.S. Antimicrobial potential of polyphenols extracted from almond skins. Lett. Appl. Microbiol. 2010, 51, 83–89. [Google Scholar] [PubMed]
- Ayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556–568. [Google Scholar] [CrossRef]
- Kuete, V.; Nana, F.; Ngameni, B.; Mbaveng, A.T.; Keumedjio, F.; Ngadjui, B.T. Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovate (Moraceae). J. Ethnopharmacol. 2009, 124, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Yin, M.C. Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2009, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Hsu, C.; Yin, M. In vitro anti-helicobacter pylori activity of diallyl sulphides and protocatechuic acid. Phytother. Res. 2008, 22, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tsao, S.; Yin, M. In vitro antibacterial activity of roselle calyx and protocatechuic acid acids. Phytother. Res. 2005, 19, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, C.G. The Pratice of Medicinal Chemistry; London Academic Press: London, UK, 2008; Volume 3, pp. 273–287. [Google Scholar]
- Bahmani, A.; Saaidpour, S.; Rostami, A. A simple, robust and efficient computational method for n-octanol/water partition coefficients of substituted aromatic drugs. Sci. Rep. 2017, 7, 5760. [Google Scholar] [CrossRef] [PubMed]
- Medina-Alarcón, K.P.; Singulani, J.L.; Voltan, A.R.; Sardi, J.C.; Petrônio, M.S.; Santos, M.B.; Polaquini, C.R.; Regasini, L.O.; Bolzani, V.S.; da Silva, D.H.; et al. Alkyl Protocatechuate loaded nanostructured lipid systems as a treatment strategy for Paracoccidioides brasiliensis and Paracoc cidioides litizii. In Vitro Front. Microbiol. 2017, 8, 1048. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhaus, J.; Addinall, S.G. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 1997, 66, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Löwe, J.; Amos, L.A. Crystal structure of the bacterial cell-division protein FtsZ. Nature 1998, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- RayChaudhuri, D.; Park, J.T. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature 1992, 17, 359–392. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P.; Stoffler, D. Protofilaments and rings, two conformations of the tubulin family conserved from bacterial FtsZ to α/β and γ tubulin. J. Cell Biol. 1996, 135, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Margalit, D.N.; Romberg, L.; Mets, R.B.; Hebert, A.M.; Mitchison, T.J.; Kirschner, M.W.; RayChaudhuri, D. Targeting cell division: Small-molecule inhibitors of ftsz gtpase perturb cytokinetic ring assembly and induce bacterial lethality. Proc. Natl. Acad. Sci. USA 2004, 101, 11821–11826. [Google Scholar] [CrossRef] [PubMed]
- Zeraik, M.L.; Petrônio, M.S.; Coelho, D.; Regasini, L.O.; Silva, D.H.; da Fonseca, L.M.; Machado, S.A.; Bolzani, V.S.; Ximenes, V.F. Improvement of pro-oxidant capacity of protocatechuic acid by esterification. PLoS ONE 2014, 9, e110277. [Google Scholar] [CrossRef] [PubMed]
- Polaquini, C.R.; Torrezan, G.S.; Santos, V.R.; Nazaré, A.C.; Campos, D.L.; Almeida, L.A.; Silva, I.C.; Ferreira, H.; Pavan, F.R.; Duque, C.; et al. Antibacterial and antitubercular activities of cinnamylideneacetophenones. Molecules 2017, 22, 1685. [Google Scholar] [CrossRef] [PubMed]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistence in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 8. [Google Scholar] [CrossRef]
- da Silva, A.R.; Ferro, J.A.; Reinach, F.C.; Farah, C.S.; Furlan, L.R.; Quaggio, R.B.; Monteiro-Vitorello, C.B.; Van Sluys, M.A.; Almeida, N.A.; Alves, L.M.; et al. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 2002, 417, 459. [Google Scholar] [CrossRef] [PubMed]
- Morão, L.G.; Polaquini, C.R.; Santos, M.B.; Regasini, L.O.; Ferreira, H. Atividade anti-Xanthomonas citri subsp citri de hibridos moleculares curcumina-cinamaldeido: Uma alternativa para a citricultura. Cienc. Tecnol. 2016, 8, 1–2. [Google Scholar]
- Mosman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Ingerman, E.; Nunnari, J. A Continuous, Regenerative Coupled GTPase Assay for Dynamin-Related Proteins. Methods Enzymol. 2005, 404, 611–619. [Google Scholar] [PubMed]






| Entry | R | Purity | log Po/w | MIC μg mL−1 (MIC in μM) | MBC μg mL−1 | |
|---|---|---|---|---|---|---|
Series I | 2,4 DHB acid | H | 85.7 | 2.6 | >100 (>648) | >100 |
| 1 | CH2CH3 | 87.6 | 2.8 | >100 (>548) | >100 | |
| 2 | (CH2)3CH3 | 85.6 | 3.9 | 39.8 (189) | 50 | |
| 3 | (CH2)5CH3 | 81.7 | 5.0 | 20.0 (84) | 50 | |
| 4 | (CH2)6CH3 | 83.8 | 5.1 | 13.2 (52) | 25 | |
| 5 | (CH2)7CH3 | 79.6 | 6.1 | >100 (>375) | >100 | |
| 6 | (CH2)8CH3 | 99.0 | 6.2 | >100 (>356) | >100 | |
| 7 | (CH2)9CH3 | 99.5 | 6.9 | >100 (>340) | >100 | |
| 8 | (CH2)11CH3 | >99.9 | 7.0 | >100 (>310) | >100 | |
| 9 |  | 93.2 | 2.6 | >100 (>510) | >100 | |
| 10 |  | >99.9 | 2.2 | >100 (>450) | >100 | |
| 11 |  | >99.9 | 4.0 | 40.5 (171) | 100 | |
Series II | 2,5 DHB acid | H | 97.3 | 2.8 | >100 (>648) | >100 |
| 13 | CH2CH3 | 98.3 | 2.4 | >100 (>549) | >100 | |
| 14 | (CH2)3CH3 | 82.0 | 3.4 | >100 (>475) | >100 | |
| 15 | (CH2)5CH3 | 80.1 | 4.5 | 33.5 (140) | 50 | |
| 16 | (CH2)6CH3 | 99.3 | 5.0 | 20.2 (80) | 25 | |
| 17 | (CH2)7CH3 | 79.8 | 5.2 | >100 (>375) | >100 | |
| 18 | (CH2)8CH3 | >99.9 | 5.7 | >100 (>357) | >100 | |
| 19 | (CH2)9CH3 | 76.5 | 6.3 | >100 (>340) | >100 | |
| 20 | (CH2)11CH3 | >99.9 | 6.8 | >100 (>310) | >100 | |
| 21 |  | >99.9 | 2.2 | >100 (>510) | >100 | |
| 22 |  | >99.9 | 3.0 | 83.5 (375) | 100 | |
| 23 |  | >99.9 | 3.5 | 90.4 (382) | 100 | |
| 24 |  | >99.9 | 3.1 | 59.7 (231) | 100 | |
Series III | 3,4 DHB acid | H | 99.6 | 0.8 | >100 (>648) | >100 |
| 25 | CH2CH3 | 99.1 | 1.7 | >100 (>549) | >100 | |
| 26 | (CH2)3CH3 | 99.1 | 2.6 | >100 (>475) | >100 | |
| 27 | (CH2)5CH3 | 99.1 | 3.7 | 44.4 (186) | 50 | |
| 28 | (CH2)6CH3 | 98.5 | 4.3 | 22.2 (88) | 50 | |
| 29 | (CH2)7CH3 | 98.5 | 4.9 | 31.2 (117) | 50 | |
| 30 | (CH2)8CH3 | 97.9 | 5.5 | 26.7 (95) | 50 | |
| 31 | (CH2)9CH3 | 91.3 | 6.0 | >100 (>340) | 100 | |
| 32 | (CH2)11CH3 | >99.9 | 6.9 | >100 (>310) | >100 | |
| 33 |  | >99.9 | 1.4 | >100 (>510) | >100 | |
| 34 |  | >99.9 | 2.2 | >100 (>450) | >100 | |
| 35 |  | >99.9 | 2.7 | >100 (>423) | >100 | |
| 36 |  | >99.9 | 2.4 | 89.1 (345) | 100 | |
| copper oxychloride | 43.1 | - | ||||
| Kanamycin | 19.9 (40) | - |
| Compounds | HaCaT | Huh-7,5 | MRC-5 | VERO | ACHN |
|---|---|---|---|---|---|
| 4 | 64.86 ± 8.91 a | 70.63 ± 5.52 a | 127.45 ± 4.59 b,c,h | 111.4 ± 8.34 c,f,h | 152.9 ± 7.07 d |
| 16 | 69.81 ± 4.87 a | 20.60 ± 0.23 e | 144.8 ± 6.29 b,d | 93.55 ± 1.93 f | 140.35 ± 7.56 b,d |
| 28 | 74.02 ± 11.74 a,g | 64.84 ± 14.91 a | 128.7 ± 2.12 b,c,h | 92.31 ± 9.34 f,g | 114.05 ± 9.97 g |
| Kanamycin | >200 i | >200 i | >200 i | >200 i | >200 i |
| copper oxychloride | >200 i | >200 i | >200 i | >200 i | >200 i |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazaré, A.C.; Polaquini, C.R.; Cavalca, L.B.; Anselmo, D.B.; Saiki, M.D.F.C.; Monteiro, D.A.; Zielinska, A.; Rahal, P.; Gomes, E.; Scheffers, D.-J.; et al. Design of Antibacterial Agents: Alkyl Dihydroxybenzoates against Xanthomonas citri subsp. citri. Int. J. Mol. Sci. 2018, 19, 3050. https://doi.org/10.3390/ijms19103050
Nazaré AC, Polaquini CR, Cavalca LB, Anselmo DB, Saiki MDFC, Monteiro DA, Zielinska A, Rahal P, Gomes E, Scheffers D-J, et al. Design of Antibacterial Agents: Alkyl Dihydroxybenzoates against Xanthomonas citri subsp. citri. International Journal of Molecular Sciences. 2018; 19(10):3050. https://doi.org/10.3390/ijms19103050
Chicago/Turabian StyleNazaré, Ana Carolina, Carlos Roberto Polaquini, Lúcia Bonci Cavalca, Daiane Bertholin Anselmo, Marilia De Freitas Calmon Saiki, Diego Alves Monteiro, Aleksandra Zielinska, Paula Rahal, Eleni Gomes, Dirk-Jan Scheffers, and et al. 2018. "Design of Antibacterial Agents: Alkyl Dihydroxybenzoates against Xanthomonas citri subsp. citri" International Journal of Molecular Sciences 19, no. 10: 3050. https://doi.org/10.3390/ijms19103050