Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies
Abstract
:1. Introduction
2. Side Effects of Platinum-Based Cancer Therapies
3. Strategies for Reducing the Toxicity of Metal-Based Cancer Therapies
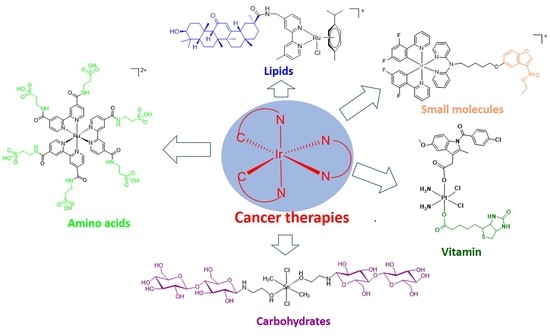
4. Natural Product-Conjugated Metal Complex Cancer Therapies
4.1. Small Molecules
4.2. Amino Acids
4.3. Lipids
4.4. Carbohydrates
4.5. Vitamin
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| HSA | human serum albumin |
| PPT | podophyllotoxin |
| ROS | reactive oxygen species |
| CNS | central nervous system |
| GA | glycyrrhetinic acid |
References
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Pasetto, L.M.; D’Andrea, M.R.; Brandes, A.A.; Rossi, E.; Monfardini, S. The development of platinum compounds and their possible combination. Crit. Rev. Oncol. Hematol. 2006, 60, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Apps, M.G.; Choi, E.H.; Wheate, N.J. The state-of-play and future of platinum drugs. Endocr. Relat. Cancer. 2015, 22, R219–R233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalayda, G.V.; Wagner, C.H.; Jaehde, U. Relevance of copper transporter 1 for cisplatin resistance in human ovarian carcinoma cells. J. Inorg. Biochem. 2012, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.F.; Tian, Q.; Setyawati, M.I.; Fang, W.; Tan, E.S.Q.; Leong, D.T.; Ang, W.H. Tuning the activity of platinum(IV) anticancer complexes through asymmetric acylation. J. Med. Chem. 2012, 55, 7571–7582. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Kapdi, A.R.; Fairlamb, I.J. Anti-cancer palladium complexes: A focus on PdX2L2, palladacycles and related complexes. Chem. Soc. Rev. 2014, 43, 4751–4777. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.; Li, C.; Xu, Z.; Chan, C.-Y.; Tse, M.-K.; Shi, P.; Zhu, G. A cancer cell-selective and low-toxic bifunctional heterodinuclear Pt(IV)–Ru(II) anticancer prodrug. Inorg. Chem. 2018, 57, 2917–2924. [Google Scholar] [CrossRef]
- Arjmand, F.; Parveen, S.; Tabassum, S.; Pettinari, C. Organotin antitumor compounds: Their present status in drug development and future perspectives. Inorg. Chim. Acta. 2014, 423, 26–37. [Google Scholar] [CrossRef]
- Machiels, J.-P.H.; Haddad, R.I.; Fayette, J.; Licitra, L.F.; Tahara, M.; Vermorken, J.B.; Clement, P.M.; Gauler, T.; Cupissol, D.; Grau, J.J. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck): An open-label, randomised phase 3 trial. Lancet Oncol. 2015, 16, 583–594. [Google Scholar] [PubMed]
- Allardyce, C.S.; Dyson, P.J. Ruthenium in medicine: Current clinical uses and future prospects. Platin. Met. Rev. 2001, 45, 62–69. [Google Scholar]
- Landier, W. Ototoxicity and cancer therapy. Cancer 2016, 122, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Margiotta, N.; Marzano, C.; Gandin, V.; Osella, D.; Ravera, M.; Gabano, E.; Platts, J.A.; Petruzzella, E.; Hoeschele, J.D.; Natile, G. Revisiting [PtCl2(cis-1, 4-DACH)]: An underestimated antitumor drug with potential application to the treatment of oxaliplatin-refractory colorectal cancer. J. Med. Chem. 2012, 55, 7182–7192. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Dreisbach, L.; Ho, M.; Reid, E.; Siegel, J. Effects of oxaliplatin, carboplatin, and cisplatin across treatment on high-frequency objective and subjective auditory measures in adults. Perspect ASHA Spec Interest Groups 2017, 2, 17–36. [Google Scholar] [CrossRef]
- Abu-Surrah, A.S.; Kettunen, M. Platinum group antitumor chemistry: Design and development of new anticancer drugs complementary to cisplatin. Curr. Med. Chem. 2006, 13, 1337–1357. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010, 188, 759–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mital, M.; Ziora, Z. Biological applications of Ru(II) polypyridyl complexes. Coord. Chem. Rev. 2018, 375, 434–458. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) compounds: Next-generation anticancer metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef] [PubMed]
- Galanski, M.; Jakupec, M.A.; Keppler, B.K. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure–activity relationships for ruthenium and osmium anticancer agents–towards clinical development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, J.P.; Romero-Canelón, I.; Sanchez-Cano, C.; Clarkson, G.J.; Habtemariam, A.; Wills, M.; Sadler, P.J. Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells. Nat. Chem. 2018, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Foltinová, V.; Švihálková Šindlerová, L.; Horváth, V.; Sova, P.; Hofmanova, J.; Janisch, R.; Kozubík, A. Mechanisms of effects of platinum(II) and platinum(IV) complexes. Comparison of cisplatin and oxaliplatin with satraplatin and LA-12, new Pt(IV)-based drugs. A Minireview. Scr. Med. 2008, 81, 105–116. [Google Scholar]
- Choi, S.; Vastag, L.; Larrabee, Y.C.; Personick, M.L.; Schaberg, K.B.; Fowler, B.J.; Sandwick, R.K.; Rawji, G. Importance of platinum(II)-assisted platinum(IV) substitution for the oxidation of guanosine derivatives by platinum(IV) complexes. Inorg. Chem. 2008, 47, 1352–1360. [Google Scholar] [CrossRef]
- Hall, M.D.; Amjadi, S.; Zhang, M.; Beale, P.J.; Hambley, T.W. The mechanism of action of platinum(IV) complexes in ovarian cancer cell lines. J. Inorg. Biochem. 2004, 98, 1614–1624. [Google Scholar] [CrossRef]
- Yang, G.; Zhong, H.-J.; Ko, C.-N.; Wong, S.-Y.; Vellaisamy, K.; Ye, M.; Ma, D.-L.; Leung, C.-H. Identification of a Rhodium(III) complex as a Wee1 inhibitor against TP53-mutated triple-negative breast cancer cells. Chem. Commun. 2018, 54, 2463–2466. [Google Scholar] [CrossRef]
- Liu, L.-J.; Wang, W.; Huang, S.-Y.; Hong, Y.; Li, G.; Lin, S.; Tian, J.; Cai, Z.; Wang, H.-M.D.; Ma, D.-L. Inhibition of the Ras/Raf interaction and repression of renal cancer xenografts in vivo by an enantiomeric iridium(III) metal-based compound. Chem. Sci. 2017, 8, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Liang, J.-X.; Li, G.; Vellaisamy, K.; Wong, C.-Y.; Ma, D.-L.; Leung, C.-H. A rhodium(III)-based inhibitor of lysine-specific histone demethylase 1 as an epigenetic modulator in prostate cancer cells. J. Med. Chem. 2017, 60, 2597–2603. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Chen, L.; Liang, J.; Lin, S.; Lee, M.-Y.; Ma, D.-L.; Leung, C.-H. Discovery of a VHL and HIF1α interaction inhibitor with in vivo angiogenic activity via structure-based virtual screening. Chem. Commun. 2016, 52, 12837–12840. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.-J.; Lu, L.; Leung, K.-H.; Wong, C.C.; Peng, C.; Yan, S.-C.; Ma, D.-L.; Cai, Z.; Wang, H.-M.D.; Leung, C.-H. An iridium(III)-based irreversible protein–protein interaction inhibitor of BRD4 as a potent anticancer agent. Chem. Sci. 2015, 6, 5400–5408. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-X.; Zhong, H.-J.; Yang, G.; Vellaisamy, K.; Ma, D.-L.; Leung, C.-H. Recent development of transition metal complexes with in vivo antitumor activity. J. Inorg. Biochem. 2017, 177, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sadler, P.J. Redox-active metal complexes for anticancer therapy. Eur. J. Inorg. Chem. 2017, 27, 1541–1548. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Lin, W. Nanoscale metal–organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Ma, D.L.; He, H.Z.; Leung, K.H.; Chan, D.S.H.; Leung, C.H. Bioactive luminescent transition-metal complexes for biomedical applications. Angew. Chem. Int. Ed. Engl. 2013, 52, 7666–7682. [Google Scholar] [CrossRef]
- Schmitt, F.; Kasparkova, J.; Brabec, V.; Begemann, G.; Schobert, R.; Biersack, B. New arene ruthenium(II) complexes of 4-aryl-4H-naphthopyrans with anticancer and anti-vascular activities. J. Inorg. Biochem. 2018, 184, 69–78. [Google Scholar] [CrossRef]
- Choi, S.-D.; Kim, M.-S.; Kim, S.K.; Lincoln, P.; Tuite, E.; Nordén, B. Binding mode of [ruthenium(II)(1, 10-phenanthroline)2L]2+ with poly (dT* dA-dT) triplex. Ligand size effect on third-strand stabilization. Biochemistry 1997, 36, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; Danishefsky, A.; Goldberg, J. Tris (phenanthroline) ruthenium(II): Stereoselectivity in binding to DNA. J. Am. Chem. Soc. 1984, 106, 2172–2176. [Google Scholar] [CrossRef]
- Pascu, G.I.; Hotze, A.C.; Sanchez-Cano, C.; Kariuki, B.M.; Hannon, M.J. Dinuclear Ruthenium (II) Triple-Stranded Helicates: Luminescent Supramolecular Cylinders That Bind and Coil DNA and Exhibit Activity against Cancer Cell Lines. Angew. Chem. Int. Ed. 2007, 46, 4374–4378. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Rossi, M.; Benson, A.; Opazo, C.; Freedman, D.; Monti, E.; Gariboldi, M.B.; Shaulky, J.; Marchetti, F.; Pettinari, R. Ruthenium–arene complexes of curcumin: X-ray and density functional theory structure, synthesis, and spectroscopic characterization, in vitro antitumor activity, and DNA docking studies of (p-cymene)-Ru-(curcuminato)-chloro. J. Med. Chem. 2012, 55, 1072–1081. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravarty, A.R. Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity. Acc. Chem. Res. 2015, 48, 2075–2083. [Google Scholar] [CrossRef]
- Kandioller, W.; Balsano, E.; Meier, S.M.; Jungwirth, U.; Göschl, S.; Roller, A.; Jakupec, M.A.; Berger, W.; Keppler, B.K.; Hartinger, C.G. Organometallic anticancer complexes of lapachol: Metal centre-dependent formation of reactive oxygen species and correlation with cytotoxicity. Chem. Commun. 2013, 49, 3348–3350. [Google Scholar] [CrossRef] [PubMed]
- Man, B.Y.-W.; Chan, H.-M.; Leung, C.-H.; Chan, D.S.-H.; Bai, L.-P.; Jiang, Z.-H.; Li, H.-W.; Ma, D.-L. Group 9 metal-based inhibitors of β-amyloid (1–40) fibrillation as potential therapeutic agents for Alzheimer′s disease. Chem. Sci. 2011, 2, 917–921. [Google Scholar] [CrossRef]
- Wenzel, M.; Bertrand, B.; Eymin, M.-J.; Comte, V.; Harvey, J.A.; Richard, P.; Groessl, M.; Zava, O.; Amrouche, H.; Harvey, P.D. Multinuclear cytotoxic metallodrugs: Physicochemical characterization and biological properties of novel heteronuclear gold–titanium complexes. Inorg. Chem. 2011, 50, 9472–9480. [Google Scholar] [CrossRef]
- Leung, C.-H.; Yang, H.; Ma, V.P.-Y.; Chan, D.S.-H.; Zhong, H.-J.; Li, Y.-W.; Fong, W.-F.; Ma, D.-L. Inhibition of Janus kinase 2 by cyclometalated rhodium complexes. MedChemComm 2012, 3, 696–698. [Google Scholar] [CrossRef]
- He, Y.; Xu, J.; Yu, Z.-H.; Gunawan, A.M.; Wu, L.; Wang, L.; Zhang, Z.-Y. Discovery and evaluation of novel inhibitors of mycobacterium protein tyrosine phosphatase B from the 6-Hydroxy-benzofuran-5-carboxylic acid scaffold. J. Med. Chem. 2013, 56, 832–842. [Google Scholar] [CrossRef]
- Kang, T.-S.; Wang, W.; Zhong, H.-J.; Dong, Z.-Z.; Huang, Q.; Mok, S.W.F.; Leung, C.-H.; Wong, V.K.W.; Ma, D.-L. An anti-prostate cancer benzofuran-conjugated iridium(III) complex as a dual inhibitor of STAT3 and NF-κB. Cancer Lett. 2017, 396, 76–84. [Google Scholar] [CrossRef]
- Tabrizi, L.; Chiniforoshan, H. Ruthenium(II) p-cymene complexes of naphthoquinone derivatives as antitumor agents: A structure−activity relationship study. J. Organomet. Chem. 2016, 822, 211–220. [Google Scholar] [CrossRef]
- Oliveira, K.M.; Corrêa, R.S.; Barbosa, M.I.; Ellena, J.; Cominetti, M.R.; Batista, A.A. Ruthenium(II)/triphenylphosphine complexes: An effective way to improve the cytotoxicity of lapachol. Polyhedron 2017, 130, 108–114. [Google Scholar] [CrossRef]
- Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Moyano, E.; Bonfill, M.; Cusido, R.M.; Palazon, J. Podophyllotoxin: Current approaches to its biotechnological production and future challenges. Eng. Life Sci. 2010, 10, 281–292. [Google Scholar] [CrossRef]
- Xu, H.; Lv, M.; Tian, X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins. Curr. Med. Chem. 2009, 16, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Beauperin, M.; Polat, D.; Roudesly, F.; Top, S.; Vessières, A.; Oble, J.; Jaouen, G.; Poli, G. Approach to ferrocenyl-podophyllotoxin analogs and their evaluation as anti-tumor agents. J. Organomet. Chem. 2017, 839, 83–90. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Manolova, Y.; Deneva, V.; Antonov, L.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin: Miniperspective. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Chandra, S.; Basu, D. Synthesis and antiarthritic study of a new orally active diferuloyl methane (curcumin) gold complex. Inorg. Chim. Acta 1987, 135, 47–48. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin–synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [PubMed]
- Pettinari, R.; Marchetti, F.; Condello, F.; Pettinari, C.; Lupidi, G.; Scopelliti, R.; Mukhopadhyay, S.; Riedel, T.; Dyson, P.J. Ruthenium(II)–Arene RAPTA Type Complexes Containing Curcumin and Bisdemethoxycurcumin Display Potent and Selective Anticancer Activity. Organometallics 2014, 33, 3709–3715. [Google Scholar] [CrossRef]
- Du, E.; Hu, X.; Roy, S.; Wang, P.; Deasy, K.; Mochizuki, T.; Zhang, Y. Taurine-modified Ru(II)-complex targets cancerous brain cells for photodynamic therapy. Chem. Commun. 2017, 53, 6033–6036. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.R.; Graminha, A.E.; Schultz, M.S.; Correia, I.; Selistre-de-Araújo, H.S.; Corrêa, R.S.; Ellena, J.; Elisângela de Paula, S.L.; Pessoa, J.C.; Batista, A.A. Cytotoxic activity and structural features of Ru (II)/phosphine/amino acid complexes. J. Inorg. Biochem. 2018, 182, 48–60. [Google Scholar] [CrossRef]
- Dos Santos, E.R.; Corrêa, R.S.; Pozzi, L.V.; Graminha, A.E.; Selistre-de-Araújo, H.S.; Pavan, F.R.; Batista, A.A. Antitumor and anti-Mycobacterium tuberculosis agents based on cationic ruthenium complexes with amino acids. Inorg. Chim. Acta. 2017, 463, 1–6. [Google Scholar] [CrossRef]
- Ruiz, J.; Rodríguez, V.; Cutillas, N.; Espinosa, A.; Hannon, M.J. A potent ruthenium(II) antitumor complex bearing a lipophilic levonorgestrel group. Inorg. Chem. 2011, 50, 9164–9171. [Google Scholar] [CrossRef]
- Lin, K.-W.; Huang, A.-M.; Hour, T.-C.; Yang, S.-C.; Pu, Y.-S.; Lin, C.-N. 18β-Glycyrrhetinic acid derivatives induced mitochondrial-mediated apoptosis through reactive oxygen species-mediated p53 activation in NTUB1 cells. Bioorg. Med. Chem. 2011, 19, 4274–4285. [Google Scholar] [CrossRef]
- Vicker, N.; Su, X.; Lawrence, H.; Cruttenden, A.; Purohit, A.; Reed, M.J.; Potter, B.V. A novel 18β-glycyrrhetinic acid analogue as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase. Bioorg. Med. Chem. Lett. 2004, 14, 3263–3267. [Google Scholar]
- Kong, Y.; Chen, F.; Su, Z.; Qian, Y.; Wang, F.-x.; Wang, X.; Zhao, J.; Mao, Z.-W.; Liu, H.-K. Bioactive ruthenium(II)-arene complexes containing modified 18β-glycyrrhetinic acid ligands. J. Inorg. Biochem. 2018, 182, 194–199. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Conklin, J.; Hogan, V.; Raz, A. Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends Mol. Med. 2002, 8, 187–192. [Google Scholar] [CrossRef]
- Khan, R.A.; Yadav, S.; Hussain, Z.; Arjmand, F.; Tabassum, S. Carbohydrate linked organotin(IV) complexes as human topoisomerase Iα inhibitor and their antiproliferative effects against the human carcinoma cell line. Dalton Trans. 2014, 43, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Fang, L.; Hua, W.; Gou, S. Biotin-Pt(IV)-indomethacin hybrid: A targeting anticancer prodrug providing enhanced cancer cellular uptake and reversing cisplatin resistance. J. Inorg. Biochem. 2017, 175, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Vellaisamy, K.; Li, G.; Wang, W.; Leung, C.-H.; Ma, D.-L. A long-lived peptide-conjugated iridium (iii) complex as a luminescent probe and inhibitor of the cell migration mediator, formyl peptide receptor 2. Chem. Sci. 2018, 9, 8171–8177. [Google Scholar] [CrossRef]
- Copeland, K.D.; Lueras, A.M.; Stemp, E.D.; Barton, J.K. DNA cross-linking with metallointercalator− peptide conjugates. Biochemistry 2002, 41, 12785–12797. [Google Scholar] [CrossRef]
- Copeland, K.D.; Fitzsimons, M.P.; Houser, R.P.; Barton, J.K. DNA hydrolysis and oxidative cleavage by metal-binding peptides tethered to rhodium intercalators. Biochemistry 2002, 41, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharmaceutics 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Bendas, G. Immunoliposomes. BioDrugs 2001, 15, 215–224. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, Y.Y.; Jon, S. A drug-loaded aptamer−gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008. [Google Scholar] [CrossRef]
- Dhar, S.; Daniel, W.L.; Giljohann, D.A.; Mirkin, C.A.; Lippard, S.J. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J. Am. Chem. Soc. 2009, 131, 14652–14653. [Google Scholar] [CrossRef] [PubMed]











| Reference | Natural Moiety | Metal Center | Mechanism of Action or Target | Cytotoxicity against Target Cells (IC50) | Cytotoxicity against Normal Cells (IC50) | Reference Compound | Demonstrated Application in |
|---|---|---|---|---|---|---|---|
| Kang et al., 2017 [51] | Benzofuran | Iridium(III) | Transcription factors NF-κB and STAT3 | 4.34 μM | 29.21 μM (LO2 cells); 32.24 μM (HEK293 cells) | Cisplatin and doxorubicin | Prostate cancer cells (DU145) |
| Oliveira et al., 2017 [53] | Lapachol | Ruthenium(II) | Bovine serum albumin (BSA) and human serum albumin (HSA) | 0.086 μM; 0.09 μM | 0.72 μM (V79 cells) | Cisplatin | Breast cancer cells (MDA-MB-231); lung cancer cells (A549) |
| Beauperin et al., 2017 [56] | Podophyllotoxin | Iron(III) | Reactive oxygen species (ROS) | 0.93 μM; 0.43 μM | NA | Podophyllotoxin | Breast cancer cells (MCF-7 and MDA-MB-231) |
| Pettinari et al., 2014 [63] | Curcumin | Ruthenium(II) | Hydrolysis | 0.20 μM; 0.27 μM | 13.0 μM (HEK293 cells) | Cisplatin | Ovarian carcinoma cells (A2780 and A2780R) |
| Du et al., 2017 [64] | Taurine | Ruthenium(II) | Reactive oxygen species (ROS) | NA | NA | Cisplatin and non-natural product conjugates | Brain cancer cells (F98, A375, HeLa, and A549) |
| Santos et al., 2018 [65] | l-tryptophan (Trp) | Ruthenium(II) | Human serum albumin (HSA) | 3.0 μM | 29.9 μM (MCF-10A cells) | Cisplatin and non-natural product conjugates | Breast cancer cells (MDA-MB-231) |
| Santos et al., 2017 [66] | l-tyrosine (Tyr) | Ruthenium(II) | N/A | 3.04 μM | NA | Cisplatin | Breast cancer cells (MDA-MB-231) |
| Ruiz et al., 2011 [67] | Levonorgestrel | Ruthenium(II) | DNA | 7.4 μM; 3.7 μM | NA | Cisplatin | Breast cancer cells (T47D); ovarian cancer cells (A2780) |
| Kong et al., 2018 [70] | Glycyrrhetinic acid | Ruthenium(II) | DNA and ROS | 24.2 μM; 34.6 μM; 63.7 μM | NA | Cisplatin | Cervical cancer cells (HeLa)o breast cancer cells (MCF-7); ovarian cancer cells (A278) |
| Khan et al., 2014 [72] | Carbohydrates | Organotin(IV) | Human topoisomerase Iα | 30 μM | NA | NA | Hepatoma cancer cells (Huh7) |
| Hu et al., 2017 [73] | Vitamin | Platinum(IV) | Endogenous reducing molecules | 42.73 μM | 59.64 μM (LO-2 cells) | Cisplatin | Umbilical vein endothelial cell (EA. hy926) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.-L.; Wu, C.; Cheng, S.-S.; Lee, F.-W.; Han, Q.-B.; Leung, C.-H. Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies. Int. J. Mol. Sci. 2019, 20, 341. https://doi.org/10.3390/ijms20020341
Ma D-L, Wu C, Cheng S-S, Lee F-W, Han Q-B, Leung C-H. Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies. International Journal of Molecular Sciences. 2019; 20(2):341. https://doi.org/10.3390/ijms20020341
Chicago/Turabian StyleMa, Dik-Lung, Chun Wu, Sha-Sha Cheng, Fu-Wa Lee, Quan-Bin Han, and Chung-Hang Leung. 2019. "Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies" International Journal of Molecular Sciences 20, no. 2: 341. https://doi.org/10.3390/ijms20020341