Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key
Abstract
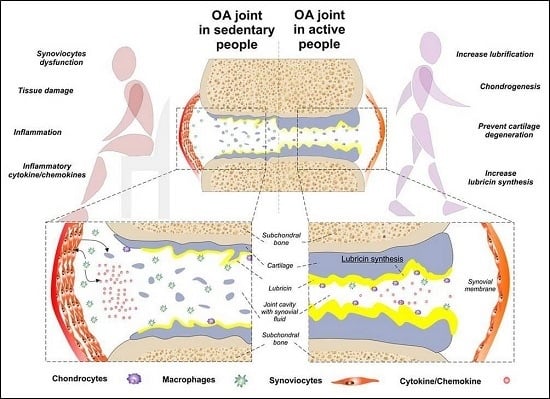
:1. Introduction
2. Results
2.1. Histology
2.2. Histomorphometric Analyses
2.3. Immunohistochemistry (IHC) and Statistical Analysis
- •
- •
- IL-4 immunolabeling was moderate in the synovium of Group 1 (ES = +; IS = 2) (Figure 2F). IL-4-immunostaining was weak (ES = +; IS = 1) in the synovium of Group 3 (Figure 2H) and it was strong in the synovial membrane of both Group 2 (ES = ++; IS = 3) (Figure 2G) and Group 4 (ES = ++; IS = 3) (Figure 2I).
- •
- IL-6 immunolabeling was moderate in the synovium of Group 1 (ES = +; IS = 2) (Figure 2K). IL-6-immunostaining was strong (ES = +; IS = 3) in the synovium of Group 2 (Figure 2L). IL-6-immunostaining was very strong (ES = +++; IS = 4) in the synovium of both Group 3 (Figure 2M) and Group 4 (Figure 2N).
- •
- •
- •
- •
- •
- IL-1β immunolabeling was much higher in Group 3 (16.92 ± 1.077) with respect to Group 1 (10.43 ± 1.139), Group 2 (10.56 ± 0.97), and Group 4 (12.08 ± 1.225). Group 1 and 2 had similar values (p ≥ 0.05, ns), whereas there was a statistically significant difference among groups (Groups 1 and 2 vs. 3, p < 0.0001; Groups 1 and 2 vs. 4, p < 0.0001; Group 3 vs. Group 4, p < 0.0001 (Figure 2E).
- •
- IL-4 immunolabeling was higher both in Group 2 and 4 (respectively, 18.91 ± 0.768 and 18.59 ± 0.632) when compared to both Group 1 (17.55 ± 0.562) and Group 3 (16.67 ± 0.375). There was a statistically significant difference between Group 1 vs. Group 2, p < 0.0001; Group 1 vs. Group 3, p < 0.0001; Group 1 vs. Group 4, p < 0.0001; Group 2 vs. Group 3, p < 0.0001; Group 2 vs. Group 4, p = 0.0196; Group 3 vs. Group 4, p < 0.0001, (Figure 2J).
- •
- IL-6 immunolabeling manifested a statistically significant difference between Group 1 (17.96 ± 0.786) vs. Group 3 (18.36 ± 1.106) (p = 0.0321) and Group 1 vs. Group 4 (18.39 ± 0.829) (p = 0.0188) with a higher IL-6 expression in both Group 3 and 4. Also, the differences between Group 2 (17.99 ± 0.638) vs. Group 3 and Group 2 vs. Group 4 were statistically significant, respectively p = 0.0468 and 0.0281. Conversely, the difference between Group 1 vs. Group 2 and between Group 3 vs. Group 4 was not statistically significant (p ≥ 0.05, ns) (Figure 2O).
- •
- IL-10 immunolabeling was evident and similar in both Group 2 (17.76 ± 0.953) and Group 4 (17.68 ± 0.979) (p = ns). A statistically significant difference was evident among all groups and in particular: Group 1 (17.07 ± 0.817) vs. Group 2 had a p = 0.0001; Group 1 vs. Group 3 (16.59 ± 0.686) had a p = 0.0145; Group 1 vs. Group 4 had a p = 0.0009; Group 2 vs. Group 3 had a p < 0.0001; Group 3 vs. Group 4 had a p < 0.0001 (Figure 2T).
- •
- TNF-α immunolabeling was higher in Group 3 (17.10 ± 0.608) when compared to all other groups, Group 1 (11.73 ± 0.968), Group 2 (11.69 ± 0.956) and Group 4 (14.39 ± 1.726) (Group 1 vs. Group 3, p < 0.0001; Group 2 vs. Group 3, p < 0.0001; Group 3 vs. Group 4, p < 0.0001). There was a statistically significant difference also between Group 1 vs. Group 4 (p < 0.0001); no statistical significance was found between Group 1 vs. Group 2 (p ≥ 0.05, ns), (Figure 3E).
- •
- MMP-13-immunostaining was much higher in Group 3 (16.65 ± 0.664) when compared to Group 1 (11.09 ± 0.869) (p < 0.0001), Group 2 (11.42 ± 0.963) (p < 0.0001) and Group 4 (15.05 ± 0.646) (p < 0.0001). Furthermore, a statistically significant difference was shown also between Group 1 vs. Group 4 (p < 0.0001) and Group 2 vs. Group 4 (p < 0.0001). No statistically significant difference was found between Group 1 vs. Group 2 (p ≥ 0.05, ns) (Figure 3J).
- •
- Lubricin immunolabeling in Group 3 (20.39 ± 0.275) was lower than in other groups. In particular: Group 1 (20.99 ± 0.226) vs. Group 3, p < 0.0001; Group 2 (21.19 ± 0.529) vs. Group 3, p < 0.0001; Group 3 vs. Group 4 (20.91 ± 0.267), p < 0.0001). Moreover, Group 1 vs. Group 2 had a statistically significant difference (p = 0.0066), whereas lubricin-immunostaining was similar in Group 1 and Group 4, hence showing no statistical difference (p ≥ 0.05, ns) (Figure 3O).
3. Discussion
4. Materials and Methods
4.1. Breeding, Housing of Animals and Experimental Design
4.2. Treadmill Training Exercise
4.3. Histology Analysis
4.4. Histomorphometric Analysis
4.5. Immunohistochemistry (IHC) Analysis
4.6. Evaluation of Immunohistochemistry
4.7. Computerized Densitometric Measurements and Image Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rios, J.L.; Boldt, K.R.; Mather, J.W.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Quantifying the Effects of Different Treadmill Training Speeds and Durations on the Health of Rat Knee Joints. Sport. Med. Open 2018, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Engstrom, C.; Friis, P. Exercise: An essential evidence-based medicine. Med. J. Aust. 2018, 208, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Castorina, S.; Guglielmino, C.; Castrogiovanni, P.; Szychlinska, M.A.; Ioppolo, F.; Massimino, P.; Leonardi, P.; Maci, C.; Iannuzzi, M.; Di Giunta, A.; et al. Clinical evidence of traditional vs fast track recovery methodologies after total arthroplasty for osteoarthritic knee treatment. A retrospective observational study. Muscles Ligaments Tendons J. 2018, 7, 504–513. [Google Scholar] [PubMed]
- Musumeci, G. The Effect of Mechanical Loading on Articular Cartilage. J. Funct. Morphol. Kinesiol. 2016, 1, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, T.A.; Craig, E. Knee anatomy. A brief review. Phys. Ther. 1980, 60, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Shikichi, M.; Kitamura, H.P.; Yanase, H.; Konno, A.; Takahashi-Iwanaga, H.; Iwanaga, T. Three-dimensional Ultrastructure of Synoviocytes in the Horse Joint as Revealed by the Scanning Electron Microscope. Arch. Histol. Cytol. 1999, 62, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Nio, J.; Yokoyama, A.; Okumura, M.; Iwanaga, T. Three-dimensional ultrastructure of synoviocytes in the knee joint of rabbits and morphological changes in osteoarthritis model. Arch. Histol. Cytol. 2002, 65, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Blewis, M.E.; Lao, B.J.; Jadin, K.D.; McCarty, W.J.; Bugbee, W.D.; Firestein, G.S.; Sah, R.L. Semi-permeable membrane retention of synovial fluid lubricants hyaluronan and proteoglycan 4 for a biomimetic bioreactor. Biotechnol. Bioeng. 2010, 106, 149–160. [Google Scholar] [CrossRef]
- Hui, A.Y.; McCarty, W.J.; Masuda, K.; Firestein, G.S.; Sah, R.L. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 15–37. [Google Scholar] [CrossRef]
- Ingram, K.R.; Wann, A.K.T.; Angel, C.K.; Coleman, P.J.; Levick, J.R. Cyclic movement stimulates hyaluronan secretion into the synovial cavity of rabbit joints. J. Physiol. 2008, 586, 1715–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estell, E.G.; Murphy, L.A.; Silverstein, A.M.; Tan, A.R.; Shah, R.P.; Ateshian, G.A.; Hung, C.T. Fibroblast-like synoviocyte mechanosensitivity to fluid shear is modulated by interleukin-1α. J. Biomech. 2017, 60, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Leonardi, R.; Al-Qahtani, M.; Mobasheri, A.; Musumeci, G. Altered joint tribology in osteoarthritis: Reduced lubricin synthesis due to the inflammatory process. New horizons for therapeutic approaches. Ann. Phys. Rehabil. Med. 2016, 59, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramson, S.B.; Attur, M. Developments in the scientific understanding of osteoarthritis. Arthritis Res. Ther. 2009, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musumeci, G.; Loreto, C.; Leonardi, R.; Castorina, S.; Giunta, S.; Carnazza, M.L.; Trovato, F.M.; Pichler, K.; Weinberg, A.M. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J. Bone Miner. Metab. 2013, 31, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. Effects of exercise on physical limitations and fatigue in rheumatic diseases. World J. Orthop. 2015, 6, 762. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Imbesi, R.; Giunta, S.; Szychlinska, M.A.; Loreto, C.; Castorina, S.; Mobasheri, A. Physical activity ameliorates cartilage degeneration in a rat model of aging: A study on lubricin expression. Scand. J. Med. Sci. Sport. 2015, 25, e222–e230. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Maria Trovato, F.; Imbesi, R.; Castrogiovanni, P. Effects of dietary extra-virgin olive oil on oxidative stress resulting from exhaustive exercise in rat skeletal muscle: A morphological study. Acta Histochem. 2014, 116, 61–69. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Trovato, F.M.; Di Rosa, M.; Malaguarnera, L.; Puzzo, L.; Leonardi, R.; Castrogiovanni, P.; Musumeci, G. Co-expression and co-localization of cartilage glycoproteins CHI3L1 and lubricin in osteoarthritic cartilage: Morphological, immunohistochemical and gene expression profiles. Int. J. Mol. Sci. 2016, 17, 359. [Google Scholar] [CrossRef]
- Di Rosa, M.; Szychlinska, M.A.; Tibullo, D.; Malaguarnera, L.; Musumeci, G. Expression of CHI3L1 and CHIT1 in osteoarthritic rat cartilage model. A morphological study. Eur. J. Histochem. 2014, 58, 2423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Liu, H.Q.; Xu, X.Z.; Zhi, J.; Geng, J.J.; Chen, J. Effects of exercise therapy on knee joint function and synovial fluid cytokine levels in patients with knee osteoarthritis. Mol. Med. Rep. 2013, 7, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Castrogiovanni, P.; Trovato, F.M.; Nsir, H.; Zarrouk, M.; Lo Furno, D.; Di Rosa, M.; Imbesi, R.; Musumeci, G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lapaj, L.; Markuszewski, J.; Wierusz-Kozlowska, M. Current views on the pathogenesis of osteoarthritis. Chir. Narzadow Ruchu Ortop. Pol. 2010, 75, 248–260. [Google Scholar] [PubMed]
- Xie, X.W.; Wan, R.Z.; Liu, Z.P. Recent Research Advances in Selective Matrix Metalloproteinase-13 Inhibitors as Anti-Osteoarthritis Agents. ChemMedChem. 2017, 12, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Roos, H.; Dahlberg, L.; Hoerrner, L.A.; Lark, M.W.; Thonar, E.J.M.A.; Shinmei, M.; Lindqvist, U.; Stefan Lohmander, L. Markers of cartilage matrix metabolism in human joint fluid and serum: The effect of exercise. Osteoarthr. Cartil. 1995, 3, 7–14. [Google Scholar] [CrossRef]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Muniz Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Jay, G.D.; Fleming, B.C.; Watkins, B.A.; McHugh, K.A.; Anderson, S.C.; Zhang, L.X.; Teeple, E.; Waller, K.A.; Elsaid, K.A. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010, 62, 2382–2391. [Google Scholar] [CrossRef] [Green Version]
- Elsaid, K.A.; Zhang, L.; Waller, K.; Tofte, J.; Teeple, E.; Fleming, B.C.; Jay, G.D. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthr. Cartil. 2012, 20, 940–948. [Google Scholar] [CrossRef]
- Leonardi, R.; Musumeci, G.; Sicurezza, E.; Loreto, C. Lubricin in human temporomandibular joint disc: An immunohistochemical study. Arch. Oral Biol. 2012, 57, 614–619. [Google Scholar] [CrossRef]
- Musumeci, G.; Loreto, C.; Carnazza, M.L.; Strehin, I.; Elisseeff, J. Oa cartilage derived chondrocytes encapsulated in poly(ethylene glycol) diacrylate (PEGDA) for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol. Histopathol. 2011, 26, 1265–1278. [Google Scholar] [PubMed]
- Loeser, R.F. Osteoarthritis year in review 2013: Biology. Osteoarthr. Cartil. 2013, 21, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castrogiovanni, P.; Trovato, F.M.; Weinberg, A.M.; Al-Wasiyah, M.K.; Alqahtani, M.H.; Mobasheri, A. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int. J. Mol. Sci. 2015, 16, 20560–20575. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Trovato, F.M.; Loreto, C.; Leonardi, R.; Szychlinska, M.A.; Castorina, S.; Mobasheri, A. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: A morphological, immunohistochemical and biochemical study. Acta Histochem. 2014, 116, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.C.; Martel-Pelletier, J.; Pelletier, J.P. The role of cytokines in osteoarthritis pathophysiology. Biorheology 2002, 39, 237–246. [Google Scholar] [PubMed]
- Pearson, M.J.; Herndler-Brandstetter, D.; Tariq, M.A.; Nicholson, T.A.; Philp, A.M.; Smith, H.L.; Davis, E.T.; Jones, S.W.; Lord, J.M. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci. Rep. 2017, 7, 3451. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, Immunity, And disease. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Voiriot, G.; Razazi, K.; Amsellem, V.; Tran Van Nhieu, J.; Abid, S.; Adnot, S.; Mekontso Dessap, A.; Maitre, B. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir. Res. 2017, 18, 64. [Google Scholar] [CrossRef]
- Nandi, D.; Mishra, M.K.; Basu, A.; Bishayi, B. Protective effects of interleukin-6 in lipopolysaccharide (LPS)-induced experimental endotoxemia are linked to alteration in hepatic anti-oxidant enzymes and endogenous cytokines. Immunobiology 2010, 215, 443–451. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohidnezhad, M.; Bayer, A.; Rasuo, B.; Hock, J.V.P.; Kweider, N.; Fragoulis, A.; Sönmez, T.T.; Jahr, H.; Pufe, T.; Lippross, S. Platelet-Released Growth Factors Modulate the Secretion of Cytokines in Synoviocytes under Inflammatory Joint Disease. Mediat. Inflamm. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, J.S.; Frémont, P.; Khan, K.; Poirier, P.; Fowles, J.; Wells, G.D.; Frankovich, R.J. Physical activity prescription: A critical opportunity to address a modifiable risk factor for the prevention and management of chronic disease: A position statement by the Canadian Academy of Sport and Exercise Medicine. Br. J. Sports Med. 2016, 50, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Moehl, K.; Navas-Enamorado, I.; Mitchell, S.J.; Zhang, Y.; Lehrmann, E.; Aon, M.A.; Cortassa, S.; Becker, K.G.; Mattson, M.P. Metabolic and molecular framework for the enhancement of endurance by intermittent food deprivation. FASEB J. 2018, 32, 3844–3858. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Musumeci, G. Which is the Best Physical Treatment for Osteoarthritis? J. Funct. Morphol. Kinesiol. 2016, 1, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Siebelt, M.; Groen, H.C.; Koelewijn, S.J.; de Blois, E.; Sandker, M.; Waarsing, J.H.; Müller, C.; van Osch, G.; de Jong, M.; Weinans, H. Increased physical activity severely induces osteoarthritic changes in knee joints with papain induced sulfate-glycosaminoglycan depleted cartilage. Arthritis Res. Ther. 2014, 16, R32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonyadi Rad, E.; Musumeci, G.; Pichler, K.; Heidary, M.; Szychlinska, M.A.; Castrogiovanni, P.; Marth, E.; Böhm, C.; Srinivasaiah, S.; Krönke, G.; et al. Runx2 mediated Induction of Novel Targets ST2 and Runx3 Leads to Cooperative Regulation of Hypertrophic Differentiation in ATDC5 Chondrocytes. Sci. Rep. 2017, 7, 17947. [Google Scholar] [CrossRef] [Green Version]
- Ostergaard, K.; Petersen, J.; Andersen, C.B.; Benutzen, K.; Salter, D.M. Histologic/histochemical grading system for osteoarthritic articular cartilage: Reproducibility and validity. Arthritis Rheum. 1997, 40, 1766–1771. [Google Scholar] [CrossRef]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18, S24–S34. [Google Scholar] [CrossRef]
- Giunta, S.; Castorina, A.; Marzagalli, R.; Szychlinska, M.A.; Pichler, K.; Mobasheri, A.; Musumeci, G. Ameliorative effects of PACAP against cartilage degeneration. Morphological, immunohistochemical and biochemical evidence from in vivo and in vitro models of rat osteoarthritis. Int. J. Mol. Sci. 2015, 16, 5922–5944. [Google Scholar] [CrossRef]
- Leonardi, R.; Rusu, M.C.; Loreto, F.; Loreto, C.; Musumeci, G. Immunolocalization and expression of lubricin in the bilaminar zone of the human temporomandibular joint disc. Acta Histochem. 2012, 114, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gardner, O.F.W.; Musumeci, G.; Neumann, A.J.; Eglin, D.; Archer, C.W.; Alini, M.; Stoddart, M.J. Asymmetrical seeding of MSCs into fibrin–poly(ester-urethane) scaffolds and its effect on mechanically induced chondrogenesis. J. Tissue Eng. Regen. Med. 2017, 11, 2912–2921. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Mobasheri, A.; Trovato, F.M.; Szychlinska, M.A.; Graziano, A.C.E.; Lo Furno, D.; Avola, R.; Mangano, S.; Giuffrida, R.; Cardile, V. Biosynthesis of collagen I, II, RUNX2 and lubricin at different time points of chondrogenic differentiation in a 3D in vitro model of human mesenchymal stem cells derived from adipose tissue. Acta Histochem. 2014, 116, 1407–1417. [Google Scholar] [CrossRef] [PubMed]





| Group | IL-1β | IL-4 | IL-6 | IL-10 | TNF-α | MMP-13 | Lubricin |
|---|---|---|---|---|---|---|---|
| 1 | ES = 0 IS = 1 | ES = + IS = 2 | ES = + IS = 2 | ES = ++ IS = 2 | ES = 0 IS = 1 | ES = 0 IS = 1 | ES = +++ IS = 3 |
| 2 | ES = 0 IS = 1 | ES = ++ IS = 3 | ES = + IS = 3 | ES = +++ IS = 3 | ES = 0 IS = 1 | ES = 0 IS = 1 | ES = ++++ IS = 4 |
| 3 | ES = ++ IS = 2 | ES = + IS = 1 | ES = +++ IS = 4 | ES = + IS = 1 | ES = +++ IS = 3 | ES = ++ IS = 3 | ES = ++ IS = 2 |
| 4 | ES = + IS = 1 | ES = ++ IS = 3 | ES = +++ IS = 4 | ES = +++ IS = 3 | ES = + IS = 1 | ES = + IS = 1 | ES = +++ IS = 3 |
| Week | Speed (m/min) | Session/Week | Session/Day | Duration (min) |
|---|---|---|---|---|
| 1 | 5 | 3 | 1 | 5 |
| 2 | 10 | 3 | 1 | 10 |
| 3–6 | 15 | 3 | 1 | 15 |
| 7–12 | 20 | 3 | 1 | 25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. Int. J. Mol. Sci. 2019, 20, 511. https://doi.org/10.3390/ijms20030511
Castrogiovanni P, Di Rosa M, Ravalli S, Castorina A, Guglielmino C, Imbesi R, Vecchio M, Drago F, Szychlinska MA, Musumeci G. Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key. International Journal of Molecular Sciences. 2019; 20(3):511. https://doi.org/10.3390/ijms20030511
Chicago/Turabian StyleCastrogiovanni, Paola, Michelino Di Rosa, Silvia Ravalli, Alessandro Castorina, Claudia Guglielmino, Rosa Imbesi, Michele Vecchio, Filippo Drago, Marta Anna Szychlinska, and Giuseppe Musumeci. 2019. "Moderate Physical Activity as a Prevention Method for Knee Osteoarthritis and the Role of Synoviocytes as Biological Key" International Journal of Molecular Sciences 20, no. 3: 511. https://doi.org/10.3390/ijms20030511