Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate
Abstract
:1. Introduction
2. Pathogenetic and Neurobiological Mechanism of Subcortical Vascular Cognitive Impairment
3. Rat Model of Chronic Cerebral Hypoperfusion
3.1. Method
3.2. Cerebral Blood Flow
3.3. White Matter Lesions
3.4. Cortical Lesions
3.5. Behavioral Analysis
3.6. Sensory/Motor Function
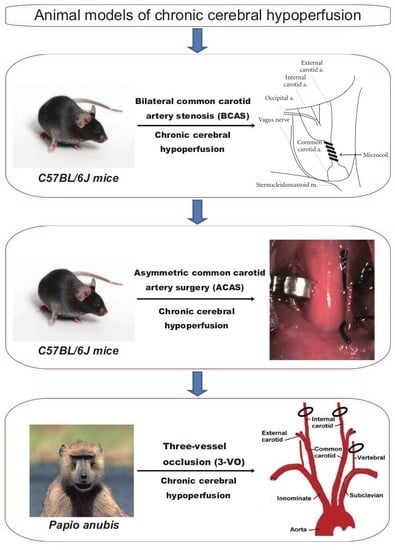
4. Mouse Model of Chronic Cerebral Hypoperfusion (Bilateral Common Carotid Artery Stenosis)
4.1. Method
4.2. Cerebral Blood Flow
4.3. White Matter Lesions
4.4. Cortical Lesions
4.5. Behavioral Analysis
4.6. Sensory/Motor Function
4.7. Pharmacological Experiments and Applications to Genetically Modified Animals
5. Mouse Model of Chronic Cerebral Hypoperfusion (Asymmetric Common Carotid Artery Surgery)
5.1. Method
5.2. Cerebral Blood Flow
5.3. White Matter Lesions
5.4. Cortical Lesions
5.5. Behavioral Analysis
5.6. Sensory/Motor Function
6. Non-Human Primate Model of Chronic Cerebral Hypoperfusion
6.1. Method
6.2. Cerebral Blood Flow
6.3. White Matter Lesions
6.4. Cortical Lesions
6.5. Behavioral Analysis
6.6. Sensory/Motor Function
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| VCI | Vascular Cognitive Impairment |
| CBF | Cerebral Blood Flow |
| CCA | Common Carotid Artery |
| 3VO | Three-Vessel Occlusion |
| BCAO | Bilateral Common Carotid Artery Occlusion |
| BCAS | Bilateral Common Carotid Artery Stenosis |
| MMP | Matrix Metalloproteinase |
| ACAS | Asymmetric Common Carotid Artery Surgery |
References
- O’Brien, J.T.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef] [Green Version]
- Matsui, Y.; Tanizaki, Y.; Arima, H.; Yonemoto, K.; Doi, Y.; Ninomiya, T.; Sasaki, K.; Iida, M.; Iwaki, T.; Kanba, S.; et al. Incidence and survival of dementia in a general population of Japanese elderly: The Hisayama study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Korczyn, A.D. The underdiagnosis of the vascular contribution to dementia. J. Neurol. Sci. 2005, 229, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. The pathology and pathophysiology of vascular dementia. Neuropharmacology 2018, 134, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Pathology and pathophysiology of vascular cognitive impairment. A critical update. Panminerva Med. 2004, 46, 217–226. [Google Scholar] [PubMed]
- Korczyn, A.D.; Vakhapova, V.; Grinberg, L.T. Vascular dementia. J. Neurol. Sci. 2012, 322, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Jiwa, N.S.; Garrard, P.; Hainsworth, A.H. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. J. Neurochem. 2010, 115, 814–828. [Google Scholar] [CrossRef]
- Gooch, J.; Wilcock, D.M. Animal Models of Vascular Cognitive Impairment and Dementia (VCID). Cell. Mol. Neurobiol. 2016, 36, 233–239. [Google Scholar] [CrossRef]
- Helman, A.M.; Murphy, M.P. Vascular cognitive impairment: Modeling a critical neurologic disease in vitro and in vivo. Biochim. Et Biophys. Acta 2016, 1862, 975–982. [Google Scholar] [CrossRef]
- Kalaria, R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016, 131, 659–685. [Google Scholar] [CrossRef] [Green Version]
- Ihara, M.; Tomimoto, H.; Ishizu, K.; Mukai, T.; Yoshida, H.; Sawamoto, N.; Inoue, M.; Doi, T.; Hashikawa, K.; Konishi, J.; et al. Decrease in cortical benzodiazepine receptors in symptomatic patients with leukoaraiosis: A positron emission tomography study. Stroke 2004, 35, 942–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edrissi, H.; Schock, S.C.; Cadonic, R.; Hakim, A.M.; Thompson, C.S. Cilostazol reduces blood brain barrier dysfunction, white matter lesion formation and motor deficits following chronic cerebral hypoperfusion. Brain Res. 2016, 1646, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Ohtani, R.; Ihara, M.; Tomimoto, H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke 2004, 35, 2598–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, Y.; Enmi, J.; Kitamura, A.; Yamamoto, Y.; Saito, S.; Takahashi, Y.; Iguchi, S.; Tsuji, M.; Yamahara, K.; Nagatsuka, K.; et al. A novel mouse model of subcortical infarcts with dementia. J. Neurosci 2015, 35, 3915–3928. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Akinyemi, R.O.; Hase, Y.; Firbank, M.J.; Ndung’u, M.N.; Foster, V.; Craggs, L.J.; Washida, K.; Okamoto, Y.; Thomas, A.J.; et al. Frontal white matter hyperintensities, clasmatodendrosis and gliovascular abnormalities in ageing and post-Stroke dementia. Brain 2016, 139, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Hainsworth, A.H.; Markus, H.S. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J. Cereb. Blood Flow Metab. 2008, 28, 1877–1891. [Google Scholar] [CrossRef] [Green Version]
- Bink, D.I.; Ritz, K.; Aronica, E.; Van Der Weerd, L.; Daemen, M.J. Mouse models to study the effect of cardiovascular risk factors on brain structure and cognition. J. Cereb. Blood Flow Metab. 2013, 33, 1666–1684. [Google Scholar] [CrossRef] [Green Version]
- Gorelick, P.B.; Counts, S.E.; Nyenhuis, D. Vascular cognitive impairment and dementia. Biochim. Et Biophys. Acta 2016, 1862, 860–868. [Google Scholar] [CrossRef]
- Venkat, P.; Chopp, M.; Chen, J. Models and mechanisms of vascular dementia. Exp. Neurol. 2015, 272, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Poulet, R.; Gentile, M.T.; Vecchione, C.; Distaso, M.; Aretini, A.; Fratta, L.; Russo, G.; Echart, C.; Maffei, A.; De Simoni, M.G.; et al. Acute hypertension induces oxidative stress in brain tissues. J. Cereb. Blood Flow Metab. 2006, 26, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnevale, D.; Mascio, G.; Ajmone-Cat, M.A.; D’Andrea, I.; Cifelli, G.; Madonna, M.; Cocozza, G.; Frati, A.; Carullo, P.; Carnevale, L.; et al. Role of neuroinflammation in hypertension-Induced brain amyloid pathology. Neurobiol. Aging 2012, 33, e219–e229. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, D.; Mascio, G.; D’Andrea, I.; Fardella, V.; Bell, R.D.; Branchi, I.; Pallante, F.; Zlokovic, B.; Yan, S.S.; Lembo, G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 2012, 60, 188–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Montgolfier, O.; Pincon, A.; Pouliot, P.; Gillis, M.A.; Bishop, J.; Sled, J.G.; Villeneuve, L.; Ferland, G.; Levy, B.I.; Lesage, F.; et al. High Systolic Blood Pressure Induces Cerebral Microvascular Endothelial Dysfunction, Neurovascular Unit Damage, and Cognitive Decline in Mice. Hypertension 2019, 73, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Noyan-Ashraf, M.H.; Meissner, A.; Voigtlaender-Bolz, J.; Kroetsch, J.T.; Foltz, W.; Jaffray, D.; Kapoor, A.; Momen, A.; Heximer, S.P.; et al. Proximal cerebral arteries develop myogenic responsiveness in heart failure via tumor necrosis factor-alpha-dependent activation of sphingosine-1-Phosphate signaling. Circulation 2012, 126, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okamoto, K.; Hazama, F.; Yamori, Y.; Haebara, H.; Nagaoka, A. Pathogenesis and prevention of stroke in spontaneously hypertensive rats. Clin. Sci. Mol. Med. 1975, 2, 161s–163s. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.F.; McCabe, C.; Khatun, H.; Kaushal, N.; Bridges, L.R.; Holmes, W.M.; Barrick, T.R.; Graham, D.; Dominiczak, A.F.; Mhairi Macrae, I.; et al. An MRI-Histological study of white matter in stroke-Free SHRSP. J. Cereb. Blood Flow Metab. 2013, 33, 760–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemper, T.L.; Blatt, G.J.; Killiany, R.J.; Moss, M.B. Neuropathology of progressive cognitive decline in chronically hypertensive rhesus monkeys. Acta Neuropathol. 2001, 101, 145–153. [Google Scholar]
- Grootendorst, J.; De Kloet, E.R.; Dalm, S.; Oitzl, M.S. Reversal of cognitive deficit of apolipoprotein E knockout mice after repeated exposure to a common environmental experience. Neuroscience 2001, 108, 237–247. [Google Scholar] [CrossRef]
- Lee, E.S.; Yoon, J.H.; Choi, J.; Andika, F.R.; Lee, T.; Jeong, Y. A mouse model of subcortical vascular dementia reflecting degeneration of cerebral white matter and microcirculation. J. Cereb. Blood Flow Metab. 2019, 39, 44–57. [Google Scholar] [CrossRef]
- Mulder, M.; Jansen, P.J.; Janssen, B.J.; Van De Berg, W.D.; Van Der Boom, H.; Havekes, L.M.; De Kloet, R.E.; Ramaekers, F.C.; Blokland, A. Low-Density lipoprotein receptor-Knockout mice display impaired spatial memory associated with a decreased synaptic density in the hippocampus. Neurobiol. Dis. 2004, 16, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Alexis, N.E.; Dietrich, W.D.; Green, E.J.; Prado, R.; Watson, B.D. Nonocclusive common carotid artery thrombosis in the rat results in reversible sensorimotor and cognitive behavioral deficits. Stroke 1995, 26, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ding, F.; Deng, S.; Guo, X.; Wang, W.; Iliff, J.J.; Nedergaard, M. Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J. Neurosci. 2017, 37, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, V.L.; Goldberg, A.P.; Rogers, M.W.; Anthony, L.; Terrin, M.L.; Guralnik, J.M.; Blackwelder, W.C.; Lam, D.F.H.; Sikdar, S.; Lal, B.K. Asymptomatic carotid stenosis is associated with mobility and cognitive dysfunction and heightens falls in older adults. J. Vasc. Surg. 2019. [Google Scholar] [CrossRef]
- Demarin, V.; Zavoreo, I.; Kes, V.B. Carotid artery disease and cognitive impairment. J. Neurol. Sci. 2012, 322, 107–111. [Google Scholar] [CrossRef]
- Chopard, R.; Piazza, G.; Gale, S.A.; Campia, U.; Albertsen, I.E.; Kim, J.; Goldhaber, S.Z. Dementia and Atrial Fibrillation: Pathophysiological Mechanisms and Therapeutic Implications. Am. J. Med. 2018, 131, 1408–1417. [Google Scholar] [CrossRef]
- De Bruijn, R.F.; Heeringa, J.; Wolters, F.J.; Franco, O.H.; Stricker, B.H.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Association Between Atrial Fibrillation and Dementia in the General Population. JAMA Neurol. 2015, 72, 1288–1294. [Google Scholar] [CrossRef]
- Adelborg, K.; Horvath-Puho, E.; Ording, A.; Pedersen, L.; Sorensen, H.T.; Henderson, V.W. Heart failure and risk of dementia: A Danish nationwide population-based cohort study. Eur. J. Heart Fail. 2017, 19, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Wolters, F.J.; Zonneveld, H.I.; Hofman, A.; Van Der Lugt, A.; Koudstaal, P.J.; Vernooij, M.W.; Ikram, M.A. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation 2017, 136, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Fan, S.; Li, J.; Wang, Y.L. Bilateral Common Carotid Artery Occlusion in the Rat as a Model of Retinal Ischaemia. Neuro Ophthalmol. 2014, 38, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choy, M.; Ganesan, V.; Thomas, D.L.; Thornton, J.S.; Proctor, E.; King, M.D.; Van Der Weerd, L.; Gadian, D.G.; Lythgoe, M.F. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J. Cereb. Blood Flow Metab. 2006, 26, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Tomimoto, H.; Ihara, M.; Wakita, H.; Ohtani, R.; Lin, J.X.; Akiguchi, I.; Kinoshita, M.; Shibasaki, H. Chronic cerebral hypoperfusion induces white matter lesions and loss of oligodendroglia with DNA fragmentation in the rat. Acta Neuropathol. 2003, 106, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, N.; Li, S.; Han, R.; Huang, Q.; Hu, J.; Jin, K.; Ji, X. Limb Ischemic Conditioning Improved Cognitive Deficits via eNOS-Dependent Augmentation of Angiogenesis after Chronic Cerebral Hypoperfusion in Rats. Aging Dis. 2018, 9, 869–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.X.; Zhang, J.J.; Zheng, P.; Zhang, Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res. 2005, 139, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Yamasaki, N.; Miyakawa, T.; Kalaria, R.N.; Fujita, Y.; Ohtani, R.; Ihara, M.; Takahashi, R.; Tomimoto, H. Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke 2007, 38, 2826–2832. [Google Scholar] [CrossRef] [Green Version]
- Ihara, M.; Taguchi, A.; Maki, T.; Washida, K.; Tomimoto, H. A mouse model of chronic cerebral hypoperfusion characterizing features of vascular cognitive impairment. Methods Mol. Biol. 2014, 1135, 95–102. [Google Scholar]
- Kim, G.H.; Lee, J.H.; Seo, S.W.; Kim, J.H.; Seong, J.K.; Ye, B.S.; Cho, H.; Noh, Y.; Kim, H.J.; Yoon, C.W.; et al. Hippocampal volume and shape in pure subcortical vascular dementia. Neurobiol. Aging 2015, 36, 485–491. [Google Scholar] [CrossRef]
- Fleischman, D.A.; Yang, J.; Arfanakis, K.; Arvanitakis, Z.; Leurgans, S.E.; Turner, A.D.; Barnes, L.L.; Bennett, D.A.; Buchman, A.S. Physical activity, motor function, and white matter hyperintensity burden in healthy older adults. Neurology 2015, 84, 1294–1300. [Google Scholar] [CrossRef] [Green Version]
- Dewar, D.; Yam, P.; McCulloch, J. Drug development for stroke: Importance of protecting cerebral white matter. Eur. J. Pharmacol. 1999, 375, 41–50. [Google Scholar] [CrossRef]
- Kitagawa, K.; Matsumoto, M.; Yang, G.; Mabuchi, T.; Yagita, Y.; Hori, M.; Yanagihara, T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: Evaluation of the patency of the posterior communicating artery. J. Cereb. Blood Flow Metab. 1998, 18, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Ihara, M.; Yamasaki, N.; Kalaria, R.N.; Maki, T.; Fujita, Y.; Ito, H.; Oishi, N.; Fukuyama, H.; Miyakawa, T.; et al. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke 2010, 41, 1278–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Kimura-Ohba, S.; Thompson, J.; Rosenberg, G.A. Rodent Models of Vascular Cognitive Impairment. Transl. Stroke Res. 2016, 7, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Enmi, J.; Iguchi, S.; Saito, S.; Yamamoto, Y.; Nagatsuka, K.; Iida, H.; Ihara, M. Substantial Reduction of Parenchymal Cerebral Blood Flow in Mice with Bilateral Common Carotid Artery Stenosis. Sci. Rep. 2016, 6, 32179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washida, K.; Ihara, M.; Nishio, K.; Fujita, Y.; Maki, T.; Yamada, M.; Takahashi, J.; Wu, X.; Kihara, T.; Ito, H.; et al. Nonhypotensive dose of telmisartan attenuates cognitive impairment partially due to peroxisome proliferator-Activated receptor-Gamma activation in mice with chronic cerebral hypoperfusion. Stroke 2010, 41, 1798–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, A.; Manso, Y.; Duncombe, J.; Searcy, J.; Koudelka, J.; Binnie, M.; Webster, S.; Lennen, R.; Jansen, M.; Marshall, I.; et al. Long-Term cilostazol treatment reduces gliovascular damage and memory impairment in a mouse model of chronic cerebral hypoperfusion. Sci. Rep. 2017, 7, 4299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, Y.; Ihara, M.; Ushiki, T.; Hirai, H.; Kizaka-Kondoh, S.; Hiraoka, M.; Ito, H.; Takahashi, R. Early protective effect of bone marrow mononuclear cells against ischemic white matter damage through augmentation of cerebral blood flow. Stroke 2010, 41, 2938–2943. [Google Scholar] [CrossRef] [Green Version]
- Hase, Y.; Craggs, L.; Hase, M.; Stevenson, W.; Slade, J.; Chen, A.; Liang, D.; Ennaceur, A.; Oakley, A.; Ihara, M.; et al. The effects of environmental enrichment on white matter pathology in a mouse model of chronic cerebral hypoperfusion. J. Cereb. Blood Flow Metab. 2018, 38, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Hase, Y.; Craggs, L.; Hase, M.; Stevenson, W.; Slade, J.; Lopez, D.; Mehta, R.; Chen, A.; Liang, D.; Oakley, A.; et al. Effects of environmental enrichment on white matter glial responses in a mouse model of chronic cerebral hypoperfusion. J. Neuroinflammation 2017, 14, 81. [Google Scholar] [CrossRef] [Green Version]
- Duncombe, J.; Kitamura, A.; Hase, Y.; Ihara, M.; Kalaria, R.N.; Horsburgh, K. Chronic cerebral hypoperfusion: A key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin. Sci. 2017, 131, 2451–2468. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Ng, G.; Tan, E.K.; Liao, P.; Kandiah, N.; Zeng, L. Chronic cerebral hypoperfusion enhances Tau hyperphosphorylation and reduces autophagy in Alzheimer’s disease mice. Sci. Rep. 2016, 6, 23964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, J.; Yamashita, T.; Zhai, Y.; Nakano, Y.; Morihara, R.; Fukui, Y.; Hishikawa, N.; Ohta, Y.; Abe, K. Strong Impact of Chronic Cerebral Hypoperfusion on Neurovascular Unit, Cerebrovascular Remodeling, and Neurovascular Trophic Coupling in Alzheimer’s Disease Model Mouse. J. Alzheimers Dis. 2016, 52, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ihara, M.; Okamoto, Y.; Maki, T.; Washida, K.; Kitamura, A.; Hase, Y.; Ito, H.; Takao, K.; Miyakawa, T.; et al. The influence of chronic cerebral hypoperfusion on cognitive function and amyloid beta metabolism in APP overexpressing mice. PLoS ONE 2011, 6, e16567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, Y.; Okamoto, Y.; Maki, T.; Yamamoto, Y.; Oishi, N.; Yamahara, K.; Nagatsuka, K.; Takahashi, R.; Kalaria, R.N.; Fukuyama, H.; et al. Silent information regulator 2 homolog 1 counters cerebral hypoperfusion injury by deacetylating endothelial nitric oxide synthase. Stroke 2014, 45, 3403–3411. [Google Scholar] [CrossRef] [Green Version]
- Bannai, T.; Mano, T.; Chen, X.; Ohtomo, G.; Ohtomo, R.; Tsuchida, T.; Koshi-Mano, K.; Hashimoto, T.; Okazawa, H.; Iwatsubo, T.; et al. Chronic cerebral hypoperfusion shifts the equilibrium of amyloid beta oligomers to aggregation-Prone species with higher molecular weight. Sci. Rep. 2019, 9, 2827. [Google Scholar] [CrossRef]
- Maki, T.; Takahashi, Y.; Miyamoto, N.; Liang, A.C.; Ihara, M.; Lo, E.H.; Arai, K. Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-Basic-Protein expressing oligodendrocytes under pathological conditions in vitro. Stem Cell Res. 2015, 15, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Maki, T.; Ihara, M.; Fujita, Y.; Nambu, T.; Miyashita, K.; Yamada, M.; Washida, K.; Nishio, K.; Ito, H.; Harada, H.; et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke 2011, 42, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, K.; Itoh, H.; Arai, H.; Suganami, T.; Sawada, N.; Fukunaga, Y.; Sone, M.; Yamahara, K.; Yurugi-Kobayashi, T.; Park, K.; et al. The neuroprotective and vasculo-Neuro-Regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology 2006, 147, 1642–1653. [Google Scholar] [CrossRef] [Green Version]
- Kondoh, T.; Ueta, Y.; Torii, K. Pre-Treatment of adrenomedullin suppresses cerebral edema caused by transient focal cerebral ischemia in rats detected by magnetic resonance imaging. Brain Res. Bull. 2011, 84, 69–74. [Google Scholar] [CrossRef]
- Stroke Therapy Academic Industry Roundtable (STAIR). Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999, 30, 2752–2758. [Google Scholar] [CrossRef]
- Madigan, J.B.; Wilcock, D.M.; Hainsworth, A.H. Vascular Contributions to Cognitive Impairment and Dementia: Topical Review of Animal Models. Stroke 2016, 47, 1953–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndung’u, M.; Hartig, W.; Wegner, F.; Mwenda, J.M.; Low, R.W.; Akinyemi, R.O.; Kalaria, R.N. Cerebral amyloid beta(42) deposits and microvascular pathology in ageing baboons. Neuropathol. Appl. Neurobiol. 2012, 38, 487–499. [Google Scholar] [CrossRef] [PubMed]




| Animal Models | Rat BCAO | Mouse BCAS | Mouse ACAS | Baboon 3-VO |
|---|---|---|---|---|
| CBF reduction (% preoperative level) | Rapid reduction to 30–50% of the original level with gradual recovery | Rapid reduction to 70% of the original level with gradual recovery by 0.18 mm microcoils | Gradual reduction to 60% (AC side) and 70% (microcoil side) of the original level without recovery | Under investigation |
| White matter lesion | Demyelination appears 14 days after surgery | Demyelination appears 14 days after surgery | Multiple infarcts and demyelination appears 14 days after surgery | Demyelination appears 14 days after surgery |
| Gray matter lesion | Rare | No gray matter infarction by microcoils with an inner diameter of 0.18 mm or more | Multiple infarcts only on the AC side | Rare |
| Cognitive dysfunction | Spatial working memory impairment | Spatial working memory impairment | Spatial working/reference memory impairment | Under investigation |
| Motor dysfunction | No motor deficits | No motor deficits before 3 months | Muscle weakness and gait disturbance | Temporal hemiparesis after surgery |
| Animal Models | Rat BCAO | Mouse BCAS | Mouse ACAS | Baboon 3-VO |
|---|---|---|---|---|
| Difficulty of surgery | Relatively easy, ligation of both CCAs | Moderate, placement of microcoils on both CCAs | Relarively high, placement of AC on the right CCA and microcoil on the left CCA | Difficult, ligation of both ICAs and left VA |
| Surgery time | 10 min | 10 min | 10 min | Not established |
| Cost | No cost | $50 (microcoil $25 × 2) | $125 (AC $100 + microcoil $25) | Not established |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washida, K.; Hattori, Y.; Ihara, M. Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate. Int. J. Mol. Sci. 2019, 20, 6176. https://doi.org/10.3390/ijms20246176
Washida K, Hattori Y, Ihara M. Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate. International Journal of Molecular Sciences. 2019; 20(24):6176. https://doi.org/10.3390/ijms20246176
Chicago/Turabian StyleWashida, Kazuo, Yorito Hattori, and Masafumi Ihara. 2019. "Animal Models of Chronic Cerebral Hypoperfusion: From Mouse to Primate" International Journal of Molecular Sciences 20, no. 24: 6176. https://doi.org/10.3390/ijms20246176