Love at First Taste: Induction of Larval Settlement by Marine Microbes
Abstract
:1. Introduction
2. Meta-Analysis of Recent Publications
3. Induction of Larval Settlement by Bacteria and Biofilms
3.1. Multispecies Biofilms and Macrofouler Settlement
3.2. Monospecies Bacterial Films and Macrofouler Settlement
4. Microbial Eukaryotes and Larval Settlement
5. Bioactive Compounds from Biofilms Inducing Settlement and Metamorphosis
5.1. Bacterial Chemical Signals
5.2. Quorum Sensing and Settlement
6. Molecular Aspects of Induction of Settlement
7. Impact of Climate Change on Biofilms and Larval Settlement
8. Future Studies
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wahl, M. Marine epibiosis. I. Fouling and antifouling: Some basic aspects. Mar. Ecol. Prog. Ser. 1989, 58, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Clare, A.S.; Rittschof, D.; Gerhart, D.J.; Maki, J.S. Molecular approaches to nontoxic antifouling. Invertebr. Reprod. Dev. 1992, 22, 67–76. [Google Scholar] [CrossRef]
- Salta, M.; Wharton, J.A.; Blache, Y.; Stokes, K.R.; Briand, J.-F. Marine biofilms on artificial surfaces: Structure and dynamics. Environ. Microbiol. 2013, 15, 2879–2893. [Google Scholar] [CrossRef]
- Antunes, J.; Leão, P.; Vasconcelos, V. Marine biofilms: Diversity of communities and of chemical cues. Environ. Microbiol. Rep. 2019, 11, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Neu, T.R.; Wozniak, D.J. The EPS matrix: The “house of biofilm cells ”. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [Green Version]
- Dobretsov, S.; Dahms, H.-U.; Qian, P.-Y. Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 2006, 22, 43–54. [Google Scholar] [CrossRef]
- Rittschof, D. Trypsins: Keystone enzymes in marine communities. JSM Enzymol. Protein Sci. 2017, 2, 1009. [Google Scholar]
- Zobell, C.E.; Allen, E.C. The significance of marine bacteria in the fouling of submerged surfaces. J. Bacteriol. 1935, 29, 239–251. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, G.A. Classic spotlight: Before they were biofilms. J. Bacteriol. 2015, 198, 5. [Google Scholar] [CrossRef] [Green Version]
- Qian, P.-Y.; Lau, S.C.K.; Dahms, H.-U.; Dobretsov, S.; Harder, T. Marine biofilms as mediators of colonization by marine macroorganisms: Implications for antifouling and aquaculture. Mar. Biotechnol. 2007, 9, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.J.; Chang, C.; Akiyama, T.; Bothner, B. New technologies for studying biofilms. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briand, J.-F.; Pochon, X.; Wood, S.A.; Bressy, C.; Garnier, C.; Réhel, K.; Urvois, F.; Culioli, G.; Zaiko, A. Metabarcoding and metabolomics offer complementarity in deciphering marine eukaryotic biofouling community shifts. Biofouling 2018, 34, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Brauer, J.I.; Makama, Z.; Bonifay, V.; Aydin, E.; Kaufman, E.D.; Beech, I.B.; Sunner, J. Mass spectrometric metabolomic imaging of biofilms on corroding steel surfaces using laser ablation and solvent capture by aspiration. Biointerphases 2015, 10, 019003. [Google Scholar] [CrossRef]
- Hmelo, L.R. Quorum sensing in marine microbial environments. Annu. Rev. Mar. Sci. 2017, 9, 257–281. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Paul, V. Mini-review: Quorum sensing in the marine environment and its relationship to biofouling. Biofouling 2009, 25, 413–427. [Google Scholar] [CrossRef]
- Kjelleberg, S.; Molin, S. Is there a role for quorum sensing signals in bacterial biofilms? Curr. Opin. Microbiol. 2002, 5, 254–258. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Bayer, M.; Gunasekera, S.; Proksch, P.; Paul, V.J. Inhibition of marine biofouling by bacterial quorum sensing inhibitors. Biofouling 2011, 27, 893–905. [Google Scholar] [CrossRef]
- Grandclément, C.; Tannières, M.; Moréra, S.; Dessaux, Y.; Faure, D. Quorum quenching: Role in nature and applied developments. FEMS Microbiol. Rev. 2016, 40, 86–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, B.; Lu, Y.; Guo, Y.; Sun, J.; Wei, B.; Zhang, H.; Wang, H. Quorum sensing inhibitors from marine microorganisms and their synthetic derivatives. Mar. Drugs 2019, 17, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisp, D.J. The larval stages of balanus hameri (ascanius, 1767). Crustaceana 1962, 4, 123–130. [Google Scholar] [CrossRef]
- Thorson, G. Reproductive and larval ecology of marine bottom invertebrates. Biol. Rev. 1950, 25, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, M.G. Biofilms and marine invertebrate larvae: What bacteria produce that larvae use to choose settlement sites. Annu. Rev. Mar. Sci. 2011, 3, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, S.K.; Todd, C.D. Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling 1998, 12, 81–118. [Google Scholar] [CrossRef]
- Mullineaux, L.S. The role of settlement in structuring a hard-substratum community in the deep sea. J. Exp. Mar. Biol. Ecol. 1988, 120, 247–261. [Google Scholar] [CrossRef]
- Rittschof, D.; Forward, R.B., Jr.; Cannon, G.; Welch, J.M.; McClary, M., Jr.; Holm, E.R.; Clare, A.S.; Conova, S.; McKelvey, L.M.; Bryan, P.; et al. Cues and context: Larval responses to physical and chemical cues. Biofouling 1998, 12, 31–44. [Google Scholar] [CrossRef]
- Rittschof, D.; Hooper, I.R.; Costlow, J.D. Barnacle settlement inhibitors from sea pansies, Renilla reniformis. Bull. Mar. Sci. 1986, 39, 376–382. [Google Scholar]
- Roberts, D.; Rittschof, D.; Holm, E.; Schmidt, A.R. Factors influencing initial larval settlement: Temporal, spatial and surface molecular components. J. Exp. Mar. Biol. Ecol. 1991, 150, 203–221. [Google Scholar] [CrossRef]
- Tamburri, M.N.; Luckenbach, M.W.; Breitburg, D.L.; Bonniwell, S.M. Settlement of Crassostrea ariakensis larvae: Effects of substrate, biofilms, sediment and adult chemical cues. J. Shellfish Res. 2008, 27, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Dobretsov, S.V. Effects of macroalgae and biofilm on settlement of blue mussel (Mytilus edulis L.) larvae. Biofouling 1999, 14, 153–165. [Google Scholar] [CrossRef]
- Unabia, C.R.C.; Hadfield, M.G. Role of bacteria in larval settlement and metamorphosis of the polychaete Hydroides elegans. Mar. Biol. 1999, 133, 55–64. [Google Scholar] [CrossRef]
- Alberte, R.S.; Snyder, S.; Zahuranec, B.J.; Whetstone, M. Biofouling research needs for the United States Navy: Program history and goals. Biofouling 1992, 6, 91–95. [Google Scholar] [CrossRef]
- Maréchal, J.-P.; Hellio, C. Challenges for the development of new non-toxic antifouling solutions. Int. J. Mol. Sci. 2009, 10, 4623–4637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Muthukrishnan, T.; Abed, R.M.M.; Dobretsov, S.; Kidd, B.; Finnie, A.A. Long-term microfouling on commercial biocidal fouling control coatings. Biofouling 2014, 30, 1155–1164. [Google Scholar] [CrossRef]
- Mieszkin, S.; Callow, M.E.; Callow, J.A. Interactions between microbial biofilms and marine fouling algae: A mini review. Biofouling 2013, 29, 1097–1113. [Google Scholar] [CrossRef]
- Maki, J.S.; Mitchell, R. Biofouling in the marine environment. In Encyclopedia of Environmental Microbiology; American Cancer Society: New York, NY, USA, 2003; ISBN 978-0-471-26339-5. [Google Scholar]
- Bakus, G.J.; Targett, N.M.; Schulte, B. Chemical ecology of marine organisms: An overview. J. Chem. Ecol. 1986, 12, 951–987. [Google Scholar] [CrossRef]
- Standing, J.D.; Hooper, I.R.; Costlow, J.D. Inhibition and induction of barnacle settlement by natural products present in octocorals. J. Chem. Ecol. 1984, 10, 823–834. [Google Scholar] [CrossRef]
- Dobretsov, S.; Abed, R.M.M.; Teplitski, M. Mini-review: Inhibition of biofouling by marine microorganisms. Biofouling 2013, 29, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Qian, P.-Y.; Li, Z.; Xu, Y.; Li, Y.; Fusetani, N. Mini-review: Marine natural products and their synthetic analogs as antifouling compounds: 2009–2014. Biofouling 2015, 31, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Goecke, F.; Bhadury, P. Minireview: Algal natural compounds and extracts as antifoulants. J. Appl. Phycol. 2018, 30, 1859–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satheesh, S.; Ba-akdah, M.A.; Al-Sofyani, A.A. Natural antifouling compound production by microbes associated with marine macroorganisms—A review. Electron. J. Biotechnol. 2016, 21, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-L.; Wu, Z.-H.; Wang, Y.; Wang, C.-Y.; Xu, Y. Mini-review: Antifouling natural products from marine microorganisms and their synthetic analogs. Mar. Drugs 2017, 15, 266. [Google Scholar] [CrossRef] [Green Version]
- Hay, M.E. Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annu. Rev. Mar. Sci. 2009, 1, 193–212. [Google Scholar] [CrossRef] [Green Version]
- Paul, V.J.; Ritson-Williams, R.; Sharp, K. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2011, 28, 345–387. [Google Scholar] [CrossRef]
- Paul, V.J.; Puglisi, M.P.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2006, 23, 153–180. [Google Scholar] [CrossRef]
- Paul, V.J.; Ritson-Williams, R. Marine chemical ecology. Nat. Prod. Rep. 2008, 25, 662–695. [Google Scholar] [CrossRef]
- Hadfield, M.; Paul, V. Natural chemical cues for settlement and metamorphosis of marine-invertebrate larvae. In Marine Chemical Ecology; McClintock, J., Baker, B., Eds.; CRC Press: Boca Raton, FL, USA, 2001; Volume 20015660, pp. 431–461. ISBN 978-0-8493-9064-7. [Google Scholar]
- Chandramouli, K.H.; Qian, P.-Y.; Ravasi, T. Proteomics insights: Proteins related to larval attachment and metamorphosis of marine invertebrates. Front. Mar. Sci. 2014, 1. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Qian, P.-Y. Review on molecular mechanisms of antifouling compounds: An update since 2012. Mar. Drugs 2017, 15, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, P.-Y.; Chen, L.; Xu, Y. Mini-review: Molecular mechanisms of antifouling compounds. Biofouling 2013, 29, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, V. A review on the role of chemical cues in habitat selection by barnacles: New insights from larval proteomics. J. Exp. Mar. Biol. Ecol. 2010, 392, 22–36. [Google Scholar] [CrossRef]
- Holm, E. Barnacles and biofouling. Integr. Comp. Biol. 2012, 52, 348–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, O.O.; Chung, H.C.; Yang, J.; Wang, Y.; Dash, S.; Wang, H.; Qian, P.-Y. Molecular techniques revealed highly diverse microbial communities in natural marine biofilms on polystyrene dishes for invertebrate larval settlement. Microb. Ecol. 2014, 68, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bao, W.-Y.; Gu, Z.-Q.; Li, Y.-F.; Liang, X.; Ling, Y.; Cai, S.-L.; Shen, H.-D.; Yang, J.-L. Larval settlement and metamorphosis of the mussel Mytilus coruscus in response to natural biofilms. Biofouling 2012, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.C.; Lee, O.O.; Huang, Y.-L.; Mok, S.Y.; Kolter, R.; Qian, P.-Y. Bacterial community succession and chemical profiles of subtidal biofilms in relation to larval settlement of the polychaete Hydroides elegans. ISME J. 2010, 4, 817–828. [Google Scholar] [CrossRef] [Green Version]
- Toupoint, N.; Mohit, V.; Linossier, I.; Bourgougnon, N.; Myrand, B.; Olivier, F.; Lovejoy, C.; Tremblay, R. Effect of biofilm age on settlement of Mytilus edulis. Biofouling 2012, 28, 985–1001. [Google Scholar] [CrossRef]
- Whalan, S.; Webster, N.S. Sponge larval settlement cues: The role of microbial biofilms in a warming ocean. Sci. Rep. 2014, 4, 4072. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.J.; Harder, T.; Steinberg, P.D. Sea urchin larvae decipher the epiphytic bacterial community composition when selecting sites for attachment and metamorphosis. FEMS Microbiol. Ecol. 2015, 91, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lema, K.A.; Constancias, F.; Rice, S.A.; Hadfield, M.G. High bacterial diversity in nearshore and oceanic biofilms and their influence on larval settlement by Hydroides elegans (Polychaeta). Environ. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hadfield, M.G. Composition and density of bacterial biofilms determine larval settlement of the polychaete Hydroides elegans. Mar. Ecol. Prog. Ser. 2003, 260, 161–172. [Google Scholar] [CrossRef]
- Lau, S.C.K.; Mak, K.K.W.; Chen, F.; Qian, P.-Y. Bioactivity of bacterial strains isolated from marine biofilms in Hong Kong waters for the induction of larval settlement in the marine polychaete Hydroides elegans. Mar. Ecol. Prog. Ser. 2002, 226, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.-Y.; Lee, O.-O.; Chung, H.-C.; Li, M.; Qian, P.-Y. Copper affects biofilm inductiveness to larval settlement of the serpulid polychaete Hydroides elegans (Haswell). Biofouling 2010, 26, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Sharp, K.H.; Sneed, J.M.; Ritchie, K.B.; Mcdaniel, L.; Paul, V.J. Induction of larval settlement in the reef coral Porites astreoides by a cultivated marine roseobacter strain. Biol. Bull. 2015, 228, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-L.; Shen, P.-J.; Liang, X.; Li, Y.-F.; Bao, W.-Y.; Li, J.-L. Larval settlement and metamorphosis of the mussel Mytilus coruscus in response to monospecific bacterial biofilms. Biofouling 2013, 29, 247–259. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Li, M.; Yu, Z.; Qian, P.-Y. Correlation between pigmentation and larval settlement deterrence by Pseudoalteromonas sp. sf57. Biofouling 2011, 27, 287–293. [Google Scholar] [CrossRef]
- Ghequire, M.G.K.; De Mot, R. The tailocin tale: Peeling off phage tails. Trends Microbiol. 2015, 23, 587–590. [Google Scholar] [CrossRef] [Green Version]
- Shikuma, N.J.; Antoshechkin, I.; Medeiros, J.M.; Pilhofer, M.; Newman, D.K. Stepwise metamorphosis of the tubeworm Hydroides elegans is mediated by a bacterial inducer and MAPK signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 10097–10102. [Google Scholar] [CrossRef] [Green Version]
- Shikuma, N.J.; Pilhofer, M.; Weiss, G.L.; Hadfield, M.G.; Jensen, G.J.; Newman, D.K. Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 2014, 343, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Freckelton, M.L.; Nedved, B.T.; Hadfield, M.G. Induction of invertebrate larval settlement; Different bacteria, different mechanisms? Sci. Rep. 2017, 7, 42557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Callahan, S.; Hadfield, M.G. Recruitment in the sea: Bacterial genes required for inducing larval settlement in a polychaete worm. Sci. Rep. 2012, 2, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harder, T.; Lam, C.; Qian, P.-Y. Induction of larval settlement in the polychaete Hydroides elegans by marine biofilms: An investigation of monospecific diatom films as settlement cues. Mar. Ecol. Prog. Ser. 2002, 229, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.; Harder, T.; Qian, P.-Y. Induction of larval settlement in the polychaete Hydroides elegans by extracellular polymers of benthic diatoms. Mar. Ecol. Prog. Ser. 2005, 286, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, W.G.; Buen, S.M.A. Evaluation of mucus, Navicula, and mixed diatoms as larval settlement inducers for the tropical abalone Haliotis asinina. Aquaculture 2003, 221, 357–364. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Riquelmes, C.; Silva, F.; Avendañod, M.; Irgang, R. Optimization of settlement of larval Argopecten purpuratus using natural diatom biofilms. J. Shellfish Res. 2003, 22, 393–399. [Google Scholar]
- Jouuchi, T.; Satuito, C.G.; Kitamura, H. Sugar compound products of the periphytic diatom Navicula ramosissima induce larval settlement in the barnacle, Amphibalanus amphitrite. Mar. Biol. 2007, 152, 1065–1076. [Google Scholar] [CrossRef]
- Kitamura, H.; Hirayama, K. Effect of primary films on the settlement of larvae of a bryozoan Bugula neritina. Nippon Suisan Gakkaishi 1987, 53, 1377–1381. [Google Scholar] [CrossRef] [Green Version]
- Dahms, H.-U.; Dobretsov, S.; Qian, P.-Y. The effect of bacterial and diatom biofilms on the settlement of the bryozoan Bugula neritina. J. Exp. Mar. Biol. Ecol. 2004, 313, 191–209. [Google Scholar] [CrossRef]
- Railkin, A.I. Marine Biofouling: Colonization Processes and Defenses; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-203-50323-2. [Google Scholar]
- Watson, M.G.; Scardino, A.J.; Zalizniak, L.; Shimeta, J. Inhibition of invertebrate larval settlement by biofilm ciliates. Mar. Ecol. Prog. Ser. 2016, 557, 77–90. [Google Scholar] [CrossRef]
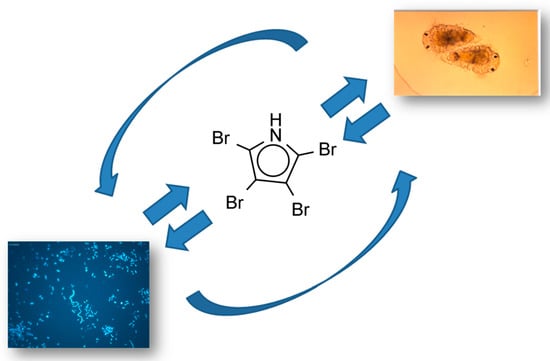
- Tebben, J.; Tapiolas, D.M.; Motti, C.A.; Abrego, D.; Negri, A.P.; Blackall, L.L.; Steinberg, P.D.; Harder, T. Induction of larval metamorphosis of the coral Acropora millepora by tetrabromopyrrole isolated from a Pseudoalteromonas Bacterium. PLoS ONE 2011, 6, e19082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sneed Jennifer, M.; Sharp Koty, H.; Ritchie Kimberly, B.; Paul Valerie, J. The chemical cue tetrabromopyrrole from a biofilm bacterium induces settlement of multiple Caribbean corals. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133086. [Google Scholar] [CrossRef] [PubMed]
- Siboni, N.; Abrego, D.; Seneca, F.; Motti, C.A.; Andreakis, N.; Tebben, J.; Blackall, L.L.; Harder, T. Using bacterial extract along with differential gene expression in Acropora millepora larvae to decouple the processes of attachment and metamorphosis. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchman, D.; Graham, S.; Reish, D.; Mitchell, R. Bacteria induce settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube (Polychaeta:Spirprbidae). J. Exp. Mar. Biol. Ecol. 1981, 56, 153–163. [Google Scholar] [CrossRef]
- Eberhard, A.; Burlingame, A.L.; Eberhard, C.; Kenyon, G.L.; Nealson, K.H.; Oppenheimer, N.J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 1981, 20, 2444–2449. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Grossart, H.-P.; Schlingloff, A.; Kiørboe, T. Possible quorum sensing in marine snow bacteria: Production of acylated homoserine lactones by Roseobacter strains isolated from marine snow. Appl. Environ. Microbiol. 2002, 68, 4111–4116. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.M.; Cicirelli, E.M.; Kan, J.; Chen, F.; Fuqua, C.; Hill, R.T. Diversity and quorum-sensing signal production of proteobacteria associated with marine sponges. Environ. Microbiol. 2008, 10, 75–86. [Google Scholar] [CrossRef]
- Taylor, M.W.; Schupp, P.J.; Baillie, H.J.; Charlton, T.S.; de Nys, R.; Kjelleberg, S.; Steinberg, P.D. Evidence for Acyl homoserine lactone signal production in bacteria associated with marine sponges. Appl. Environ. Microbiol. 2004, 70, 4387–4389. [Google Scholar] [CrossRef] [Green Version]
- Golberg, K.; Eltzov, E.; Shnit-Orland, M.; Marks, R.S.; Kushmaro, A. Characterization of quorum sensing signals in coral-associated bacteria. Microb. Ecol. 2011, 61, 783–792. [Google Scholar] [CrossRef]
- Wagner-Döbler, I.; Thiel, V.; Eberl, L.; Allgaier, M.; Bodor, A.; Meyer, S.; Ebner, S.; Hennig, A.; Pukall, R.; Schulz, S. Discovery of complex mixtures of novel long-chain quorum sensing signals in free-living and host-associated marine alphaproteobacteria. ChemBioChem 2005, 6, 2195–2206. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Dobretsov, S.; Ki, J.-S.; Yang, L.-H.; Qian, P.-Y. Presence of Acyl-homoserine lactone in subtidal biofilm and the implication in larval behavioral response in the polychaete Hydroides elegans. Microb. Ecol. 2007, 54, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Joint, I.; Tait, K.; Wheeler, G. Cross-kingdom signalling: Exploitation of bacterial quorum sensing molecules by the green seaweed Ulva. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1223–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, K.; Joint, I.; Daykin, M.; Milton, D.L.; Williams, P.; Cámara, M. Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms. Environ. Microbiol. 2005, 7, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Tait, K.; Williams, P.; Atkinson, S.; Cámara, M. Interference with the germination and growth of Ulva zoospores by quorum-sensing molecules from Ulva-associated epiphytic bacteria. Environ. Microbiol. 2014, 16, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinberger, F.; Beltran, J.; Correa, J.A.; Lion, U.; Pohnert, G.; Kumar, N.; Steinberg, P.; Kloareg, B.; Potin, P. Spore release in Acrochaetium sp. (Rhodophyta) is bacterially controlled. J. Phycol. 2007, 43, 235–241. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, Z.; He, J.; Rittschof, D.; Su, P.; Ke, C.; Feng, D. Conspecific cues that induce spore settlement in the biofouling and green tide-forming alga Ulva tepida provide a potential aggregation mechanism. Int. Biodeterior. Biodegrad. 2019, 145, 104807. [Google Scholar] [CrossRef]
- Tait, K.; Havenhand, J. Investigating a possible role for the bacterial signal molecules N-acylhomoserine lactones in Balanus improvisus cyprid settlement. Mol. Ecol. 2013, 22, 2588–2602. [Google Scholar] [CrossRef]
- Browne, K.A.; Tamburri, M.N.; Zimmer-Faust, R.K. Modelling quantitative structure-activity relationships between animal behaviour and environmental signal molecules. J. Exp. Biol. 1998, 201, 245–258. [Google Scholar]
- Crisp, D.J. Chemical factors inducing settlement in Crassostrea virginica (Gmelin). J. Anim. Ecol. 1967, 36, 329–335. [Google Scholar] [CrossRef]
- Crisp, D.J.; Meadows, P.S.; Brambell, F.W.R. Adsorbed layers: The stimulus to settlement in barnacles. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1963, 158, 364–387. [Google Scholar]
- Crisp, D.J.; Meadows, P.S.; Brambell, F.W.R. The chemical basis of gregariousness in cirripedes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1962, 156, 500–520. [Google Scholar] [CrossRef]
- Tamburri, M.N.; Zimmer-Faust, R.K.; Tamplin, M.L. Natural sources and properties of chemical inducers mediating settlement of oyster larvae: A re-examination. Biol. Bull. 1992, 183, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Rittschof, D.; Cohen, J.H. Crustacean peptide and peptide-like pheromones and kairomones. Peptides 2004, 25, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Decho, A.W.; Browne, K.A.; Zimmer-Faust, R.K. Chemical cues: Why basic peptides are signal molecules in marine environments. Limnol. Oceanogr. 1998, 43, 1410–1417. [Google Scholar] [CrossRef]
- Hidu, H. Gregarious setting in the American oyster Crassostrea virginica Gmelin. Chesap. Sci. 1969, 10, 85–92. [Google Scholar] [CrossRef]
- Zimmer-Faust, R.K.; Tamburri, M.N. Chemical identity and ecological implications of a waterborne, larval settlement cue. Limnol. Oceanogr. 1994, 39, 1075–1087. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Tait, K.; Taylor, A.; Brownlee, C.; Joint, I. Acyl-homoserine lactones modulate the settlement rate of zoospores of the marine alga Ulva intestinalis via a novel chemokinetic mechanism. Plant Cell Environ. 2006, 29, 608–618. [Google Scholar] [CrossRef]
- Tait, K.; Williamson, H.; Atkinson, S.; Williams, P.; Cámara, M.; Joint, I. Turnover of quorum sensing signal molecules modulates cross-kingdom signalling. Environ. Microbiol. 2009, 11, 1792–1802. [Google Scholar] [CrossRef]
- Singh, R.P.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Effect of quorum sensing signals produced by seaweed-associated bacteria on carpospore liberation from Gracilaria dura. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Lyu, Y.; Richlen, M.L.; Anderson, D.M.; Cai, Z. Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. Crit. Rev. Plant Sci. 2016, 35, 81–105. [Google Scholar] [CrossRef]
- Bonar, D.B.; Coon, S.L.; Walch, M.; Weiner, R.M.; Fitt, W. Control of oyster settlement and metamorphosis by endogenous and exogenous chemical cues. Bull. Mar. Sci. 1990, 46, 484–498. [Google Scholar]
- Bonar, D.B.; Weiner, R.M.; Colwell, R.R. Microbial-invertebrate interactions and potential for biotechnology. Microb. Ecol. 1986, 12, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, E.W.; Crisp, D.J. Gregariousness in barnacles in relation to the fouling of ships and to anti-fouling research. Nature 1953, 171, 1109–1110. [Google Scholar] [CrossRef] [PubMed]
- Essock-Burns, T.; Wepprich, A.; Thompson, A.; Rittschof, D. Enzymes manage biofilms on crab surfaces aiding in feeding and antifouling. J. Exp. Mar. Biol. Ecol. 2016, 479, 106–113. [Google Scholar] [CrossRef] [Green Version]
- Essock-Burns, T.; Gohad, N.V.; Orihuela, B.; Mount, A.S.; Spillmann, C.M.; Wahl, K.J.; Rittschof, D. Barnacle biology before, during and after settlement and metamorphosis: A study of the interface. J. Exp. Biol. 2017, 220, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Fears, K.P.; Orihuela, B.; Rittschof, D.; Wahl, K.J. Acorn barnacles secrete phase-separating fluid to clear surfaces ahead of cement deposition. Adv. Sci. 2018, 5, 1700762. [Google Scholar] [CrossRef]
- Dickinson, G.H.; Vega, I.E.; Wahl, K.J.; Orihuela, B.; Beyley, V.; Rodriguez, E.N.; Everett, R.K.; Bonaventura, J.; Rittschof, D. Barnacle cement: A polymerization model based on evolutionary concepts. J. Exp. Biol. 2009, 212, 3499–3510. [Google Scholar] [CrossRef] [Green Version]
- So, C.R.; Scancella, J.M.; Fears, K.P.; Essock-Burns, T.; Haynes, S.E.; Leary, D.H.; Diana, Z.; Wang, C.; North, S.; Oh, C.S.; et al. Oxidase activity of the barnacle adhesive interface involves peroxide-dependent catechol oxidase and lysyl oxidase enzymes. ACS Appl. Mater. Interfaces 2017, 9, 11493–11505. [Google Scholar] [CrossRef]
- Essock-Burns, T. Exploring the Interface between Macroorganisms and Microorganisms: Biochemical, Ecological, and Evolutionary Contexts. Ph.D. Thesis, Duke University, Durham, North Carolina, 2015. [Google Scholar]
- Hughes, M. The function of concurrent signals: Visual and chemical communication in snapping shrimp. Anim. Behav. 1996, 52, 247–257. [Google Scholar] [CrossRef]
- Caldwell, R.L. Cavity occupation and defensive behaviour in the stomatopod Gonodactylus festai: Evidence for chemically mediated individual recognition. Anim. Behav. 1979, 27, 194–201. [Google Scholar] [CrossRef]
- Rittschof, D. Oyster drills and the frontiers of chemical ecology: Unsettling ideas. Am. Malacol. Bull. 1985, 1, 111–116. [Google Scholar]
- Rittschof, D. Body odors and neutral-basic peptide mimics: A review of responses by marine organisms. Integr. Comp. Biol. 1993, 33, 487–493. [Google Scholar] [CrossRef]
- Endrizzi, B.J.; Stewart, R.J. Glueomics: An expression survey of the adhesive gland of the sandcastle worm. J. Adhes. 2009, 85, 546–559. [Google Scholar] [CrossRef]
- Essock-Burns, T.; Soderblom, E.J.; Orihuela, B.; Moseley, M.A.; Rittschof, D. Hypothesis testing with proteomics: A case study using wound healing mechanisms in fluids associated with barnacle glue. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Reports—IPCC. Available online: https://www.ipcc.ch/reports/ (accessed on 17 November 2019).
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobretsov, S.; Coutinho, R.; Rittschof, D.; Salta, M.; Ragazzola, F.; Hellio, C. The oceans are changing: Impact of ocean warming and acidification on biofouling communities. Biofouling 2019, 35, 585–595. [Google Scholar] [CrossRef]
- Chan, V.B.S.; Li, C.; Lane, A.C.; Wang, Y.; Lu, X.; Shih, K.; Zhang, T.; Thiyagarajan, V. CO2-driven ocean acidification alters and weakens integrity of the calcareous tubes produced by the serpulid tubeworm, Hydroides elegans. PLoS ONE 2012, 7, e42718. [Google Scholar] [CrossRef] [Green Version]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [Green Version]
- Lane, A.C.; Mukherjee, J.; Chan, V.B.S.; Thiyagarajan, V. Decreased pH does not alter metamorphosis but compromises juvenile calcification of the tube worm Hydroides elegans. Mar. Biol. 2013, 160, 1983–1993. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Li, C.; Li, H.; Shih, K.; He, C.; Yao, H.; Thiyagarajan, V. Recoverable impacts of ocean acidification on the tubeworm, Hydroides elegans: Implication for biofouling in future coastal oceans. Biofouling 2019, 35, 945–957. [Google Scholar] [CrossRef]
- Peck, L.S.; Clark, M.S.; Power, D.; Reis, J.; Batista, F.M.; Harper, E.M. Acidification effects on biofouling communities: Winners and losers. Glob. Chang. Biol. 2015, 21, 1907–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, S.C.K.; Thiyagarajan, V.; Cheung, S.C.K.; Qian, P.-Y. Roles of bacterial community composition in biofilms as a mediator for larval settlement of three marine invertebrates. Aquat. Microb. Ecol. 2005, 38, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.-C.; Yates, J.R. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Target Species | Source of QS | QS Molecule | Mechanism | Reference |
|---|---|---|---|---|
| Algae | ||||
| Ulva (Enteromorpha) sp. | Vibrio anguillarum | 3-oxo-C12-HSL C6-HSL | Attachment of spores | [95,96,110] |
| Ulva sp. | Schewanella sp. | C4-HSL, C6HSL, C12-HSL | Attachment of spores | [111] |
| Ulva linza | Different species | C12-HSL | Germination of spores | [97] |
| Gracilaria dura | Epibiotic bacteria | C6HSL | Spore liberation | [112] |
| Scrippsiella trochoidea | Epibiotic bacteria | ? | Growth | [113] |
| Acrochaetium | Epibiotic bacteria | C4HSL | Spore liberation | [98] |
| Polychaete | ||||
| Hydroides elegans | Biofilms | C6-HSL, C12-HSL, 3-oxo-C8-HLS | Settlement behavior | [94] |
| Barnacle | ||||
| Balanus improvisus | Vibrio anguillarum, Aeromonas hydrophila, Sulfitobacter sp. | C8-HSL, 3-oxo-C10-HSL, C12-HSL | Settlement | [100] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobretsov, S.; Rittschof, D. Love at First Taste: Induction of Larval Settlement by Marine Microbes. Int. J. Mol. Sci. 2020, 21, 731. https://doi.org/10.3390/ijms21030731
Dobretsov S, Rittschof D. Love at First Taste: Induction of Larval Settlement by Marine Microbes. International Journal of Molecular Sciences. 2020; 21(3):731. https://doi.org/10.3390/ijms21030731
Chicago/Turabian StyleDobretsov, Sergey, and Daniel Rittschof. 2020. "Love at First Taste: Induction of Larval Settlement by Marine Microbes" International Journal of Molecular Sciences 21, no. 3: 731. https://doi.org/10.3390/ijms21030731