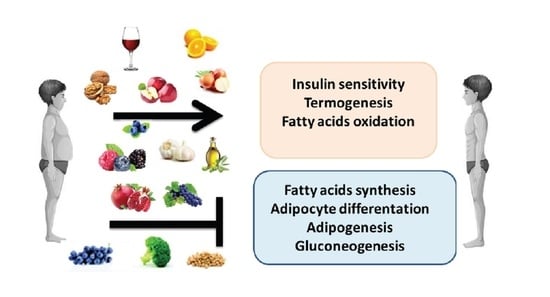
Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities
Abstract
:1. Introduction
2. Polyphenols: Bioactive Compounds
3. Anti-Obesity Properties of Polyphenols
3.1. Actions of Some Polyphenols
3.2. Actions of Some Polyphenolic-Food
4. Bioavailability and Pharmacokinetics
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| BMI | Body mass index |
| CPT-1 | Carnitine palmitoyltransferases 1 |
| CRP | C-reactive protein |
| GTC | Green tea catechins |
| IL | Interleukin |
| LDL-C | Low density lipoprotein-cholesterol |
| MCP-1 | Monocyte chemoattractant protein-1 |
| PPAR | Peroxisome proliferator-activated receptor |
| RE | Resveratrol |
| TC | Total cholesterol |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
References
- WHO. Consideration of the Evidence on Childhood Obesity for the Commission on Ending Childhood Obesity: Report of the Ad Hoc Working Group on Science and Evidence for Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Ricci, S.; Pinto, F.; Auletta, A.; Giordano, A.; Giovane, A.; Settembre, G.; Boccellino, M.; Boffo, S.; Di Carlo, A.; Di Domenico, M. The enigmatic role of matrix metalloproteinases in epithelial-to-mesenchymal transition of oral squamous cell carcinoma: Implications and nutraceutical aspects. J. Cell. Biochem. 2019, 3, 6813–6819. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic, Report of a WHO Consultation; WHO Technical Report Series, No. 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Dyson, P.A. The therapeutics of lifestyle management on obesity. Diabetes Obes. Metab. 2010, 12, 941–946. [Google Scholar] [CrossRef]
- Leitner, D.R.; Frühbeck, G. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies—EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Cuschieri, S.; Mamo, J. Getting to grips with the obesity epidemic in Europe. SAGE Open Med. 2016, 4, 2050312116670406. [Google Scholar] [CrossRef] [Green Version]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Han, J.C.; Lawlor, D.A.; Kimm, S.Y. Childhood obesity. Lancet 2010, 375, 1737–1748. [Google Scholar] [CrossRef]
- Kraak, V.I.; Vandevijvere, S. Progress achieved in restricting the marketing of high-fat, sugary and salty food and beverage products to children. Bull. World Health Organ. 2016, 94, 540–548. [Google Scholar] [CrossRef]
- Reducing the Impact of Marketing of Foods and Non-Alcoholic Beverages on Children. 2010. Available online: https://www.who.int/elena/titles/guidance_summaries/food_marketing_children/en/ (accessed on 31 July 2003).
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020, 9, 1195–1199. [Google Scholar] [CrossRef]
- Lee, A.; Cardel, M.; Donahoo, W.T. Social and Environmental Factors Influencing Obesity; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Solmi, M.; Köhler, C.A.; Stubbs, B.; Koyanagi, A.; Bortolato, B.; Monaco, F.; Vancampfort, D.; Machado, M.O.; Maes, M.; Tzoulaki, I.; et al. Environmental risk factors and nonpharmacological and nonsurgical interventions for obesity: An umbrella review of meta-analyses of cohort studies and randomized controlled trials. Eur. J. Clin. Investig. 2018, 48, e12982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddock, J. The relationship between obesity and the prevalence of fast food restaurants: State-level analysis. Am. J. Health Promot. 2004, 19, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A review. Nutr. Neurosci. 2015, 18, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Cekanova, M.; LeMieux, M.; Greer, B.; Moustaid-Moussa, N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013, 24, 613–623. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N. The adipose tissue renin-angiotensin system and metabolic disorders: A review of molecular mechanisms. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 379–390. [Google Scholar] [CrossRef]
- Boccellino, M.; Donniacuo, M.; Bruno, F.; Rinaldi, B.; Quagliuolo, L.; Ambruosi, M.; Pace, S.; De Rosa, M.; Olgaç, A.; Banoglu, E.; et al. Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors. Eur. J. Med. Chem. 2019, 180, 637–647. [Google Scholar] [CrossRef]
- Giudice, A.; Montella, M.; Boccellino, M.; Crispo, A.; D’Arena, G.; Bimonte, S.; Facchini, G.; Ciliberto, G.; Botti, G.; Quagliuolo, L.; et al. Epigenetic Changes Induced by Green Tea Catechins are Associated with Prostate Cancer. Curr. Mol. Med. 2017, 17, 405–420. [Google Scholar] [CrossRef]
- Saberi-Karimian, M.; Katsiki, N.; Caraglia, M.; Boccellino, M.; Majeed, M.; Sahebkar, A. Vascular endothelial growth factor: An important molecular target of curcumin. Crit. Rev. Food Sci. Nutr. 2019, 59, 299–312. [Google Scholar] [CrossRef]
- Motti, M.L.; D’Angelo, S.; Meccariello, R. MicroRNAs, Cancer and Diet: Facts and New Exciting Perspectives. Curr. Mol. Pharmacol. 2018, 11, 90–96. [Google Scholar] [CrossRef]
- D’Angelo, S.; Scafuro, M.; Meccariello, R. BPA and Nutraceuticals, Simultaneous Effects on Endocrine Functions. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 594–604. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, D.O. Polyphenols and the Human Brain: Plant “Secondary Metabolite” Ecologic Roles and Endogenous Signaling Functions Drive Benefits. Adv. Nutr. 2014, 5, 515–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovaskainen, M.-L.; Torronen, R.; Koponen, J.M.; Sinkko, H.; Hellstrom, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensalem, J.; Dal-Pan, A.; Gillard, E.; Calon, F.; Pallet, V. Protective Effects of Berry Polyphenols against Age- Related Cognitive Impairment. Nutr. Aging. 2016, 3, 89–106. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Curr. Nutr. Food Sci. 2020, 16, 286–299. [Google Scholar] [CrossRef]
- Zappia, V.; Galletti, P.; Manna, C.; D’Angelo, S.; Napoli, D.; De Bonis, M.L.; Capasso, G. Effects of Hydroxytyrosol on Cyclosporine Nephrotoxicity. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Oxford, UK, 2010; pp. 1245–1252. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Sci. Rep. 2019, 10, 13045. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Maluspumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751. [Google Scholar] [CrossRef]
- D’Angelo, S.; Sammartino, D. Protective effect of Annurca apple extract against oxidative damage in human erythrocytes. Curr. Nutr. Food Sci. 2015, 11, 248–256. [Google Scholar] [CrossRef]
- D’Angelo, S. Polyphenols: Potential beneficial effects of these phytochemicals in athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Dos Santos, C.N. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef] [Green Version]
- Di Domenico, M.; Pinto, F.; Quagliuolo, L.; Contaldo, M.; Settembre, G.; Romano, A.; Coppola, M.; Ferati, K.; Bexheti-Ferati, A.; Sciarra, A.; et al. The Role of Oxidative Stress and Hormones in Controlling Obesity. Front. Endocrinol. (Lausanne) 2019, 13, 540. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; Lembo, S.; Flora, F.; De Bonis, M.L.; Balato, A.; Ayala, F.; Balato, N.; Galletti, P.; Zappia, V. Abnormal isoaspartyl residues in erythrocyte membranes from psoriatic patients. Arch. Dermatol. Res. 2012, 304, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccellino, M.; Di Domenico, M.; Donniacuo, M.; Bitti, G.; Gritti, G.; Ambrosio, P.; Quagliuolo, L.; Rinaldi, B. AT1-receptor blockade: Protective effects of irbesartan in cardiomyocytes under hypoxic stress. PLoS ONE 2018, 13, e0202297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, S.; Trojsi, F.; Salvatore, A.; Daniele, L.; Raimo, M.; Galletti, P.; Monsurrò, M.R. Accumulation of altered aspartyl residues in erythrocyte membrane proteins from patients with sporadic amyotrophic lateral sclerosis. Neurochem. Int. 2013, 63, 626–634. [Google Scholar] [CrossRef]
- Toldo, S.; Boccellino, M.; Rinaldi, B.; Seropian, I.M.; Mezzaroma, E.; Severino, A.; Quagliuolo, L.; Van Tassell, B.W.; Marfella, R.; Paolisso, G.; et al. Altered oxido-reductive state in the diabetic heart: Loss of cardioprotection due to protein disulfide isomerase. Mol. Med. 2011, 17, 1012–1021. [Google Scholar] [CrossRef]
- Vanacore, D.; Messina, G.; Lama, S.; Bitti, G.; Ambrosio, P.; Tenore, G.; Messina, A.; Monda, V.; Zappavigna, S.; Boccellino, M.; et al. Effect of restriction vegan diet’s on muscle mass, oxidative status, and myocytes differentiation: A pilot study. J. Cell Physiol. 2018, 233, 9345–9353. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; Rosa, R. Oxidative stress and sport performance. Sport Sci. 2020, 13, 18–22. [Google Scholar]
- Matsuda, M.; Shimomura, I. Roles of oxidative stress, adiponectin, and nuclear hormone receptors in obesity-associated insulin resistance and cardiovascular risk. Horm. Mol. Biol. Clin. Investig. 2014, 19, 75–88. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martínez-González, M.A.; Medina-Remón, A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Portillo, M.P.; Moreno, J.J.; et al. Polyphenol Levels Are Inversely Correlated with Body Weight and Obesity in an Elderly Population after 5 Years of Follow Up (The Randomised PREDIMED Study). Nutrients 2017, 9, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox. Sign. 2008, 10, 511–545. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef] [Green Version]
- Jayarathne, S.; Koboziev, I.; Park, O.H.; Oldewage-Theron, W.; Shen, C.L.; Moustaid-Moussa, N. Anti-Inflammatory and Anti-Obesity Properties of Food Bioactive Components: Effects on Adipose Tissue. Prev. Nutr. Food Sci. 2017, 22, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Bradford, P.G. Curcumin and obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef]
- Ramirez-Bosca, A.; Soler, A.; Carrion, M.A.; Diaz-Alperi, J.; Bernd, A.; Quintanilla, C.; Quintanilla Almagro, E.; Miquel, J. An hydroalcoholic extract of curcuma longa lowers the apo B/apo A ratio. Implications for atherogenesis prevention. Mech. Ageing Dev. 2000, 119, 41–47. [Google Scholar] [CrossRef]
- Ramirez Boscáa, A.; Soler, A.; Carrión-Gutiérrez, M.A.; Pamies Mira, D.; Pardo Zapata, J.; Diaz-Alperi, J.; Bernd, A.; Quintanilla Almagro, E.; Miquel, J. An hydroalcoholic extract of Curcuma longa lowers the abnormally high values of human-plasma fibrinogen. Mech. Ageing Dev. 2000, 114, 207–210. [Google Scholar] [CrossRef]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Soflaei, S.S.; Majeed, M.; Sahebkar, A. Effects of supplementation with curcumin on serum adipokine concentrations: A randomized controlled trial. Nutrition 2016, 32, 1116–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, A.; Sahebkar, A.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of supplementation with curcuminoids on dyslipidemia in obese patients: A randomized crossover trial. Phytother. Res. 2013, 27, 374–379. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.; Ghayour-Mobarhan, M. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 2014, 898361. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Mohammadi, A.; Atabati, A.; Rahiman, S.; Tavallaie, S.; Iranshahi, M.; Akhlaghi, S.; Ferns, G.A.; Ghayour-Mobarhan, M. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother. Res. 2013, 27, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxid. Med. Cell Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef]
- WHO. Evaluation of Certain Food Additives; WHO Technical Report Series, 891; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 15, 4491–4499. [Google Scholar] [CrossRef] [Green Version]
- Jin, T.; Song, Z.; Weng, J.; Fantus, I.G. Curcumin and other dietary polyphenols: Potential mechanisms of metabolic actions and therapy for diabetes and obesity. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E201–E205. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediators Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24 h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-) hypertension: A randomised double-blinded placebo-controlled cross-over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef] [Green Version]
- Shanely, R.A.; Knab, A.M.; Nieman, D.C.; Jin, F.; McAnulty, S.R.; Landram, M.J. Quercetin supplementation does not alter antioxidant status in humans. Free Radic. Res. 2010, 44, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trials.gov C. Investigating the Use of Quercetin on Glucose Absorption in Obesity, and Obesity with Type 2 Diabetes. 2003–2020. Available online: http://clinicaltrials.gov/show/NCT00065676 (accessed on 31 July 2003).
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; García-Almagro, F.J.; Avilés-Plaza, F.; Parra, S.; Yáñez-Gascón, M.J.; Ruiz-Ros, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef]
- Pereira, S.; Park, E.; Moore, J.; Faubert, B.; Breen, D.M.; Oprescu, A.I.; Nahle, A.; Kwan, D.; Giacca, A.; Tsiani, E. Resveratrol prevents insulin resistance caused by short-term elevation of free fatty acids in vivo. Appl. Physiol. Nutr. Metab. 2015, 40, 1129–1136. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Novelle, M.G.; Wahl, D.; Dieguez, C.; Bernier, M.; de Cabo, R. Resveratrol supplementation: Where are we now and where should we go? Ageing. Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Arzola-Paniagua, M.A.; García-Salgado López, E.R.; Calvo-Vargas, C.G.; Guevara-Cruz, M. Efficacy of an orlistat-resveratrol combination for weight loss in subjects with obesity: A randomized controlled trial. Obesity 2016, 24, 1454–1463. [Google Scholar] [CrossRef]
- Kjær, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Stødkilde-Jørgensen, H.; Jessen, N.; Jørgensen, J.; Richelsen, B.; Pedersen, S.B. No Beneficial Effects of Resveratrol on the Metabolic Syndrome: A Randomized Placebo-Controlled Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 102, 1642–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; van Boekschoten, M.; de Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stodkilde-Jorgensen, H.; Møller, N.; Jessen, N.; Pedersen, S.B.; Jørgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scapagnini, G.; Davinelli, S.; Kaneko, T.; Koverech, G.; Koverech, A.; Calabrese, E.J.; Calabrese, V. Dose response biology of resveratrol in obesity. J. Cell Commun Signal. 2014, 8, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [Green Version]
- Suliburska, J.; Bogdanski, P.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol. Trace Elem. Res. 2012, 149, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef]
- Levy, Y.; Narotzki, B.; Reznick, A.Z. Green tea, weight loss and physical activity. Clin. Nutr. 2017, 36, 315. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2010, 22, 1–7. [Google Scholar] [CrossRef]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes. 2009, 33, 956–961. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [CrossRef] [Green Version]
- Jurgens, T.M.; Whelan, A.M.; Killian, L.; Doucette, S.; Kirk, S.; Foy, E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst. Rev. 2012, 12, CD008650. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Chen, C.L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu. Rev. Nutr. 2016, 36, 75–299. [Google Scholar] [CrossRef]
- Dow, C.A.; Going, S.B.; Chow, H.H.; Patil, B.S.; Thomson, C.A. The effects of daily consumption of grapefruit on body weight, lipids, and blood pressure in healthy, overweight adults. Metabolism 2012, 61, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.Q.; Dourado, G.K.; Cesar, T.B. Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. Int. J. Food Sci. Nutr. 2015, 66, 830–836. [Google Scholar] [CrossRef]
- Azzini, E.; Venneria, E.; Ciarapica, D.; Foddai, M.S.; Intorre, F.; Zaccaria, M.; Maiani, F.; Palomba, L.; Barnaba, L.; Tubili, C.; et al. Effect of red orange juice consumption on body composition and nutritional status in overweight/obese female: A pilot study. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Ribeiro, C.; Dourado, G.; Cesar, T. Orange juice allied to a reduced-calorie diet results in weight loss and ameliorates obesity-related biomarkers: A randomized controlled trial. Nutrition 2017, 38, 13–19. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Rosa, R. The impact of supplementation with Pomegranate fruit (Punica Granatum L.) on sport performance. Sport Sci. 2020, 13, 29–37. [Google Scholar]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Zhao, L. The mulberry (Morus alba L.) fruit-a review of characteristic components and health benefits. J. Agric. Food. Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Mahboubi, M. Morus alba (mulberry), a natural potent compound in management of obesity. Pharmacol. Res. 2019, 146, 104341. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, Y. Mulberry Fruit Extract Ameliorates Adipogenesis via Increasing AMPK Activity and Downregulating MicroRNA-21/143 in 3T3-L1 Adipocytes. J. Med. Food. 2020, 23, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Da Villa, G.; Ianiro, G.; Mangiola, F.; Del Toma, E.; Vitale, A.; Gasbarrini, A.; Gasbarrini, G. White mulberry supplementation as adjuvant treatment of obesity. J. Biol. Regul. Homeost. Agents 2014, 28, 141–145. [Google Scholar]
- Karlsen, A.; Paur, I.; Bøhn, S.K.; Sakhi, A.K.; Borge, G.I.; Serafini, M.; Erlund, I.; Laake, P.; Tonstad, S.; Blomhoff, R. Bilberry juice modulates plasma concentration of NF-kappaB related inflammatory markers in subjects at increased risk of CVD. Eur. J. Nutr. 2010, 49, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Keast, D.R.; O’Neil, C.E.; Jones, J.M. Dried fruit consumption is associated with improved diet quality and reduced obesity in US adults: National Health and Nutrition Examination Survey, 1999–2004. Nutr. Res. 2011, 31, 460–467. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Eisner, A.; Ramachandran, P.; Cabalbag, C.; Metti, D.; Shamloufard, P.; Kern, M.; Hong, M.Y.; Hooshmand, S. Effects of Dried Apple Consumption on Body Composition, Serum Lipid Profile, Glucose Regulation, and Inflammatory Markers in Overweight and Obese Children. J. Med. Food. 2020, 23, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.W.; Koch, T.C.L.; Watzl, B.; Dietrich, H.; Will, F.; Bub, A. Moderate effects of apple juice consumption on obesity-related markers in obese men: Impact of diet-gene interaction on body fat content. Eur. J. Nutr. 2012, 51, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Kim, S.P. The effect of onion extract intake for 12 weeks on blood lipid and obesity index in obese university women. Korean J. Sports. Sci. 2013, 22, 955–962. [Google Scholar]
- Choi, E.Y.; Lee, H.; Woo, J.S.; Jang, H.H.; Hwang, S.J.; Kim, H.S.; Kim, W.S.; Kim, Y.S.; Choue, R.; Cha, Y.J.; et al. Effect of onion peel extract on endothelial function and endothelial progenitor cells in overweight and obese individuals. Nutrition 2015, 31, 1131–1135. [Google Scholar] [CrossRef]
- Lee, J.S.; Cha, Y.J.; Lee, K.H.; Yim, J.E. Onion peel extract reduces the percentage of body fat in overweight and obese subjects: A 12-week, randomized, double-blind, placebo-controlled study. Nutr. Res. Pract. 2016, 10, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, C.W. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008, 138, 1244S–1249S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ørgaard, A.; Jensen, L. The effects of soy isoflavones on obesity. Exp. Biol. Med. 2008, 233, 1066–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscica, M.; Pavanello, C.; Gandini, S.; Gomaraschi, M.; Vitali, C.; Macchi, C.; Morlotti, B.; Aiello, G.; Bosisio, R.; Calabresi, L.; et al. Effect of soy on metabolic syndrome and cardiovascular risk factors: A randomized controlled trial. Eur. J. Nutr. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Llaneza, P.; González, C.; Fernández-Iñarrea, J.; Alonso, A.; Díaz, F.; Pérez-López, F.R. Soy isoflavones improve insulin sensitivity without changing serum leptin among postmenopausal women. Climacteric 2012, 15, 611–620. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Zare, M.; Nouripour, F. Effect of Soy and Soy Isoflavones on Obesity-Related Anthropometric Measures: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Adv Nutr. 2017, 8, 705–717. [Google Scholar] [CrossRef]
- Lee, M.; Sorn, S.R.; Park, Y.; Park, H.K. Anthocyanin Rich-Black Soybean Testa Improved Visceral Fat and Plasma Lipid Profiles in Overweight/Obese Korean Adults: A Randomized Controlled Trial. J. Med. Food. 2016, 19, 995–1003. [Google Scholar] [CrossRef]
- Oh, J.H.; Karadeniz, F.; Lee, J.I.; Seo, Y.; Kong, C.S. Artemisia princeps inhibits adipogenic differentiation of 3T3-L1 pre-adipocytes via downregulation of PPARγ and MAPK pathways. Prev. Nutr. Food Sci. 2019, 24, 299–307. [Google Scholar] [CrossRef]
- Dabe, N.E.; Kefale, A.T. Antidiabetic Effects of Artemisia Species: A Systematic Review. Anc. Sci. Life 2017, 36, 175–181. [Google Scholar] [CrossRef]
- Helvaci, S.; Kökdil, G.; Kawai, M.; Duran, N.; Duran, G.; Güvenç, A. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D. Pharm. Biol. 2010, 48, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Zhao, F.; Jiang, Z.H.; Chen, L.X.; Zhao, Q.; Liu, H.X.; Yao, X.S.; Qiu, F. Steroids and flavonoids from Physalis alkekengi var. franchetii and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2008, 71, 642–646. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Kim, M.; Irfan, M.; Kim, S.H.; Kim, S.D.; Rhee, M.H. Physalis alkekengi Exhibits Antiobesity Effects in Mice with Potential of Inducing White Adipose Tissue Browning. J. Med. Food. 2020, 23, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.Y.; Shin, M.R.; Seo, B.I.; Noh, J.S.; Roh, S.S. Young Persimmon Fruit Extract Suppresses Obesity by Modulating Lipid Metabolism in White Adipose Tissue of Obese Mice. J. Med. Food. 2020, 23, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.M.; Tran, B.Q.; Jang, Y.J.; Park, S.H.; Fondrie, W.E.; Chowdhury, K.; Yoon, S.H.; Goodlett, D.R.; Chae, S.W.; Chae, H.J.; et al. Assessment of the Therapeutic Potential of Persimmon Leaf Extract on Prediabetic Subjects. Mol. Cells 2017, 40, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [Green Version]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Chow, H.H.; Hakim, I.A. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol. Res. 2011, 64, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [Green Version]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heger, M.; van Golen, R.F.; Broekgaarden, M.; Michel, M.C. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol. Rev. 2014, 66, 222–307. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, S.; Cusano, P. Adherence to the Mediterranean diet in athletes. Sport Sci. 2020, 13, 58–63. [Google Scholar]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the Mediterranean Diet: A Review of Evidence of the Role and Sustainability of the Mediterranean Diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wruss, J.; Lanzerstorfer, P.; Huemer, S.; Himmelsbach, M.; Mangge, H.; Höglinger, O.; Weghuber, D.; Weghuber, J. Differences in pharmacokinetics of apple polyphenols after standardized oral consumption of unprocessed apple juice. Nutr. J. 2015, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| POLYPHENOL | Human Trials | Effects | References * |
|---|---|---|---|
| Curcumin | Randomized, double-blind, placebo-controlled, crossover trial 30 obese individuals 30-day treatment of curcumin (1 g/day) | = Weight, BMI, % of Body fat ↓ TG | 65 |
| Randomized crossover trial 30 obese individuals 30-day treatment of curcumin 1 g/day for 4 weeks | ↓ Serum levels of VEGF ↓ IL-1β and IL-4 | 66 | |
| Randomized double-blind placebo-controlled cross-over trial 30 obese individuals Curcumin supplementation (1 g/day for 30 days) | ↓ Oxidative stress burden | 67 | |
| Randomized, controlled study. 44 overweight subjects 30 days with curcumin | ↑ Weight loss ↓↓ % of Body fat ↑ Waistline reduction, ↓↓ Hip circumference ↓↓ BMI | 68 | |
| Randomized placebo-controlled clinical trial 60 Overweight and obese female adolescents. 500 mg tablet per day (95% curcumin) | ↓ BMI ↓ Waist circumference ↓ Hip circumference ↓ LDL | 69 | |
| Quercetin | Double-blind crossover study overweight-obese subjects 150 mg/d quercetin for 8 weeks | ↓ Waist circumference ↓ Postprandial systolic blood pressure ↓ TG | 76 |
| Double-blinded, placebo-controlled cross-over trial 70 Overweight-to-obese subjects 162 mg/d quercetin 6-week treatment periods | ↓ Ambulatory blood pressure | 77 | |
| Resveratrol | Randomized double-blind crossover study 11 obese men 150 mg/day resveratrol for 30 days | ↓ Insulin, Plasma Fatty Acids, ↓ TG, Glucose, Leptin | 85 |
| 11 healthy obese men 30 days (150 mg resveratrol/day) | ↓ Adipocyte size | 86 |
| Polyphenolic-Food | Polyphenols Contained | Effects | Reference * |
|---|---|---|---|
| Soy beans | Isoflavones | ↓ Serum leptin, TNF-α ↑ Adiponectin | 132 |
| ↓ BMI | 133 | ||
| Black soybean | Isoflavones Anthocyanins | ↓ TNF-α, MCP-1 | 134 |
| Red orange | Anthocyanins Citrus flavonoids | ↓ CRP, TNF-α | 98, 108 |
| Mulberries | Anthocyanins Quercetin Chlorogenic acids | ↓ Body weight ↓ Waist circumference ↓ Thigh circumference ↓ Insulin and glucose curves | 116 |
| Strawberry | Anthocyanins | ↓ IL-6 ↓ Oxidized -LDL | 118, 119 |
| Grapefruit | Citrus flavonoids | ↓ Body weight ↓ Waist circumference ↓ Systolic blood pressure | 105 |
| Dried apple | Flavanols | ↓ BMI, TC | 122 |
| Apple | Flavanols Phenolic acids | = BMI, waist circumferences ↓ Body weight | 123 |
| Green tea | Flavanols | ↓ BMI, waist circumference, glucose ↓ Total cholesterol, LDL-C, TG ↑ Total antioxidant level ↑ Adiponectin | 94,98 |
| Onion | Quercetin | ↓ Body weight ↓ % Body fat ↓ BMI | 124 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. https://doi.org/10.3390/ijms21165642
Boccellino M, D’Angelo S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. International Journal of Molecular Sciences. 2020; 21(16):5642. https://doi.org/10.3390/ijms21165642
Chicago/Turabian StyleBoccellino, Mariarosaria, and Stefania D’Angelo. 2020. "Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities" International Journal of Molecular Sciences 21, no. 16: 5642. https://doi.org/10.3390/ijms21165642