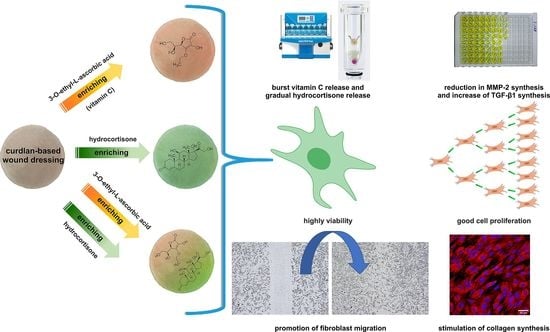
Effect of Vitamin C/Hydrocortisone Immobilization within Curdlan-Based Wound Dressings on In Vitro Cellular Response in Context of the Management of Chronic and Burn Wounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Vitamin C and Hydrocortisone Release Profile
2.2. Cytotoxicity and Proliferation Assessment
2.3. Cell Migration Assessment
2.4. Type I Collagen Production Assessment
2.5. MMP-2 and TGF-β1 Production Assessment
3. Materials and Methods
3.1. Materials
3.2. Biomaterials Fabrication
3.3. Vitamin C and Hydrocortisone Release Profile
3.4. Cell Culture Experiments
3.4.1. Cytotoxicity Assessment
3.4.2. Proliferation Assessment
3.4.3. Cell Migration Assessment
3.4.4. Type I Collagen Production Assessment
3.4.5. MMP-2 and TGF-β1 Production Assessment
3.5. Statistical Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound healing and the use of medicinal plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Granick, M.S.; Sood, A.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a wound dressing based on common wound characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunus Basha, R.; Sampath Kumar, T.S.; Selvaraj, R.; Doble, M. Silver loaded nanofibrous curdlan mat for diabetic wound healing: An in vitro and in vivo study. Macromol. Mater. Eng. 2018, 303, 1800234. [Google Scholar] [CrossRef]
- Weller, C.D.; Team, V.; Sussman, G. First-line interactive wound dressing update: A comprehensive review of the evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef] [Green Version]
- Ljiljana, D.; Martina, M.; Ana, Ć.; Jadranka, F. Composite chitosan hydrogels as advanced wound dressings with sustained ibuprofen release and suitable application characteristics. Pharm. Dev. Technol. 2020, 25, 332–339. [Google Scholar]
- Long, J.; Etxabide, A.; Nand, A.V.; Bunt, C.R.; Ray, S. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Evranos, B.; Aycan, D.; Alemdar, N. Production of ciprofloxacin loaded chitosan/gelatin/bone ash wound dressing with improved mechanical properties. Carbohydr. Polym. 2019, 222, 115007. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.I.; Lee, J.Y.; Son, B.C.; Park, C.H.; Kim, C.S. Bi-layered nanofibers membrane loaded with titanium oxide and tetracycline as controlled drug delivery system for wound dressing applications. Polymers 2019, 11, 1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzanfar, S.; Savari, G.; Babak, M. Vitamin B12-loaded polycaprolacton/gelatin nanofibrous scaffold as potential wound care material. Biomed. Eng. Lett. 2020, 10, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Vivcharenko, V.; Wojcik, M.; Palka, K.; Przekora, A. Highly porous and superabsorbent biomaterial made of marine-derived polysaccharides and ascorbic acid as an optimal dressing for exuding wound management. Materials 2021, 14, 1211. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic acid (HA)-based silk fibroin/zinc oxide core–Shell electrospun dressing for burn wound management. Macromol. Biosci. 2020, 20, 1900328. [Google Scholar] [CrossRef]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.L.; et al. Growth factors delivery system for skin regeneration: An advanced wound dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Vivcharenko, V.; Przekora, A. Modifications of wound dressings with bioactive agents to achieve improved pro-healing properties. Appl. Sci. 2021, 11, 4114. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2019, 122, 452–460. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O. The use of electrospun curcumin-loaded poly (L-lactic acid) fiber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Wojcik, M.; Kazimierczak, P.; Benko, A.; Palka, K.; Vivcharenko, V.; Przekora, A. Superabsorbent curdlan-based foam dressings with typical hydrocolloids properties for highly exuding wound management. Mater. Sci. Eng. C 2021, 124, 112068. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydrocortisone (accessed on 25 July 2021).
- Jaeger, M.; Harats, M.; Kornhaber, R.; Aviv, U.; Zerach, A.; Haik, J. Treatment of hypergranulation tissue in burn wounds with topical steroid dressings: A case series. Int. Med. Case Rep. J. 2016, 9, 241–245. [Google Scholar] [PubMed] [Green Version]
- Schönfelder, U.; Abel, M.; Wiegand, C.; Klemm, D.; Elsner, P.; Hipler, U.C. Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro. Biomaterials 2005, 26, 6664–6673. [Google Scholar] [CrossRef]
- Hofman, D.; Moore, K.; Cooper, R.; Eagle, M.; Cooper, S. Use of topical corticosteroids on chronic leg ulcers. J. Wound Care 2007, 16, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Jaimini, M.; Kothari, A.H. Sustained release matrix type drug deliery system: A review. J. Drug Deliv. Ther. 2012, 2, 142–148. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Moores, J. Vitamin C: A wound healing perspective. Br. J. Community Nurs. 2013, 18, 8–11. [Google Scholar] [CrossRef]
- Das Gupta, V. Effect of vehicles and other active ingredients on stability of hydrocortisone. J. Pharm. Sci. 1978, 67, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.R.; Harris, K.L.; Jubin, K.; Bainbridge, N.J.; Jordan, N.R. The effect of pH in modulating skin cell behaviour. Br. J. Dermatol. 2009, 161, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res. Int. 2014, 2014, 747584. [Google Scholar] [CrossRef] [Green Version]
- Rangaraj, A.; Harding, K.; Leaper, D. Role of collagen in wound management. Wounds UK 2011, 7, 54–63. [Google Scholar]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [Green Version]
- Nuutinen, P.; Autio, P.; Hurskainen, T.; Oikarinen, A. Glucocorticoid action on skin collagen: Overview on clinical significance and consequences. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 361–362. [Google Scholar]
- Nuutinen, P.; Riekki, R.; Parikka, M.; Salo, T.; Autio, P.; Risteli, J.; Oikarinen, A. Modulation of collagen synthesis and mRNA by continuous and intermittent use of topical hydrocortisone in human skin. Br. J. Dermatol. 2003, 148, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Golubitskii, G.B.; Budko, E.V.; Basova, E.M.; Kostarnoi, A.V.; Ivanov, V.M. Stability of ascorbic acid in aqueous and aqueous-organic solutions for quantitative determination. J. Anal. Chem. 2007, 62, 742–747. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and wound healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The role of matrix metalloproteinases in diabetic wound healing in relation to photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljada, A.; Ghanim, H.; Mohanty, P.; Hofmeyer, D.; Tripathy, D.; Dandona, P. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J. Clin. Endocrinol. Metab. 2001, 86, 5988–5991. [Google Scholar] [CrossRef]
- Kato, Y.; Lambert, C.A.; Colige, A.C.; Mineur, P.; Noël, A.; Frankenne, F.; Foidart, J.M.; Baba, M.; Hata, R.I.; Miyazaki, K.; et al. Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J. Biol. Chem. 2005, 280, 10938–10944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma 2012, 2, 18–28. [Google Scholar]
- Ramirez, H.; Patel, S.B.; Pastar, I. The role of TGFβ signaling in wound epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. 10993-5: 2009 Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- ISO. 10993-12 Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro? Cent. Eur. J. Biol. 2014, 9, 277–289. [Google Scholar] [CrossRef]
- Przekora, A.; Palka, K.; Ginalska, G. Biomedical potential of chitosan/HA and chitosan/β-1,3-glucan/HA biomaterials as scaffolds for bone regeneration—A comparative study. Mater. Sci. Eng. C 2016, 58, 891–899. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Ginalska, G.; Przekora, A. The cytotoxicity assessment of the novel latex urinary catheter with prolonged antimicrobial activity. J. Biomed. Mater. Res. Part A 2011, 98A, 222–228. [Google Scholar] [CrossRef]
- Vivcharenko, V.; Wojcik, M.; Przekora, A. Cellular response to vitamin C-enriched chitosan/agarose film with potential application as artificial skin substitute for chronic wound treatment. Cells 2020, 9, 1185. [Google Scholar] [CrossRef]
- Przekora, A.; Ginalska, G. Enhanced differentiation of osteoblastic cells on novel chitosan/β-1,3-glucan/bioceramic scaffolds for bone tissue regeneration. Biomed. Mater. 2015, 10, 015009. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojcik, M.; Kazimierczak, P.; Vivcharenko, V.; Koziol, M.; Przekora, A. Effect of Vitamin C/Hydrocortisone Immobilization within Curdlan-Based Wound Dressings on In Vitro Cellular Response in Context of the Management of Chronic and Burn Wounds. Int. J. Mol. Sci. 2021, 22, 11474. https://doi.org/10.3390/ijms222111474
Wojcik M, Kazimierczak P, Vivcharenko V, Koziol M, Przekora A. Effect of Vitamin C/Hydrocortisone Immobilization within Curdlan-Based Wound Dressings on In Vitro Cellular Response in Context of the Management of Chronic and Burn Wounds. International Journal of Molecular Sciences. 2021; 22(21):11474. https://doi.org/10.3390/ijms222111474
Chicago/Turabian StyleWojcik, Michal, Paulina Kazimierczak, Vladyslav Vivcharenko, Malgorzata Koziol, and Agata Przekora. 2021. "Effect of Vitamin C/Hydrocortisone Immobilization within Curdlan-Based Wound Dressings on In Vitro Cellular Response in Context of the Management of Chronic and Burn Wounds" International Journal of Molecular Sciences 22, no. 21: 11474. https://doi.org/10.3390/ijms222111474