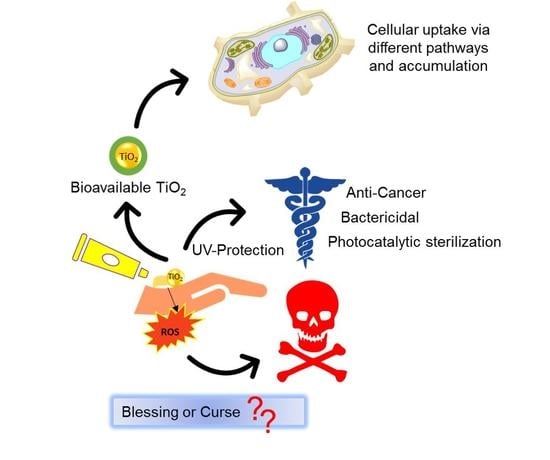
Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen
Abstract
:1. Introduction
2. Sunscreen Exploits the Semiconducting Property of TiO2
3. The Biological Use and Solubility of TiO2 and Its NPs
4. Applications of TiO2 NPs Provide Further Insight into Its Functionality
5. Elucidating the Impact that the Bioactivity of TiO2 NPs from Sunscreen Use Could Have on the Aquatic Environment and Human Health
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ratner, B.; Hoffman, A.; Schoen, F.; Lemons, J. Biomaterials Science: An Introduction to Materials in Medicine, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Nair, M.; Elizabeth, E. Applications of Titania Nanotubes in Bone Biology. J. Nanosci. Nanotechnol. 2015, 15, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Egerton, A.; Tooley, I.R. UV absorption and scattering properties of inorganic-based sunscreens. Int. J. Cosmet. Sci. 2011, 34, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, M.; Alcaraz, C.F. Why titanium is a beneficial element for plants. J. Plant Nutr. 1998, 21, 655–664. [Google Scholar] [CrossRef]
- Hruby, M.; Cigler, P.; Kuzel, S. Contribution to understanding the mechanism of titanium action in plant. J. Plant Nutr. 2002, 25, 577–598. [Google Scholar] [CrossRef]
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron Micro-XRE and Micro-XANES Confirmation of the Uptake and Translocation of TiO2 Nanoparticles in Cucumber (Cucumis sativus) Plants. Environ. Sci. Technol. 2012, 46, 7637–7643. [Google Scholar] [CrossRef]
- Istvan, P.; Feher, M.; Bokori, J.; Nagy, B. Physilogically beneficial effects of titanium. Water Air Soil Pollut. 1991, 57–58, 675–680. [Google Scholar] [CrossRef]
- Yaghoubi, S.; Schwietert, C.W.; McCue, J.P. Biological roles of titanium. Biol. Trace Elem. Res. 2000, 78, 205–217. [Google Scholar] [CrossRef]
- Schwietert, C.W.; Yaghoubi, S.; Gerber, N.C.; McSharry, J.J.; McCue, J.P. Dietary titanium and infant growth. Biol. Trace Elem. Res. 2001, 83, 149–167. [Google Scholar] [CrossRef]
- Oosthuizen, S.J. Titanium: The innovators’ metal-Historical case studies tracing titanium process and product innovation. J. S. Afr. Inst. Min. Metall. 2011, 111, 781–786. [Google Scholar]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef]
- Jung, C. About Oxygen, Cytochrome P450 and Titanium: Learning from Ron Estabrook. Drug Metab. Rev. 2007, 39, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Kang, S.-M.; Seo, K.-W.; Nahm, K.-Y.; Chung, K.-R.; Kim, S.-H.; Ahn, J.-P. Nanoscale bonding between human bone and titanium surfaces: Osseohybridization. BioMed. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nuevo-Ordonez, Y.; Montes-Bayon, M.; Blanco-Gonzalez, E.; Paz-Aparicio, J.; Raimundez, J.D.; Tejerina, J.M.; Pena, M.A.; Sanz-Medel, A. Titanium release in serum of patients with different bone fixation implants and its interaction with serum biomolecules at physiological levels. Anal. Bioanal. Chem. 2011, 401, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Woll, K.A.; Prusinskas, K.; Johnson, M.D.; Norkus, E. Metal Speciation in Health and Medicine Represented by Iron and Vanadium. Inorg. Chem. 2013, 52, 12262–12275. [Google Scholar] [CrossRef] [PubMed]
- Doucette, K.A.; Hassell, K.N.; Crans, D.C. Selective speciation improves efficacy and lowers toxicity of platinum anticancer and vanadium antidiabetic drugs. J. Inorg. Biochem. 2016, 165, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Levina, A.; Crans, D.C.; Lay, P.A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Coord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Soto-Alvaredo, J.; Blanco, E.; Bettmer, J.; Hevia, D.; Sainz, R.M.; Chaves, C.L.; Sanchez, C.; Llopis, J.; Sanz-Medel, A.; Montes-Bayon, M. Evaluation of the biological effect of Ti generated debris from metal implants: Ions and nanoparticles. Metallomics 2014, 6, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Tagger Green, N.; Machtei, E.; Horwitz, J.; Peled, M. Fracture of dental implants: Literature review and report of a case. Implant Dent. 2002, 11, 137–143. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Tasat, D.R.; Guglielmotti, M.B.; Cabrini, R.L. Biodistribution of titanium dioxide from biologic compartments. J. Mater. Sci. Mater. Med. 2008, 19, 3049–3056. [Google Scholar] [CrossRef]
- Thomas, P.; Bandl, W.D.; Maier, S.; Summer, B.; Przybilla, B. Hypersensitivity to titanium osteosynthesis with impaired fracture healing, eczema, and T-cell hyperresponsiveness in vitro: Case report and review of the literature. Contact Dermat. 2006, 55, 199–202. [Google Scholar] [CrossRef]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef] [PubMed]
- Tower, S.S. Arthroprosthetic cobaltism: Neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: A case report. J. Bone Jt. Surg. Am. 2010, 92, 2847–2851. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.J. Commentary on an article by Stephen S. Tower, MD: “Arthroprosthetic cobaltism: Neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty. A case report”. J. Bone Jt. Surg. 2010, 92, e35. [Google Scholar] [CrossRef] [PubMed]
- Sotos, J.G.; Tower, S.S. Systemic disease after hip replacement: Aeromedical implications of arthroprosthetic cobaltism. Aviat. Space Environ. Med. 2013, 84, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S.M.; Wilkinson, J.M.; Ferner, R.E. Systemic toxicity related to metal hip prostheses. Clin. Toxicol. 2014, 52, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Dirk, K. The Bleeding Edge; Netflix: Los Gatos, CA, USA, 2018. [Google Scholar]
- Golasik, M.; Herman, M.; Piekoszewski, W. Toxicological aspects of soluble titanium—A review of in vitro and in vivo studies. Metallomics 2016, 8, 1227–1242. [Google Scholar] [CrossRef]
- Christodoulou, C.V.; Eliopoulos, A.G.; Young, L.S.; Hodgkins, L.; Ferry, D.R.; Kerr, D.J. Anti-proliferative activity and mechanism of action of titanocene dichloride. Br. J. Cancer. 1998, 77, 2088–2097. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Saxena, M.; Sharma, S.; Noinaj, N.; Delgado, Y.; Gonzalez, E.P.Q.; Conklin, S.E.; Zambrana, N.; Loza-Rosas, S.A.; Parks, T.B. Unusual synergism of transferrin and citrate in the regulation of Ti(IV) speciation, transport, and toxicity. J. Am. Chem. Soc. 2016, 138, 5659–5665. [Google Scholar] [CrossRef]
- Loza-Rosas, S.A.; Saxena, M.; Delgado, Y.; Gaur, K.; Pandrala, M.; Tinoco, A.D. A ubiquitous metal, difficult to track: Towards an understanding of the regulation of titanium(iv) in humans. Metallomics 2017, 9, 346–356. [Google Scholar] [CrossRef]
- Saxena, M.; Loza Rosas, S.; Gaur, K.; Sharma, S.; Perez Otero, S.C.; Tinoco, A.D. Exploring titanium(IV) chemical proximity to iron(III) to elucidate a function for Ti(IV) in the human body. Coord. Chem. Rev. 2018, 363, 109–125. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Lucas, R.M.; Cullen, A.P.; de Gruijl, F.R.; Longstreth, J.; Takizawa, Y.; van der Leun, J.C. The human health effects of ozone depletion and interactions with climate change. Photochem. Photobiol. Sci. 2011, 2, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, C.; Kosmadaki, M.; Stratigos, A.J.; Katsambas, A.D. Sunscreens—What’s important to know. JEADV 2008, 22, 1110–1119. [Google Scholar] [PubMed]
- Dransfield, G.P. Inorganic Sunscreens. Radiat. Prot. Dosim. 2000, 91, 271–273. [Google Scholar] [CrossRef]
- Davis, J.; Wang, A.; Shtakin, J. Nanomaterial Case Studies: Nanoscale Titanium Dioxide in Water Treatment and in Topical Sunscreen; US EPA: Research Triangle Park, NC, USA, 2010.
- Morgan, B.J.; Watson, G.W. Intrinsic n-type Defect Formation in TiO2: A Comparison of Rutile and Anatase from GGA+U Calculations. J. Phys. Chem. C 2010, 114, 2321–2328. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Cantrell, A.; McGarvey, D.J.; Truscott, T.G. Comprehensive Series in Photosciences; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 495–519. [Google Scholar]
- Tanvir, S.; Pulvin, S.; Anderson, W. Toxicity associated with the photo catalytic and photo stable forms of titanium dioxide nanoparticles used in sunscreen. MOJ Toxicol. 2015, 1, 00011. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Dodd, N.J.; Jha, A.N. Photoexcitation of aqueous suspensions of titanium dioxide nanoparticles: An electron spin resonance spin trapping study of potentially oxidative reactions. Photochem. Photobiol. 2011, 87, 632–640. [Google Scholar] [CrossRef]
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Lewicka, Z.A.; William, W.Y.; Oliva, B.L.; Contreras, E.Q.; Colvin, V.L. Photochemical behavior of nanoscale TiO2 and ZnO sunscreen ingredients. J. Photochem. Photobiol. A Chem. 2013, 263, 24–33. [Google Scholar] [CrossRef]
- Armand, L.; Tarantini, A.; Beal, D.; Biola-Clier, M.; Bobyk, L.; Sorieul, S.; Pernet-Gallay, K.; Marie-Desvergne, C.; Lynch, I.; Herlin-Boime, N. Long-term exposure of A549 cells to titanium dioxide nanoparticles induces DNA damage and sensitizes cells towards genotoxic agents. Nanotoxicology 2016, 10, 913–923. [Google Scholar] [CrossRef] [PubMed]
- IARC. Carbon Black, Titanium Dioxide, and Talc. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93. [Google Scholar]
- Brezová, V.; Gabčová, S.; Dvoranová, D.; Staško, A. Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (an EPR study). J. Photochem. Photobiol. B-Biol. 2005, 79, 121–134. [Google Scholar]
- Fujiwara, R.; Luo, Y.; Sasaki, T.; Fujii, K.; Ohmori, H.; Kuniyasu, H. Cancer Therapeutic Effects of Titanium Dioxide Nanoparticles Are Associated with Oxidative Stress and Cytokine Induction. Pathobiology 2015, 82, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Martins dos Santos, V.A.P.; Fernández-García, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef] [PubMed]
- Schmets, J.; Van Muylder, J.; Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pourbaix, M., Ed.; Pergamon Press: Oxford, UK, 1996. [Google Scholar]
- Knauss, K.G.; Dibley, M.J.; Bourcier, W.L.; Shaw, H.F. Ti(IV) hydrolysis constants derived from rutile solubility measurements made from 100 to 300 degrees C. Appl. Geochem. 2001, 16, 1115–1128. [Google Scholar] [CrossRef]
- Schmidt, J.; Vogelsberger, W. Aqueous Long-Term Solubility of Titania Nanoparticles and Titanium(IV) Hydrolysis in a Sodium Chloride System Studied by Adsorptive Stripping Voltammetry. J. Solution Chem. 2009, 38, 1267–1282. [Google Scholar] [CrossRef]
- Lang, Y.; del Monte, F.; Rodriguez, B.J.; Dockery, P.; Finn, D.P.; Pandit, A. Integration of TiO2 into the diatom Thalassiosira weissflogii during frustule synthesis. Sci. Rep. 2013, 3, 3205. [Google Scholar] [CrossRef] [Green Version]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Bertini, I.; Gray, H.B.; Stiefel, E.I.; Valentine, J.S. Biological Inorganic Chemistry: Structure and Reactivity; University Science Books: Sausalit, CA, USA, 2007. [Google Scholar]
- Shabtai, Y.; Fleminger, G. Adsorption of Rhodococcus Strain GIN-1 (NCIMB 40340) on Titanium Dioxide and Coal Fly Ash Particles. Appl. Environ. Microbiol. 1994, 60, 3079–3088. [Google Scholar]
- Siegmann, A.; Komarska, A.; Betzalel, Y.; Brudo, I.; Jindou, S.; Mor, G.; Fleminger, G. The titanium binding protein of Rhodococcus ruber GIN1 (NCIMB 40340) is a cell-surface homolog of the cytosolic enzyme dihydrolipoamide dehydrogenase. J. Mol. Recognit. 2009, 22, 138–145. [Google Scholar] [CrossRef]
- Dayan, A.; Babin, G.; Ganoth, A.; Kayouf, N.S.; Nitoker Eliaz, N.; Mukkala, S.; Tsfadia, Y.; Fleminger, G. The involvement of coordinative interactions in the binding of dihydrolipoamide dehydrogenase to titanium dioxide-Localization of a putative binding site. J. Mol. Recognit. 2017, 30. [Google Scholar] [CrossRef]
- Dayan, A.; Lamed, R.; Benayahu, D.; Fleminger, G. RGD-modified dihydrolipoamide dehydrogenase as a molecular bridge for enhancing the adhesion of bone forming cells to titanium dioxide implant surfaces. J. Biomed. Mater. Res. Part A 2019, 107, 545–551. [Google Scholar] [CrossRef]
- McWhirter, M.J.; Bremer, P.J.; Lamont, I.L.; McQuillan, A.J. Siderophore-Mediated Covalent Bonding to Metal (Oxide) Surfaces during Biofilm Initiation by Pseudomonas aeruginosa Bacteria. Langmuir 2003, 19, 3575–3577. [Google Scholar] [CrossRef]
- Petrone, L. Molecular surface chemistry in marine bioadhesion. Adv. Colloid Interface Sci. 2013, 195–196, 1–18. [Google Scholar] [CrossRef]
- Horst, A.M.; Neal, A.C.; Mielke, R.E.; Sislian, P.R.; Suh, W.H.; Madler, L.; Stucky, G.D.; Holden, P.A. Dispersion of TiO2 nanoparticle agglomerates by Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2010, 76, 7292–7298. [Google Scholar] [CrossRef]
- Baramov, T.; Keijzer, K.; Irran, E.; Mosker, E.; Baik, M.H.; Sussmuth, R. Synthesis and Structural Characterization of Hexacoordinate Silicon, Germanium, and Titanium Complexes of the E-coli Siderophore Enterobactin. Chem. Eur. J. 2013, 19, 10536–10542. [Google Scholar] [CrossRef]
- Jones, K.E.; Batchler, K.L.; Zalouk, C.; Valentine, A.M. Ti(IV) and the Siderophore Desferrioxamine B: A Tight Complex Has Biological and Environmental Implications. Inorg. Chem. 2017, 56, 1264–1272. [Google Scholar] [CrossRef]
- Butler, A.; Theisen, R.M. Iron(III)-siderophore coordination chemistry: Reactivity of marine siderophores. Coord. Chem. Rev. 2010, 254, 288–296. [Google Scholar] [CrossRef]
- Dakanali, M.; Kefalas, E.T.; Raptopoulou, C.P.; Terzis, A.; Voyiatzis, G.; Kyrikou, I.; Mavromoustakos, T.; Salifoglou, A. A new dinuclear Ti(IV)-peroxo-citrate complex from aqueous solutions. Synthetic, structural, and spectroscopic studies in relevance to aqueous titanium(IV)-peroxo-citrate speciation. Inorg. Chem. 2003, 42, 4632–4639. [Google Scholar] [CrossRef]
- Deng, Y.F.; Zhou, Z.H.; Wan, H.L. pH-dependent isolations and spectroscopic, structural, and thermal studies of titanium citrate complexes. Inorg. Chem. 2004, 43, 6266–6273. [Google Scholar] [CrossRef]
- Collins, J.M.; Uppal, R.; Incarvito, C.D.; Valentine, A.M. Titanium(IV) Citrate Speciation and Structure under Environmentally and Biologically Relevant Conditions. Inorg. Chem. 2005, 44, 3431–3440. [Google Scholar] [CrossRef]
- Panagiotidis, P.; Kefalas, E.T.; Raptopoulou, C.P.; Terzis, A.; Mavromoustakos, T.; Salifoglou, A. Delving into the complex picture of Ti(IV)-citrate speciation in aqueous media: Synthetic, structural, and electrochemical considerations in mononuclear Ti(IV) complexes containing variably deprotonated citrate ligands. Inorg. Chim. Acta 2008, 361, 2210–2224. [Google Scholar] [CrossRef]
- Uppal, R.; Incarvito, C.D.; Lakshmi, K.V.; Valentine, A.M. Aqueous spectroscopy and redox properties of carboxylate-bound titanium. Inorg. Chem. 2006, 45, 1795–1804. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Eames, E.V.; Valentine, A.M. Reconsideration of serum Ti(IV) transport: Albumin and transferrin trafficking of Ti(IV) and its complexes. J. Am. Chem. Soc. 2008, 130, 2262–2270. [Google Scholar] [CrossRef]
- Silva, A.M.N.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009, 8616–8625. [Google Scholar] [CrossRef]
- Valentine, A.M. Siderophore-promoted release of titanium(IV) from metal oxide materials. Abstr. Pap. Am. Chem. Soc. 2017, 253, 1. [Google Scholar]
- Dey, R.; Mukharjee, K.; Langer, V.; Roychowdhury, P. A titanium salicylate, Na4[Ti(C7H4O3)3]2 11H2O. Acta Crystallogr. Sect. E Struct Rep. Online 2005, 61, M1495–M1497. [Google Scholar] [CrossRef]
- Loza-Rosas, S.A.; Vazquez, A.M.; Rivero, K.I.; Negrón, L.J.; Delgado, Y.; Benjamin-Rivera, J.A.; Vazquez-Maldonado, A.L.; Parks, T.B.; Munet-Colon, C.; Tinoco, A.D. Expanding the therapeutic potential of the iron chelator deferasirox in the development of aqueous stable Ti(IV) anticancer complexes. Inorg. Chem. 2017, 56, 7788–7802. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; Naud, N.; Taché, S.; Grysan, P.; Reguer, S. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci. Rep. 2017, 7, 40373. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and removal of titanium at full scale wastewater treatment plants: Implications for TiO2 nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef]
- Gondikas, A.; von der Kammer, F.; Kaegi, R.; Borovinskaya, O.; Neubauer, E.; Navratilova, J.; Praetorius, A.; Cornelis, G.; Hofmann, T. Where is the nano? Analytical approaches for the detection and quantification of TiO 2 engineered nanoparticles in surface waters. Environ. Sci. Nano. 2018, 5, 313–326. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, N.K.; Singla, M.L. Study on reflectivity and photostability of Al-doped TiO2 nanoparticles and their reflectors. J. Mater. Res. 2013, 28, 521–528. [Google Scholar] [CrossRef]
- Schug, H.; Isaacson, C.W.; Sigg, L.; Ammann, A.A.; Schirmer, K. Effect of TiO2 nanoparticles and UV radiation on extracellular enzyme activity of intact heterotrophic biofilms. Environ. Sci. Technol. 2014, 48, 11620–11628. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Carabin, A.; Drogui, P.; Robert, D. Photo-degradation of carbamazepine using TiO2 suspended photocatalysts. J. Taiwan Ins. Chem. Eng. 2015, 54, 109–117. [Google Scholar] [CrossRef]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.; Mantzavinos, D.; Fatta-Kassinos, D. UV-A and solar photodegradation of ibuprofen and carbamazepine catalyzed by TiO2. Sep. Sci. Technol. 2010, 45, 1564–1570. [Google Scholar] [CrossRef]
- Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.; Grilli, R.; Mamane, H. N-doped TiO2-coated ceramic membrane for carbamazepine degradation in different water qualities. Nanomaterials 2017, 7, 206. [Google Scholar] [CrossRef]
- Djouder, R.; Laoufi, A.; Bentahar, F. Photodegradation of salicylic acid in aqueous phase by TiO2/UV System. Revue des Energies Renouvelables 2012, 15, 179–185. [Google Scholar]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B Biol. 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Gogniat, G.; Thyssen, M.; Denis, M.; Pulgarin, C.; Dukan, S. The bactericidal effect of TiO2 photocatalysis involves adsorption onto catalyst and the loss of membrane integrity. FEMS Microbiol. Lett. 2006, 258, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Maness, P.-C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar]
- Kim, B.; Kim, D.; Cho, D.; Cho, S. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria. Chemosphere 2003, 52, 277–281. [Google Scholar] [CrossRef]
- Zane, A.; Zuo, R.; Villamena, F.A.; Rockenbauer, A.; Foushee, A.M.D.; Flores, K.; Dutta, P.K.; Nagy, A. Biocompatibility and antibacterial activity of nitrogen-doped titanium dioxide nanoparticles for use in dental resin formulations. Int. J. Nanomed. 2016, 11, 6459. [Google Scholar] [CrossRef]
- Florez, F.L.E.; Hiers, R.D.; Larson, P.; Johnson, M.; O’Rear, E.; Rondinone, A.J.; Khajotia, S.S. Antibacterial dental adhesive resins containing nitrogen-doped titanium dioxide nanoparticles. Mater. Sci. Eng. C. 2018, 93, 931–943. [Google Scholar] [CrossRef]
- Chambers, C.; Stewart, S.; Su, B.; Jenkinson, H.; Sandy, J.; Ireland, A. Silver doped titanium dioxide nanoparticles as antimicrobial additives to dental polymers. Dent. Mater. 2017, 33, e115–e123. [Google Scholar] [CrossRef]
- Lakshmi, K.D.; Rao, T.S.; Padmaja, J.S.; Raju, I.M.; Kumar, M.R. Structure, photocatalytic and antibacterial activity study of Meso porous Ni and S co-doped TiO2 nano material under visible light irradiation. Chin. J. Chem. Eng. 2018, 10, 494–504. [Google Scholar] [CrossRef]
- Zahid, M.; Papadopoulou, E.L.; Suarato, G.; Binas, V.D.; Kiriakidis, G.; Gounaki, I.; Moira, O.; Venieri, D.; Bayer, I.S.; Athanassiou, A. Fabrication of visible light-induced antibacterial and self-cleaning cotton fabrics using manganese doped TiO2 nanoparticles. ACS Appl. Bio Mater. 2018, 1, 1154–1164. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Geetha, N.; Kanimozhi, K.; Magdalane, C.M.; Sivaranjani, S.; Ayeshamariam, A.; Kennedy, J.; Maaza, M. In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of zinc oxide doped TiO2 nanocrystals: Investigation of bio-medical application by chemical method. Mater. Sci. Eng. C 2017, 74, 325–333. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.; Bartlett, J.; Nolan, M.; Pillai, S. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Guo, J.; Li, S.; Duan, L.; Guo, P.; Li, X.; Cui, Q.; Wang, H.; Jiang, Q. Preparation of Si doped molecularly imprinted TiO2 photocatalyst and its degradation to antibiotic wastewater. Integr. Ferroelectr. 2016, 168, 170–182. [Google Scholar] [CrossRef]
- Leyland, N.S.; Podporska-Carroll, J.; Browne, J.; Hinder, S.J.; Quilty, B.; Pillai, S.C. Highly efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.-S.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Bohara, R.A.; Mali, S.S.; Pawar, S.H.; Delekar, S.D. Synthesis and visible light photocatalytic antibacterial activity of nickel-doped TiO2 nanoparticles against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A Chem. 2014, 294, 130–136. [Google Scholar] [CrossRef]
- Zhang, A.-P.; Sun, Y.-P. Photocatalytic killing effect of TiO2 nanoparticles on Ls-174-t human colon carcinoma cells. World J. Gastroenterol. 2004, 10, 3191. [Google Scholar] [CrossRef]
- Wamer, W.G.; Yin, J.-J.; Wei, R.R. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic. Biol. Med. 1997, 23, 851–858. [Google Scholar] [CrossRef]
- Jaeger, A.; Weiss, D.G.; Jonas, L.; Kriehuber, R. Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology 2012, 296, 27–36. [Google Scholar] [CrossRef]
- Lagopati, N.; Kitsiou, P.; Kontos, A.; Venieratos, P.; Kotsopoulou, E.; Kontos, A.; Dionysiou, D.; Pispas, S.; Tsilibary, E.; Falaras, P. Photo-induced treatment of breast epithelial cancer cells using nanostructured titanium dioxide solution. J. Photochem. Photobiol. A Chem. 2010, 214, 215–223. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, Y.; Dong, L. A comparison of TiO2 and ZnO nanoparticles as photosensitizers in photodynamic therapy for cancer. J. Biomed. Nanotechnol. 2014, 10, 1450–1457. [Google Scholar] [CrossRef]
- Yang, C.-C.; Sun, Y.-J.; Chung, P.-H.; Chen, W.-Y.; Swieszkowski, W.; Tian, W.; Lin, F.-H. Development of Ce-doped TiO2 activated by X-ray irradiation for alternative cancer treatment. Ceram. Int. 2017, 43, 12675–12683. [Google Scholar] [CrossRef]
- Xie, J.; Pan, X.; Wang, M.; Yao, L.; Liang, X.; Ma, J.; Fei, Y.; Wang, P.-N.; Mi, L. Targeting and Photodynamic Killing of Cancer Cell by Nitrogen-Doped Titanium Dioxide Coupled with Folic Acid. Nanomaterials 2016, 6, 113. [Google Scholar] [CrossRef]
- Glass, S.; Trinklein, B.; Abel, B.; Schulze, A. TiO2 as Photosensitizer and Photoinitiator for Synthesis of Photoactive TiO2-PEGDA Hydrogel Without Organic Photoinitiator. Front. Chem. 2018, 6, 340. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Ates, M.; Demir, V.; Adiguzel, R.; Arslan, Z. Bioaccumulation, subacute toxicity, and tissue distribution of engineered titanium dioxide nanoparticles in goldfish (Carassius auratus). J. Nanomater. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Mansfield, C.; Alloy, M.; Hamilton, J.; Verbeck, G.; Newton, K.; Klaine, S.; Roberts, A. Photo-induced toxicity of titanium dioxide nanoparticles to Daphnia magna under natural sunlight. Chemosphere 2015, 120, 206–210. [Google Scholar] [CrossRef]
- Jovanovic, B.; Guzman, H.M. Effects of titanium dioxide (TiO2) nanoparticles on caribbean reef-building coral (Montastraea faveolata). Environ. Toxicol. Chem. 2014, 33, 1346–1353. [Google Scholar] [CrossRef]
- Jovanović, B.; Whitley, E.M.; Kimura, K.; Crumpton, A.; Palić, D. Titanium dioxide nanoparticles enhance mortality of fish exposed to bacterial pathogens. Environ. Pollut. 2015, 203, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Quiles, D.; Tovar-Sánchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Reed, R.B.; Lee, S.; Bi, X.; Hanigan, D.; Yang, Y.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Detection and Sizing of Ti-Containing Particles in Recreational Waters Using Single Particle ICP-MS. Bull. Environ. Contam. Toxicol. 2018, 100, 120–126. [Google Scholar] [CrossRef]
- David Holbrook, R.; Motabar, D.; Quinones, O.; Stanford, B.; Vanderford, B.; Moss, D. Titanium distribution in swimming pool water is dominated by dissolved species. Environ. Pollut. 2013, 181, 68–74. [Google Scholar] [CrossRef]
- Buettner, K.M.; Valentine, A.M. Bioinorganic chemistry of titanium. Chem. Rev. 2011, 112, 1863–1881. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding marine mussel adhesion. Mar. Biotechnol. (N. Y.) 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Sever, M.J.; Wilker, J.J. Absorption spectroscopy and binding constants for first-row transition metal complexes of a DOPA-containing peptide. Dalton Trans. 2005, 6, 813–822. [Google Scholar] [CrossRef]
- Guo, M.; Harvey, I.; Campopiano, D.J.; Sadler, P.J. Short Oxo–Titanium (iv) Bond in Bacterial Transferrin: A Protein Target for Metalloantibiotics. Angew. Chem. Int. Edit. 2006, 45, 2758–2761. [Google Scholar] [CrossRef]
- Sugumaran, M.; Robinson, W.E. Structure, biosynthesis and possible function of tunichromes and related compounds. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 163, 1–25. [Google Scholar] [CrossRef]
- Uppal, R.; Israel, H.P.; Incarvito, C.D.; Valentine, A.M. Titanium(IV) Complexes with N,N′-Dialkyl-2,3-dihydroxyterephthalamides and 1-Hydroxy-2(1H)-pyridinone as Siderophore and Tunichrome Analogues. Inorg. Chem. 2009, 48, 10769–10779. [Google Scholar] [CrossRef]
- Black, W.A.P.; Mitchell, R.L. Trace elements in the common brown algae and in sea water. J. Mar. Biol. Assoc. UK 1952, 30, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Levine, E.P. Occurrence of titanium, vanadium, chromium, and sulfuric acid in ascidian Eudistoma ritteri. Science 1961, 133, 1352–1353. [Google Scholar] [CrossRef]
- Orians, K.J.; Boyle, E.A.; Bruland, K.W. Dissolved titanium in the open ocean. Nature. 1990, 348, 322–325. [Google Scholar] [CrossRef]
- Butler, A. Acquisition and utilization of transition metal ions by marine organisms. Science 1998, 281, 207–210. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Dufour, E.K.; Roberts, M.S. Nanotechnology, cosmetics and the skin: Is there a health risk? Skin Pharmacol. Physiol. 2008, 21, 136–149. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Xue, C.; Zhou, S.; Lan, F.; Bi, L.; Xu, H.; Yang, X.; Zeng, F.D. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol. Lett. 2009, 191, 1–8. [Google Scholar] [CrossRef]
- Jani, P.U.; McCarthy, D.E.; Florence, A.T. Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int. J. Pharm. 1994, 105, 157–168. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Tasat, D.; Guglielmotti, M.B.; Cabrini, R.L. Titanium transport through the blood stream. An experimental study on rats. J. Mater. Sci. Mater. Med. 2003, 14, 1099–1103. [Google Scholar] [CrossRef]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotechnol. 2013, 11, 26. [Google Scholar] [CrossRef]
- Tedja, R.; Lim, M.; Amal, R.; Marquis, C. Effects of Serum Adsorption on Cellular Uptake Profile and Consequent Impact of Titanium Dioxide Nanoparticles on Human Lung Cell Lines. ACS Nano 2012, 6, 4083–4093. [Google Scholar] [CrossRef]
- Allouni, Z.E.; Gjerdet, N.R.; Cimpan, M.R.; Høl, P.J. The effect of blood protein adsorption on cellular uptake of anatase TiO2 nanoparticles. Int. J. Nanomed. 2015, 10, 687. [Google Scholar]
- Preissner, K.T. Structure and biological role of vitronectin. Annu. Rev. Cell Biol. 1991, 7, 275–310. [Google Scholar] [CrossRef]
- Boylan, A.M.; Sanan, D.A.; Sheppard, D.; Broaddus, V.C. Vitronectin enhances internalization of crocidolite asbestos by rabbit pleural mesothelial cells via the integrin alpha v beta 5. J. Clin. Investig. 1995, 96, 1987–2001. [Google Scholar] [CrossRef]
- Pande, P.; Mosleh, T.A.; Aust, A.E. Role of αvβ5 integrin receptor in endocytosis of crocidolite and its effect on intracellular glutathione levels in human lung epithelial (A549) cells. Toxicol. Appl. Pharmacol. 2006, 210, 70–77. [Google Scholar] [CrossRef]
- Chen, P.; Kanehira, K.; Taniguchi, A. Role of toll-like receptors 3, 4 and 7 in cellular uptake and response to titanium dioxide nanoparticles. Sci. Technol. Adv. Mater. 2013, 14, 015008. [Google Scholar] [CrossRef] [Green Version]
- Pinsino, A.; Russo, R.; Bonaventura, R.; Brunelli, A.; Marcomini, A.; Matranga, V. Titanium dioxide nanoparticles stimulate sea urchin immune cell phagocytic activity involving TLR/p38 MAPK-mediated signalling pathway. Sci. Rep. 2015, 5, 14492. [Google Scholar] [CrossRef] [Green Version]
- Sweet, M.J.; Chessher, A.; Singleton, I. Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2012; Volume 80, pp. 113–142. [Google Scholar]
- Sund, J.; Alenius, H.; Vippola, M.; Savolainen, K.; Puustinen, A. Proteomic characterization of engineered nanomaterial–protein interactions in relation to surface reactivity. ACS Nano 2011, 5, 4300–4309. [Google Scholar] [CrossRef]
- Deng, Z.J.; Mortimer, G.; Schiller, T.; Musumeci, A.; Martin, D.; Minchin, R.F. Differential plasma protein binding to metal oxide nanoparticles. Nanotechnology 2009, 20, 455101. [Google Scholar] [CrossRef]
- Maurer, M.M.; Donohoe, G.C.; Maleki, H.; Yi, J.; McBride, C.; Nurkiewicz, T.R.; Valentine, S.J. Comparative plasma proteomic studies of pulmonary TiO2 nanoparticle exposure in rats using liquid chromatography tandem mass spectrometry. J. Proteom. 2016, 130, 85–93. [Google Scholar] [CrossRef]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.; Godwin, H. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef]
- Givens, B.E.; Xu, Z.; Fiegel, J.; Grassian, V.H. Bovine serum albumin adsorption on SiO2 and TiO2 nanoparticle surfaces at circumneutral and acidic pH: A tale of two nano-bio surface interactions. J. Colloid Interface Sci. 2017, 493, 334–341. [Google Scholar] [CrossRef]
- Zaqout, M.S.; Sumizawa, T.; Igisu, H.; Higashi, T.; Myojo, T. Binding of human serum proteins to titanium dioxide particles in vitro. J. Occup. Health 2011, 53, 75. [Google Scholar] [CrossRef]
- Afaq, F.; Abidi, P.; Matin, R.; Rahman, Q. Cytotoxicity, pro-oxidant effects and antioxidant depletion in rat lung alveolar macrophages exposed to ultrafine titanium dioxide. J. Appl. Toxicol. 1998, 18, 307–312. [Google Scholar] [CrossRef]
- Jin, C.Y.; Zhu, B.S.; Wang, X.F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 1–33. [Google Scholar] [CrossRef]
- Food and Drug Administration. Sunscreen Drug Products for Over-the-Counter Human Use; Food and Drug Administration: Silver Spring, MD, USA, 2019; pp. 6204–6275.
- Xu, Y.; Wei, M.-T.; Ou-Yang, H.D.; Walker, S.G.; Wang, H.Z.; Gordon, C.R.; Guterman, S.; Zawacki, E.; Applebaum, E.; Brink, P.R. Exposure to TiO2 nanoparticles increases Staphylococcus aureus infection of HeLa cells. J. Nanobiotechnol. 2016, 14, 34. [Google Scholar] [CrossRef]
- Huang, C.; Sun, M.; Yang, Y.; Wang, F.; Ma, X.; Li, J.; Wang, Y.; Ding, Q.; Ying, H.; Song, H. Titanium dioxide nanoparticles prime a specific activation state of macrophages. Nanotoxicology 2017, 11, 737–750. [Google Scholar] [CrossRef]
- Chakraborty, S.; Castranova, V.; Perez, M.K.; Piedimonte, G. Nanoparticles increase human bronchial epithelial cell susceptibility to respiratory syncytial virus infection via nerve growth factor-induced autophagy. Physiol. Rep. 2017, 5, e13344. [Google Scholar] [CrossRef]
- Li, J.; Yang, S.; Lei, R.; Gu, W.; Qin, Y.; Ma, S.; Chen, K.; Chang, Y.; Bai, X.; Xia, S. Oral administration of rutile and anatase TiO 2 nanoparticles shifts mouse gut microbiota structure. Nanoscale 2018, 10, 7736–7745. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell. 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kuroda, E.; Hirai, T.; Tsutsumi, Y.; Ishii, K.J. Allergic Responses Induced by the Immunomodulatory Effects of Nanomaterials upon Skin Exposure. Front. Immunol. 2017, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Adachi, T.; Kobayashi, T.; Sugihara, E.; Yamada, T.; Ikuta, K.; Pittaluga, S.; Saya, H.; Amagai, M.; Nagao, K. Hair follicle-derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat. Med. 2015, 21, 1272–1279. [Google Scholar] [CrossRef]
- Nagao, K.; Kobayashi, T.; Moro, K.; Ohyama, M.; Adachi, T.; Kitashima, D.Y.; Ueha, S.; Horiuchi, K.; Tanizaki, H.; Kabashima, K.; et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012, 13, 744–752. [Google Scholar] [CrossRef] [Green Version]
- Larsen, S.T.; Roursgaard, M.; Jensen, K.A.; Nielsen, G.D. Nano titanium dioxide particles promote allergic sensitization and lung inflammation in mice. Basic Clin. Pharmacol. Toxicol. 2010, 106, 114–117. [Google Scholar] [CrossRef]
- Schreiver, I.; Hesse, B.; Seim, C.; Castillo-Michel, H.; Villanova, J.; Laux, P.; Dreiack, N.; Penning, R.; Tucoulou, R.; Cotte, M.; et al. Synchrotron-based ν-XRF mapping and μ-FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin. Sci. Rep. 2017, 7, 11395. [Google Scholar] [CrossRef]
- Paradies, J.; Crudass, J.; MacKay, F.; Yellowlees, L.J.; Montgomery, J.; Parsons, S.; Oswald, I.; Robertson, N.; Sadler, P.J. Photogeneration of titanium(III) from titanium(IV) citrate in aqueous solution. J. Inorg. Biochem. 2006, 100, 1260–1264. [Google Scholar] [CrossRef]
- Zehnder, A.; Wuhrmann, K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science 1976, 194, 1165–1166. [Google Scholar] [CrossRef]
- Sun, H.Z.; Li, H.Y.; Weir, R.A.; Sadler, P.J. The first specific Ti-IV-protein complex: Potential relevance to anticancer activity of titanocenes. Angew. Chem. Int. Edit. 1998, 37, 1577–1579. [Google Scholar] [CrossRef]
- Guo, M.L.; Sun, H.Z.; McArdle, H.J.; Gambling, L.; Sadler, P.J. Ti(IV) uptake and release by human serum transferrin and recognition of Ti(IV) transferrin by cancer cells: Understanding the mechanism of action of the anticancer drug titanocene dichloride. Biochemistry 2000, 39, 10023–10033. [Google Scholar] [CrossRef]
- Messori, L.; Orioli, P.; Banholzer, V.; Pais, I.; Zatta, P. Formation of titanium(IV) transferrin by reaction of human serum apotransferrin with titanium complexes. FEBS Lett. 1999, 442, 157–161. [Google Scholar] [CrossRef]
- Moshtaghie, A.A.; Ani, M.; Arabi, M.H. Spectroscopic Studies on Titanium Ion Binding to the Apolactoferrin. Iran. Biomed. J. 2006, 10, 93–98. [Google Scholar]
- Tinoco, A.D.; Incarvito, C.D.; Valentine, A.M. Calorimetric, Spectroscopic, and Model Studies Provide Insight into the Transport of Ti(IV) by Human Serum Transferrin. J. Am. Chem. Soc. 2007, 129, 3444–3454. [Google Scholar] [CrossRef]
- Sekyere, E.; Richardson, D.R. The membrane-bound transferrin homologue melanotransferrin: Roles other than iron transport? FEBS Lett. 2000, 483, 11–16. [Google Scholar] [CrossRef]
- Sekyere, E.O.; Dunn, L.L.; Richardson, D.R. Examination of the distribution of the transferrin homologue, melanotransferrin (tumour antigen p97), in mouse and human. Biochim. Biophys. Acta 2005, 1722, 131–142. [Google Scholar] [CrossRef]
- Suryo Rahmanto, Y.; Bal, S.; Loh, K.H.; Yu, Y.; Richardson, D.R. Melanotransferrin: Search for a function. Biochim. Biophys. Acta 2012, 1820, 237–243. [Google Scholar] [CrossRef]
- Taira, M.; Sasaki, K.; Saitoh, S.; Nezu, T.; Sasaki, M.; Kimura, S.; Terasaki, K.; Sera, K.; Narushima, T.; Araki, Y. Accumulation of Element Ti in Macrophage-like RAW264 Cells Cultured in Medium with 1 ppm Ti and Effects on Cell Viability, SOD Production and TNF-α Secretion. Dent. Mater. J. 2006, 25, 726–732. [Google Scholar] [CrossRef]
- Guo, M.L.; Guo, Z.J.; Sadler, P.J. Titanium(IV) targets phosphoesters on nucleotides: Implications for the mechanism of action of the anticancer drug titanocene dichloride. J. Biol. Inorg. Chem. 2001, 6, 698–707. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Sharma, R.K.; Gaur, K.; Cátala Torres, J.F.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials 2019, 12, 2317. https://doi.org/10.3390/ma12142317
Sharma S, Sharma RK, Gaur K, Cátala Torres JF, Loza-Rosas SA, Torres A, Saxena M, Julin M, Tinoco AD. Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials. 2019; 12(14):2317. https://doi.org/10.3390/ma12142317
Chicago/Turabian StyleSharma, Shweta, Rohit K. Sharma, Kavita Gaur, José F. Cátala Torres, Sergio A. Loza-Rosas, Anamaris Torres, Manoj Saxena, Mara Julin, and Arthur D. Tinoco. 2019. "Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen" Materials 12, no. 14: 2317. https://doi.org/10.3390/ma12142317