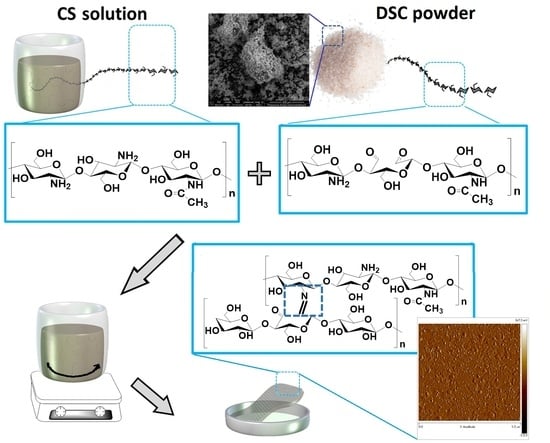
Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dialdehyde Starch Synthesis
2.3. Dialdehyde Chitosan Synthesis
2.4. Cross-Linked Chitosan Films Preparation
2.5. Analysis and Characterization
2.5.1. Determination of the Content of Aldehyde Groups (ALD, %)
2.5.2. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.5.3. Scanning Electron Microscopy (SEM)
2.5.4. Contact Angle Measurement
2.5.5. Thermal Analysis
2.5.6. Mechanical Properties
2.5.7. Atomic Force Microscopy (AFM)
2.5.8. Swelling Ability
2.5.9. Toxicity Assessment
3. Results and Discussion
3.1. Synthesis of Dialdehyde Chitosan and Its Oxidation Degree
3.2. Characterization of Dialdehyde Chitosan
3.2.1. ATR-FTIR Spectroscopy
3.2.2. Scanning Electron Microscopy (SEM)
3.2.3. Contact Angle Measurement
3.2.4. Thermal Analysis
3.2.5. X-ray Diffraction (XRD)
3.3. Formation of Chitosan Films by Cross-Linking with Various Cross-Linkers
3.3.1. ATR-FTIR Spectroscopy
3.3.2. Scanning Electron Microscopy (SEM)
3.3.3. Atomic Force Microscopy (AFM)
3.3.4. Contact Angle Measurement
3.3.5. Thermal Analysis
3.3.6. Mechanical Properties
3.3.7. Swelling Ability
3.3.8. Microtox® Toxicity Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muhamad, I.I.; Asmak, N.; Lazim, M.; Selvakumaran, S. Natural polysaccharide-based composites for drug delivery and biomedical applications. In Natural Polysaccharides in Drug Delivery and Biomedical Applications, 1st ed.; Hasnain, M.S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 419–440. [Google Scholar]
- Nady, N.; Kandil, S.H. Novel blend for producing porous chitosan-based films suitable for biomedical applications. Membranes 2018, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Borowska, M.; Mylkie, K.; Kozlowska, M.; Nowak, P.; Chelminiak-Dudkiewicz, D.; Kozakiewicz, A.; Ilnicka, A.; Kaczmarek-Kedziera, A. Effect of geometrical structure, drying, and synthetic method on aminated chitosan-coated magnetic nanoparticles utility for HSA effective immobilization. Molecules 2019, 24, 1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziegler-Borowska, M. Magnetic nanoparticles coated with aminated starch for HSA immobilization—Simple and fast polymer surface functionalization. Int. J. Biol. Macromol. 2019, 136, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Chetouania, A.; Follainb, N.; Marais, S.; Rihouey, C.; Elkolli, M.; Bounekhel, M.; Benachour, D.; Cerf, D.L. Physicochemical properties and biological activities of novel blend films using oxidized pectin/chitosan. Int. J. Biol. Macromol. 2017, 97, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, Z.; Peng, Y.; Han, B.; Li, Z.; Li, X.; Liu, W. Preparation, characterization and feasibility study of dialdehyde carboxymethyl cellulose as a novel cross-linking reagent. Carbohydr. Polym. 2016, 137, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.F.; Panzavolta, S.; Albertini, B.; Bonvicini, F.; Gentilomi, G.; Orlacchio, R.; Passerini, N.; Bigi, A.; Dolci, L.S. Functional properties of chitosan films modified by snail mucus extract. Int. J. Biol. Macromol. 2020, 143, 126–135. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial chitosan conjugates: Current synthetic strategies and potential applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Zhao, H.; Xu, J.; Xie, Z.; Li, G.; Gan, Z.; Wang, X. Borneol-modified chitosan: Antimicrobial adhesion properties and application in skin flora protection. Carbohydr. Polym. 2020, 228, 115378. [Google Scholar] [CrossRef]
- Nataraj, D.; Sakkara, S.; Meghwal, M.; Reddy, N. Chitin and chitosan: Properties and applications. Int. J. Biol. Macromol. 2018, 120, 1256–1264. [Google Scholar] [CrossRef]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, characterization and antimicrobial activity of a novel chitosan schiff bases based on heterocyclic moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug. Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Ju, J. Effect of cross-linking agents on drug distribution in chitosan hydrogel for targeted drug delivery to treat cancer. J. Polym. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Li, P.; Zhao, J.; Chen, Y.; Cheng, B.; Yu, Z.; Zhao, Y.; Yan, X.; Tong, Z.; Jin, S. Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydr. Polym. 2016, 157, 1383–1392. [Google Scholar] [CrossRef]
- Nešović, K.; Janković, A.; Radetić, T.; Perić-Grujić, A.; Vukašinović-Sekulić, M.; Kojić, V.; Rhee, K.Y.; Mišković-Stanković, V. Chitosan-based hydrogel wound dressings with electrochemically incorporated silver nanoparticles—In vitro study. Eur. Polym. J. 2019, 121, 109257. [Google Scholar] [CrossRef]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Chitosan as a potential alternative to collagen for the development of genipin-crosslinked scaffolds. React. Funct. Polym. 2020, 146, 104414. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan based scaffolds and their applications in wound healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.V.; Gomes, P.S.; Fernandes, M.H.; Costa, M.E.V.; Almeida, M.M. Glutaraldehyde-crosslinking chitosan scaffolds reinforced with calcium phosphate spray-dried granules for bone tissue applications. Mater. Sci. Eng. C 2020, 109, 110557. [Google Scholar] [CrossRef]
- Kanauchi, O.; Deuchi, K.; Imasato, Y.; Shizukuishi, M.; Kobayashi, E. Mechanism for the inhibition of fat digestion by chitosan and for the synergistic effect of ascorbate. Biosci. Biotechnol. Biochem. 1995, 59, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Liu, K.; Liu, D.; Guo, X.; Ge, Y.; Li, J.; Cui, L.; et al. HTCC as a highly effective polymeric inhibitor of SARS-CoV-2 and MERS-CoV. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Pellá, C.G.M.; Lima-Tenório, K.M.; Tenório-Neto, T.E.; Guilherme, R.M.; Muniz, C.E.; Rubira, F.A. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar]
- Mahdavinia, G.R.; Pourjavadi, A.; Zohuriaan-Mehr, M.J. Synthesis and properties of highly swelling PAAm/chitosan semi-IPN hydrogels. Macromol. Symp. 2008, 274, 171–176. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, Z.; Qian, Q.; Zhang, Z.; Wang, X. Improving the pervaporation performance of the glutaraldehyde cross-linked chitosan membrane by simultaneously changing its surface and bulk structure. J. Membr. Sci. 2010, 348, 213–223. [Google Scholar] [CrossRef]
- Wei, Y.C.; Hudson, S.M.; Mayer, J.M.; Kaplan, D.L. The cross-linking of chitosan fibers. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 2187–2193. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Andrady, A.L. Permeability of vitamin B-12 in chitosan membranes. Effect of cross-linking and blending with poly(vinyl alcohol) on permeability. J. Appl. Polym. Sci. 1992, 44, 17–28. [Google Scholar] [CrossRef]
- De Angelis, A.A.; Capitani, D.; Crescenzi, V. Synthesis and 13C CP-MAS NMR characterization of a new chitosan-based polymeric network. Macromolecules 1998, 31, 1595–1601. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Ahmad, Z.; Akil, H.M. Preparation and characterization of 1,2,4,5-benzenetetra carboxylic-chitosan. e-Polymers 2010, 10, 77. [Google Scholar] [CrossRef]
- Jin, J.; Song, M.; Hourston, D.J. Novel chitosan-based films cross-linked by genipin with improved physical properties. Biomacromolecules 2004, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Kuboe, Y.; Tonegawa, H.; Ohkawa, K.; Yamamoto, H. Quinone cross-linked polysaccharide hybrid fiber. Biomacromolecules 2004, 5, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.R.; Schauer, C.L.; Qadri, S.B.; Price, R.R. Chitosan cross-linking with a water-soluble, blocked diisocyanate. 1. solid state. Biomacromolecules 2002, 3, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Lin-Gibson, S.; Walls, H.J.; Kennedy, S.B.; Welsh, E.R. Reaction kinetics and gel properties of blocked diisocyinate cross-linked chitosan hydrogels. Carbohydr. Polym. 2003, 54, 193–199. [Google Scholar] [CrossRef]
- Ganguly, K.; Kulkarni, A.R.; Aminabhavi, T.M. In vitro cytotoxicity and in vivo efficacy of 5-fluorouracil-loaded enteric-coated PEG-cross-linked chitosan microspheres in colorectal cancer therapy in rats. Drug Deliv. 2016, 23, 2838–2851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, A.R.; Hukkeri, V.I.; Sung, H.-W.; Liang, H.-F. A novel method for the synthesis of the peg-crosslinked chitosan with a ph-independent swelling behavior. Macromol. Biosci. 2005, 5, 925–928. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Baier, J.M. Acellular vascular tissues: Natural biomaterials for tissue repair and tissue engineering. Biomaterials 2000, 21, 2215–2231. [Google Scholar] [CrossRef]
- Münster, L.; Capáková, Z.; Fišera, M.; Kuřitka, I.; Vícha, L. Biocompatible dialdehyde cellulose/poly(vinyl alcohol) hydrogels with tunable properties. Carbohydr. Polym. 2019, 218, 333–342. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D printed chitosan dressing cross-linked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is dialdehyde starch a valuable cross-linking agent for collagen/elastin based materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef] [Green Version]
- Kamoun, K.A. N-Succinyl chitosan-dialdehyde starch hybrid hydrogels for biomedical applications. J. Adv. Res. 2015, 7, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gu, Z.; Qin, H.; Li, L.; Yang, X. Crosslinking effect of dialdehyde starch (DAS) on decellularized porcine aortas for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Long, Z.; Song, C.; Dai, L.; He, H.; Wang, P. Preparation of dialdehyde chitosan and its application in green synthesis of silver nanoparticles. Bioresources 2013, 8, 6161–6172. [Google Scholar] [CrossRef] [Green Version]
- Keshk, S.M.A.S.; Ramadan, A.M.; Al-Sehemi, A.G.; Irfan, A.; Bondock, S. An unexpected reactivity during periodate oxidation of chitosan and the affinity of its 2,3-di-aldehyde toward sulfa drugs. Carbohydr. Polym. 2017, 175, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dan, N.; Dan, W.; Gong, J. Feasibility study of the natural derived chitosan dialdehyde for chemical modification of collagen. Int. J. Biol. Macromol. 2016, 82, 989–997. [Google Scholar] [CrossRef]
- Bam, P.; Bhatta, A.; Krishnamoorthy, G. Design of biostable scaffold based on collagen cross-linked by dialdehyde chitosan with presence of gallic acid. Int. J. Biol. Macromol. 2019, 130, 836–844. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Wegrzynowska-Drzymalska, K.; Chelminiak-Dudkiewicz, D.; Kowalonek, J.; Kaczmarek, H. Photochemical reactions in dialdehyde starch. Molecules 2018, 23, 3358. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Shen, Y.; Xie, A.; Zhang, W. Preparation and protein immobilization of magnetic dialdehyde starch nanoparticles. J. Phys. Chem. B 2013, 117, 3720–3725. [Google Scholar] [CrossRef]
- Owens, D.; Wendt, R. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Kuderko, J.; Bajek, A.; Maj, M.; Sionkowska, A.; Ziegler-Borowska, M. Collagen/elastin hydrogels cross-linked by squaric acid. Mater. Sci. Eng. C 2016, 60, 100–108. [Google Scholar] [CrossRef]
- Johnson, B.T. Microtox® acute toxicity test. In Small-Scale Freshwater Toxicity Investigations; Blaise, C., Férard, J.-F., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 1, pp. 69–105. [Google Scholar]
- Recillas, S.; García, A.; González, E.; Casals, E.; Puntes, V.; Sánchez, A.; Font, X. Use of CeO2, TiO2 and Fe3O4 nanoparticles for the removal of lead from water: Toxicity of nanoparticles and derived compounds. Desalination 2011, 277, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.F.; Geroni, G.M.; Lloyd, D.; Choi, H.; Clark, N.; Pirog, A.; Lees, J.; Porch, A. Bioluminescence of vibrio fischeri: Bacteria respond quickly and sensitively to pulsed microwave electric (but not magnetic) fields. J. Biomed. Opt. 2019, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ezati, P.; Rhim, J.-W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.-S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [Green Version]
- Bashir, S.; Teo, Y.Y.; Naeem, S.; Ramesh, S.; Ramesh, K. pH responsive N-succinyl chitosan/Poly (acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PLoS ONE 2017, 12, e0179250. [Google Scholar]
- Saito, T.; Isogai, A. Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 219–225. [Google Scholar] [CrossRef]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Hameedha Beevi, A. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- López-Pérez, P.M.; Marques, A.P.; Da Silva, R.M.P.; Pashkuleva, I.; Reis, R.L. Effect of chitosan membrane surface modification via plasma induced polymerization on the adhesion of osteoblast-like cells. J. Mater. Chem. 2007, 17, 4064–4071. [Google Scholar] [CrossRef] [Green Version]
- Charhouf, I.; Bennamara, A.; Abourriche, A.; Chenite, A.; Zhu, J.; Berrada, M. Characterization of a dialdehyde chitosan generated by periodate oxidation. Int. J. Sci. Basic Appl. Res. 2014, 16, 336–348. [Google Scholar]
- Saud, R.; Pokhrel, S.; Yadav, P.N. Synthesis, characterization and antimicrobial activity of maltol functionalized chitosan derivatives. J. Macromol. Sci. Part A 2019, 56, 375–383. [Google Scholar] [CrossRef]
- Tang, R.; Du, Y.; Fan, L. Dialdehyde starch-crosslinked chitosan films and their antimicrobial effects. J. Polym. Sci. Pol. Phys. 2003, 41, 993–997. [Google Scholar] [CrossRef]
- Yap, W.F.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. X-ray photoelectron spectroscopy and atomic force microscopy studies on cross-linked chitosan thin film. Int. J. Phys. Sci. 2011, 6, 2744–2749. [Google Scholar]
- Chełminak-Dudkiewicz, D.; Ziegler-Borowska, M.; Stolarska, M.; Sobotta, Ł.; Falkowski, M.; Mielcarek, J.; Goslinski, T.; Kowalonek, J.; Wegrzynowska-Drzymalska, K.; Kaczmarek, H. The chitosan—Porphyrazine hybrid materials and their photochemical properties. J. Photochem. Photobiol B 2018, 181, 1–13. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kedziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Qin, C.; Wang, L.; Li, W. Volatile compounds formed from the pyrolysis of chitosan. Carbohydr. Polym. 2011, 83, 1553–1557. [Google Scholar] [CrossRef]
- Mucha, M.; Pawlak, A. Thermal analysis of chitosan and its blends. Thermochim. Acta. 2005, 427, 69–76. [Google Scholar] [CrossRef]
- Arianita, A.; Cahyaningtyas; Amalia, B.; Pudjiastuti, W.; Melanie, S.; Fauzia, V.; Imawan, C. Effect of glutaraldehyde to the mechanical properties of chitosan/nanocellulose. J. Phys. Conf. Ser. 2019, 1317, 012045. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Timotius, D.; Rochmadi, R.; Yuni, K. Preparation and characterization of local indonesian chitosan-graft-maleic anhydride as drug carrier. IOP Conf. Ser. Mater. Sci. Eng. 2019, 599, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Escárcega-Galaz, A.A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Sanches-Silva, A.; Madera-Santana, T.J.; Paseiro-Losada, P. Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. Int. J. Biol. Macromol. 2018, 116, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Takigawa, T.; Endo, Y. Effects of glutaraldehyde exposure on human health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Naushad, M.; Kumar, A.; Kumar, A.; Ahamad, T.; Stadler, F.J. Facile fabrication of chitosan-cl-poly(AA)/ZrPO4 nanocomposite for remediation of rhodamine B and antimicrobial activity. J. King Saud Univ. Sci. 2020, 32, 1359–1365. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Menazea, A.A.; Eid, M.M.; Ahmed, M.K. Synthesis, characterization, and evaluation of antimicrobial activity of novel chitosan/tigecycline composite. Int. J. Biol. Macromol. 2020, 147, 194–199. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Wootthikanokkhan, J.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Mechanical and antibacterial properties of the chitosan coated cellulose paper for packaging applications: Effects of molecular weight types and concentrations of chitosan. Int. J. Biol. Macromol. 2020, 155, 1510–1519. [Google Scholar] [CrossRef]
- Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-free and non-crosslinked chitosan scaffolds with efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. Carbohydr. Polym. 2020, 241, 116386. [Google Scholar] [CrossRef]
- Rathinam, S.; Ólafsdóttir, S.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Másson, M. Selective synthesis of N,N,N-trimethylated chitosan derivatives at different degree of substitution and investigation of structure-activity relationship for activity against P. aeruginosa and MRSA. Int. J. Biol. Macromol. 2020, 160, 548–557. [Google Scholar] [CrossRef]
- Chung, Y.C.; Su, Y.P.; Chen, C.C.; Jia, G.; Wang, H.L.; Wu, J.C.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar] [PubMed]
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial polymers. Adv. Healthc. Mater. 2014, 3, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Sharma, N.; Sharma, V.; Abrol, V.; Shankar, R.; Jaglan, S. An update on polysaccharide-based nanomaterials for antimicrobial applications. Appl. Microbiol. Biotechnol. 2016, 100, 2603–2615. [Google Scholar] [CrossRef]
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Mansilla, A.Y.; Albertengo, L.; Rodríguez, M.S.; Debbaudt, A.; Zúñiga, A.; Casalongué, C.A. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C. V Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Takahashi, T.; Imai, M.; Suzuki, I.; Sawai, J. Growth inhibitory effect on bacteria of chitosan membranes regulated with deacetylation degree. Biochem. Eng. J. 2008, 40, 485–491. [Google Scholar] [CrossRef]
- Chen, K.-S.; Ku, Y.-A.; Lee, C.-H.; Lin, H.-R.; Lin, F.-H.; Chen, T.-M. Immobilization of chitosan gel with cross-linking reagent on PNIPAAm gel/PP nonwoven composites surface. Mater. Sci. Eng. C 2005, 25, 472–478. [Google Scholar] [CrossRef]
- Kim, K.W.; Min, B.J.; Kim, Y.-T.; Kimmel, R.M.; Cooksey, K.; Park, S.I. Antimicrobial activity against foodborne pathogens of chitosan biopolymer films of different molecular weights. LWT Food Sci. Technol. 2011, 44, 565–569. [Google Scholar] [CrossRef]



















| Sample | CS:OA | ALD, % |
|---|---|---|
| DACS1 | 1:0.5 | 22 |
| DACS2 | 1:0.7 | 29 |
| DACS3 | 1:0.9 | 35 |
| DACS4 | 1:1 | 58 |
| Sample | Average Contact Angle (θ, °) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|
| Measuring Liquid | |||||
| Glycerin | Diiodomethane | γs | γsd | γsp | |
| CS | 82.0 | 56.0 | 30.70 | 27.46 | 3.23 |
| DACS | 70.0 | 51.8 | 35.10 | 27.26 | 7.84 |
| Sample | –OH | –NH | C–H | C=N | –NH | C–N | C–O | =C–H |
|---|---|---|---|---|---|---|---|---|
| CS | 3342 | 3278 | 2877 | 1636 | 1578 | 1381 | 1064 | - |
| CS-DACS | 3335 | 3228 | 2876 | 1636 | 1552 | 1380 | 1062 | 776 |
| CS-DAS | 3345 | 3284 | 2867 | 1637 | 1564 | 1379 | 1071 | 772 |
| CS-Glu | 3346 | 3284 | 2868 | 1636 | 1565 | 1379 | 1067 | - |
| Roughness Parameters (nm) | Sample | |||
|---|---|---|---|---|
| CS | CS-5DACS | CS-10DACS | CS-15DACS | |
| Rq | 9.24 | 21.9 | 20.3 | 5.57 |
| Ra | 7.49 | 17.0 | 15.4 | 4.38 |
| Rmax | 89.9 | 132.0 | 119.0 | 56.6 |
| CS-5DAS | CS-10DAS | CS-15DAS | ||
| Rq | - | 13.7 | 16.1 | 27.4 |
| Ra | - | 10.8 | 13.8 | 21.6 |
| Rmax | - | 79.5 | 87.9 | 201.0 |
| CS-5Glu | CS-10Glu | CS-15Glu | ||
| Rq | - | 9.4 | 5.75 | 2.69 |
| Ra | - | 7.93 | 4.55 | 1.64 |
| Rmax | - | 46.1 | 47.7 | 76.9 |
| Sample | Average Contact Angle (θ, °) | Surface Free Energy (mJ/m2) | |||
|---|---|---|---|---|---|
| Measuring Liquid | |||||
| Glycerin | Diiodomethane | γs | γsd | γsp | |
| CS | 82.0 | 56.0 | 30.70 | 27.46 | 3.23 |
| CS-5%DACS | 76.1 | 67.8 | 27.31 | 18.59 | 8.73 |
| CS-10%DACS | 76.0 | 73.9 | 25.72 | 14.98 | 10.74 |
| CS-15%DACS | 69.3 | 67.7 | 30.37 | 17.33 | 13.04 |
| CS-5%DAS | 80.6 | 70.0 | 25.00 | 18.50 | 6.84 |
| CS-10%DAS | 81.4 | 64.9 | 26.70 | 21.51 | 5.20 |
| CS-15%DAS | 78.3 | 72.0 | 25.20 | 16.50 | 8.70 |
| CS-5%Glu | 72.5 | 70.3 | 28.21 | 16.41 | 11.80 |
| CS-10%Glu | 65.3 | 67.6 | 32.51 | 16.67 | 15.86 |
| CS-15%Glu | 53.7 | 67.6 | 39.78 | 14.78 | 25.00 |
| Sample | First Stage | Second Stage | Third Stage | Residue 600 °C, (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tmax (°C) | Δm (%) | To (°C) | Tmax (°C) | Δm (%) | To (°C) | Tmax (°C) | Δm (%) | ||
| CS | 30, 60 | 8 | - | - | - | 145 | 286 | 51 | 40 |
| DACS | 52 | 11 | - | - | - | 160 | 281 | 45 | 41 |
| DAS | 72 | 10 | - | - | - | 178 | 292 | 70 | 20 |
| CS-5%DACS | 53 | 13 | - | - | - | 175 | 278 | 49 | 37 |
| CS-10%DACS | 56 | 12 | 126 | 148 | 3 | 175 | 285 | 46 | 39 |
| CS-15%DACS | 51 | 10 | 127 | 149 | 5 | 169 | 275 | 44 | 40 |
| CS-5%DAS | 47 | 13 | 120 | 145 | - | 180 | 279 | 47 | 39 |
| CS-10%DAS | 65 | 10 | 117 | 148 | 4 | 174 | 264 | 44 | 42 |
| CS-15%DAS | 52 | 9 | 113 | 149 | 5 | 173 | 269 | 45 | 40 |
| CS-5%Glu | * | 13 | - | - | - | 164 | 278 | 47 | 39 |
| CS-10%Glu | 54 | 12 | - | - | - | 140 | 261 | 51 | 36 |
| CS-15%Glu | 54 | 14 | - | - | - | 146 | 257 | 52 | 34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegrzynowska-Drzymalska, K.; Grebicka, P.; Mlynarczyk, D.T.; Chelminiak-Dudkiewicz, D.; Kaczmarek, H.; Goslinski, T.; Ziegler-Borowska, M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials 2020, 13, 3413. https://doi.org/10.3390/ma13153413
Wegrzynowska-Drzymalska K, Grebicka P, Mlynarczyk DT, Chelminiak-Dudkiewicz D, Kaczmarek H, Goslinski T, Ziegler-Borowska M. Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications. Materials. 2020; 13(15):3413. https://doi.org/10.3390/ma13153413
Chicago/Turabian StyleWegrzynowska-Drzymalska, Katarzyna, Patrycja Grebicka, Dariusz T. Mlynarczyk, Dorota Chelminiak-Dudkiewicz, Halina Kaczmarek, Tomasz Goslinski, and Marta Ziegler-Borowska. 2020. "Crosslinking of Chitosan with Dialdehyde Chitosan as a New Approach for Biomedical Applications" Materials 13, no. 15: 3413. https://doi.org/10.3390/ma13153413