Accuracy Evaluation of Additively and Subtractively Fabricated Palatal Plate Orthodontic Appliances for Newborns and Infants–An In Vitro Study
Abstract
:1. Introduction
- The compared technologies do not differ in accuracy from the conventional solution.
- Layer thickness does not influence the accuracy for DLP samples.
- There is no significant difference in accuracy among the tested SM materials.
2. Materials and Methods
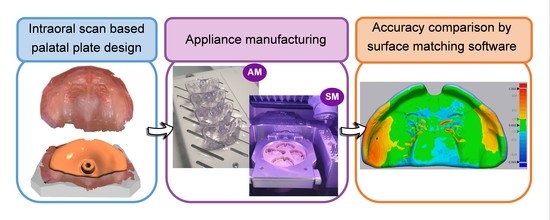
2.1. Sample Design and Manufacturing
2.2. Comparison of Manufacturing Times and Material Cost
2.3. Acquisition and Measurement of Data
2.4. Statistical Analyses
3. Results
3.1. Material and Technology Effect on Accuracy
3.2. Influence of Layer Thickness for DLP
3.3. Comparison of Manufacturing Times and Material Costs
4. Discussion
4.1. Material and Technology Effect on Accuracy
4.1.1. Vat Polymerization Techniques
4.1.2. Milling
4.1.3. Conventional
4.1.4. Comparison Problems and Influence of Background Noise throughout the Measuring Workflow
4.2. Clinical Interpretation and Acceptance
4.3. Limitations
5. Conclusions
- All tested materials and technologies are within the clinically acceptable range and are appropriate for their use.
- A significant difference for the accuracy between two SM materials was found.
- SM offered superior trueness and accuracy than AM.
- DLP offered a superior trueness and precision values compared to SLA.
- All CAD/CAM groups showed superior trueness and precision to conventional manufacturing; except for SLA, which exhibited lower trueness but higher precision.
- DLP with 100 µm layer thickness showed the highest efficiency, obtaining high trueness and precision within the shortest manufacturing time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henprasert, P.; Dawson, D.V.; El-Kerdani, T.; Song, X.; Couso-Queiruga, E.; Holloway, J.A. Comparison of the Accuracy of Implant Position Using Surgical Guides Fabricated by Additive and Subtractive Techniques. J. Prosthodont. 2020, 29, 534–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reymus, M.; Hickel, R.; Keßler, A. Accuracy of CAD/CAM-fabricated bite splints: Milling vs 3D printing. Clin. Oral Investig. 2020, 24, 4607–4615. [Google Scholar] [CrossRef]
- Jin, M.C.; Yoon, H.I.; Yeo, I.S.; Kim, S.H.; Han, J.S. The effect of build angle on the tissue surface adaptation of maxillary and mandibular complete denture bases manufactured by digital light processing. J. Prosthet. Dent. 2020, 123, 473–482. [Google Scholar] [CrossRef]
- Favero, C.S.; English, J.D.; Cozad, B.E.; Wirthlin, J.O.; Short, M.M.; Kasper, F.K. Effect of print layer height and printer type on the accuracy of 3-dimensional printed orthodontic models. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 557–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartaglia, G.M.; Mapelli, A.; Maspero, C.; Santaniello, T.; Serafin, M.; Farronato, M.; Caprioglio, A. Direct 3D Printing of Clear Orthodontic Aligners: Current State and Future Possibilities. Materials 2021, 14, 1799. [Google Scholar] [CrossRef] [PubMed]
- Hotz, M.; Gnoinski, W. Comprehensive care of cleft lip and palate children at Zurich University; A preliminary report. Am. J. Orthod. 1976, 70, 481–504. [Google Scholar] [CrossRef]
- Xepapadeas, A.B.; Weise, C.; Frank, K.; Spintzyk, S.; Poets, C.F.; Wiechers, C.; Arand, J.; Koos, B. Technical note on introducing a digital workflow for newborns with craniofacial anomalies based on intraoral scans—Part I: 3D printed and milled palatal stimulation plate for trisomy 21. BMC Oral Health 2020, 20, 20. [Google Scholar] [CrossRef]
- Castillo-Morales, R.; Crotti, E.; Avalle, C.; Limbrock, G. Orofaziale regulation beim Down-syndrom durch gaumenplatte. Sozialpadiatrie 1982, 1, 10–17. [Google Scholar]
- Castillo-Morales, R.; Brondo, J.; Hoyer, H.; Limbrock, G. Die Behandlung von Kau-, Schluck-und Sprechstörungen bei behinderten Kindern mit der orofazialen Regulationstherapie nach Castillo-Morales: Aufgabe für Pädiater und Zahnarzt. Zahnärztl Mitt 1985, 75, 935–942. [Google Scholar]
- Limbrock, G.; Hesse, A.; Hoyer, H. Orofaziale Regulationstherapie nach Castillo-Morales bei Kindern mit zerebralen Läsionen. Fortschr. Kieferorthopädie 1987, 48, 335–339. [Google Scholar] [CrossRef]
- Aretxabaleta, M.; Xepapadeas, A.B.; Poets, C.F.; Koos, B.; Spintzyk, S. Fracture Load of an Orthodontic Appliance for Robin Sequence Treatment in a Digital Workflow. Materials 2021, 14, 344. [Google Scholar] [CrossRef] [PubMed]
- Xepapadeas, A.B.; Weise, C.; Frank, K.; Spintzyk, S.; Poets, C.F.; Wiechers, C.; Arand, J.; Koos, B. Technical note on introducing a digital workflow for newborns with craniofacial anomalies based on intraoral scans—Part II: 3D printed Tubingen palatal plate prototype for newborns with Robin sequence. BMC Oral Health 2020, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Chate, R.A. A report on the hazards encountered when taking neonatal cleft palate impressions (1983–1992). Br. J. Orthod. 1995, 22, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Weise, C.; Frank, K.; Weise, H.; Reinert, S.; Koos, B.; Xepapadeas, A.B. Intraoral scanning of neonates, infants, and small children with craniofacial disorders: Evaluation of feasibility, scanning duration and clinical experience. Eur. J. Orthod. 2021. Under review. [Google Scholar]
- Gallardo, Y.R.; Bohner, L.; Tortamano, P.; Pigozzo, M.N.; Lagana, D.C.; Sesma, N. Patient outcomes and procedure working time for digital versus conventional impressions: A systematic review. J. Prosthet. Dent. 2018, 119, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chebib, N.; Kalberer, N.; Srinivasan, M.; Maniewicz, S.; Perneger, T.; Muller, F. Edentulous jaw impression techniques: An In Vivo comparison of trueness. J. Prosthet. Dent. 2019, 121, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J. Prosthet. Dent. 2016, 116, 249–256. [Google Scholar] [CrossRef]
- Aretxabaleta, M.; Xepapadeas, A.B.; Poets, C.F.; Koos, B.; Spintzyk, S. Comparison of additive and subtractive CAD/CAM materials for their potential use as Tübingen Palatal Plate: An in-vitro study on flexural strength. Addit. Manuf. 2021, 37. [Google Scholar] [CrossRef]
- Lee, S.; Hong, S.J.; Paek, J.; Pae, A.; Kwon, K.R.; Noh, K. Comparing accuracy of denture bases fabricated by injection molding, CAD/CAM milling, and rapid prototyping method. J. Adv. Prosthodont. 2019, 11, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.Y.; Yang, T.C.; Wang, T.M.; Lin, L.D. Effects of fabrication techniques on denture base adaptation: An In Vitro study. J. Prosthet. Dent. 2020, 124, 740–747. [Google Scholar] [CrossRef]
- Steinmassl, O.; Dumfahrt, H.; Grunert, I.; Steinmassl, P.A. CAD/CAM produces dentures with improved fit. Clin. Oral Investig. 2018, 22, 2829–2835. [Google Scholar] [CrossRef] [Green Version]
- Einarsdottir, E.R.; Geminiani, A.; Chochlidakis, K.; Feng, C.; Tsigarida, A.; Ercoli, C. Dimensional stability of double-processed complete denture bases fabricated with compression molding, injection molding, and CAD-CAM subtraction milling. J. Prosthet. Dent. 2020, 124, 116–121. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 5725-1:1994 Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 1: General Principles and Definitions; International Organization for Standardization (ISO): Geneva, Switzerland, 1994. [Google Scholar]
- Hwang, H.J.; Lee, S.J.; Park, E.J.; Yoon, H.I. Assessment of the trueness and tissue surface adaptation of CAD-CAM maxillary denture bases manufactured using digital light processing. J. Prosthet. Dent. 2019, 121, 110–117. [Google Scholar] [CrossRef]
- Yoon, H.I.; Hwang, H.J.; Ohkubo, C.; Han, J.S.; Park, E.J. Evaluation of the trueness and tissue surface adaptation of CAD-CAM mandibular denture bases manufactured using digital light processing. J. Prosthet. Dent. 2018, 120, 919–926. [Google Scholar] [CrossRef]
- Emir, F.; Ayyildiz, S. Accuracy evaluation of complete-arch models manufactured by three different 3D printing technologies: A three-dimensional analysis. J. Prosthodont. Res. 2020. [Google Scholar] [CrossRef]
- VOCO GmbH. V-Print Splint—Instructions of Use; VOCO GmbH: Cuxhavem, Germany, 2019. [Google Scholar]
- Formlabs. Dental LT Clear V1—Instructions of Use; Formlabs: Somerville, MA, USA, 2020. [Google Scholar]
- Formlabs. Dental Model V2—Instructions of Use; Formlabs: Somerville, MA, USA, 2021. [Google Scholar]
- Dentaurum GmbH & Co. KG. Orthocryl—Instructions of Use; Dentaurum GmbH & Co. KG: Ispringen, Germany, 2016. [Google Scholar]
- 3Shape A/S. 3Shape Dental System Catalog—Innovative 3D Scanning and CAD Solutions; 3Shape: Cophenagen, Denmark, 2017; Available online: https://www.3shape.com/en/scanners/lab (accessed on 15 January 2021).
- Jeong, Y.G.; Lee, W.S.; Lee, K.B. Accuracy evaluation of dental models manufactured by CAD/CAM milling method and 3D printing method. J. Adv. Prosthodont. 2018, 10, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unkovskiy, A.; Schmidt, F.; Beuer, F.; Li, P.; Spintzyk, S.; Kraemer Fernandez, P. Stereolithography vs. Direct Light Processing for Rapid Manufacturing of Complete Denture Bases: An In Vitro Accuracy Analysis. J. Clin. Med. 2021, 10, 70. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Li, P.L.; Chu, F.T.; Shen, G. Influence of the three-dimensional printing technique and printing layer thickness on model accuracy. J. Orofac. Orthop. 2019, 80, 194–204. [Google Scholar] [CrossRef]
- Xu, Y.; Xepapadeas, A.B.; Koos, B.; Geis-Gerstorfer, J.; Li, P.; Spintzyk, S. Effect of post-rinsing time on the mechanical strength and cytotoxicity of a 3D printed orthodontic splint material. Dent. Mater. 2021, 37, e314–e327. [Google Scholar] [CrossRef] [PubMed]
- Ammoun, R.; Dalal, N.; Abdulmajeed, A.A.; Deeb, G.R.; Bencharit, S. Effects of two postprocessing methods onto surface dimension of in-office fabricated stereolithographic implant surgical guides. J. Prosthodont. 2021, 30, 71–75. [Google Scholar] [CrossRef]
- McLaughlin, J.B.; Ramos, V., Jr.; Dickinson, D.P. Comparison of Fit of Dentures Fabricated by Traditional Techniques Versus CAD/CAM Technology. J. Prosthodont. 2019, 28, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Shin, Y.S.; Jung, H.D.; Hwang, C.J.; Baik, H.S.; Cha, J.Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, R.B.; Alharbi, N.; Wismeijer, D. Build Angle: Does It Influence the Accuracy of 3D-Printed Dental Restorations Using Digital Light-Processing Technology? Int. J. Prosthodont. 2017, 30, 182–188. [Google Scholar] [CrossRef]












| Measured Value | Conventional | AM-SLA | AM-DLP 1 | SM-PEEK | SM-PMMA | |
|---|---|---|---|---|---|---|
| Trueness (RMS, mm) | Mean | 0.058 | 0.066 | 0.045 | 0.040 | 0.029 |
| SD | 0.005 | 0.006 | 0.003 | 0.002 | 0.001 | |
| Precision (RMS, mm) | Mean | 0.023 | 0.018 | 0.016 | 0.011 | 0.020 |
| SD | 0.005 | 0.004 | 0.004 | 0.002 | 0.008 | |
| Average + (mm) | Mean | 0.043 | 0.053 | 0.037 | 0.037 | 0.024 |
| SD | 0.004 | 0.030 | 0.004 | 0.002 | 0.002 | |
| Average − (mm) | Mean | −0.081 | −0.047 | −0.035 | −0.019 | −0.016 |
| SD | 0.120 | 0.008 | 0.003 | 0.002 | 0.002 | |
| Max + (mm) | Mean | 0.200 | 0.264 | 0.168 | 0.171 | 0.123 |
| SD | 0.071 | 0.053 | 0.017 | 0.009 | 0.006 | |
| Min − (mm) | Mean | −0.201 | −0.153 | −0.146 | −0.076 | −0.088 |
| SD | 0.027 | 0.050 | 0.030 | 0.005 | 0.019 |
| Measured Value | DLP Layer Thickness | |||
|---|---|---|---|---|
| 25 µm | 50 µm | 100 µm | ||
| Trueness (RMS) | Mean | 0.043 | 0.041 | 0.045 |
| SD | 0.005 | 0.002 | 0.003 | |
| Precision (RMS) | Mean | 0.024 | 0.022 | 0.016 |
| SD | 0.008 | 0.005 | 0.004 | |
| Average + (mm) | Mean | 0.037 | 0.031 | 0.037 |
| SD | 0.004 | 0.003 | 0.004 | |
| Average − (mm) | Mean | −0.035 | −0.034 | −0.035 |
| SD | 0.003 | 0.002 | 0.003 | |
| Max + (mm) | Mean | 0.148 | 0.146 | 0.168 |
| SD | 0.023 | 0.022 | 0.017 | |
| Min − (mm) | Mean | −0.180 | −0.160 | −0.146 |
| SD | 0.039 | 0.029 | 0.030 | |
| Technology | Material | Layer Thickness | Layer Count | Fabrication Time | Post-Processing Time | Total Time | Material Cost |
|---|---|---|---|---|---|---|---|
| Conventional | Orthocryl Clear | / | / | 1 h 10 min | 45 min | 1 h 55 min | EUR 0.24 |
| SLA | Dental LT Clear | 100 µm | 236 | 1 h 40 min | 1 h 20 min | 3 h | EUR 0.78 |
| DLP | V-Print Splint | 25 µm | 914 | 1 h 55 min | 27 min | 2 h 22 min | EUR 0.77 |
| 50 µm | 457 | 1 h 1 min | 1 h 28 min | ||||
| 100 µm | 228 | 37 min | 1 h 4 min | ||||
| Milling | Yamahachi PMMA | / | / | 2 h 38 min | 7 min | 2 h 45 min | EUR 8.23 |
| Smile PEEK | / | / | 3 h 18 min | 3 h 25 min | EUR 39.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aretxabaleta, M.; Unkovskiy, A.; Koos, B.; Spintzyk, S.; Xepapadeas, A.B. Accuracy Evaluation of Additively and Subtractively Fabricated Palatal Plate Orthodontic Appliances for Newborns and Infants–An In Vitro Study. Materials 2021, 14, 4103. https://doi.org/10.3390/ma14154103
Aretxabaleta M, Unkovskiy A, Koos B, Spintzyk S, Xepapadeas AB. Accuracy Evaluation of Additively and Subtractively Fabricated Palatal Plate Orthodontic Appliances for Newborns and Infants–An In Vitro Study. Materials. 2021; 14(15):4103. https://doi.org/10.3390/ma14154103
Chicago/Turabian StyleAretxabaleta, Maite, Alexey Unkovskiy, Bernd Koos, Sebastian Spintzyk, and Alexander B. Xepapadeas. 2021. "Accuracy Evaluation of Additively and Subtractively Fabricated Palatal Plate Orthodontic Appliances for Newborns and Infants–An In Vitro Study" Materials 14, no. 15: 4103. https://doi.org/10.3390/ma14154103