The Influence of Dry Hydrated Limes on the Fresh and Hardened Properties of Architectural Injection Grout
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Composition
2.2. Methods
3. Results and Discussion
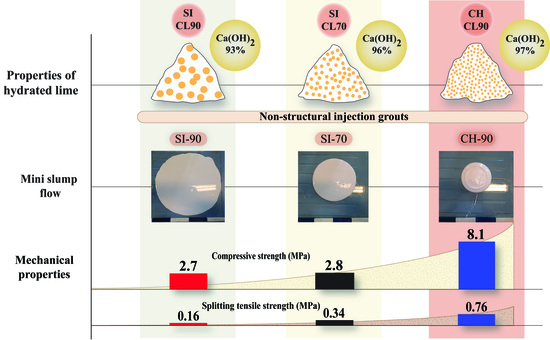
3.1. Fresh State Properties
3.2. Properties in Hardened State
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venice Charter. International Charter for the Conservation and Restoration of Monuments and Sites; Committee for Drafting the International Charter for the Conservation and Restoration of Monuments: Venice, Italy, 1964.
- Padovnik, A.; Piqué, F.; Jornet, A.; Bokan-Bosiljkov, V. Injection grouts for the re-attachment of architectural surfaces with historic value—Measures to improve the properties of hydrated lime grouts in Slovenia. Int. J. Archit. Herit. Conserv. Anal. Restor. 2016, 10, 993–1007. [Google Scholar] [CrossRef]
- Padovnik, A. Consolidation of Detached Plaster Layers of Mural Paintings with Non-Structural Grouting. Ph.D. Thesis, Faculty of Civil and Geodetic Engineering, University of Ljubljana, Ljubljana, Slovenia, 26 May 2016. [Google Scholar]
- González-Sánchez, J.F.; Taşcı, B.; Fernández, J.M.; Navarro-Blasco, Í.; Alvarez, J.I. Combination of polymeric superplasticizers, water repellents and pozzolanic agents to improve air lime-based grouts for historic masonry repair. Polymers 2020, 12, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zacharopoulou, G. Interpreting chemistry and technology of lime binders and implementing it in the conservation field. Conserv. Património 2009, 10, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Aggelakopoulou, E.; Bakolas, A.; Moropoulou, A. Lime putty versus hydrated lime powder: Physicochemical and mechanical characteristics of lime based mortars. Constr. Build. Mater. 2019, 225, 633–641. [Google Scholar] [CrossRef]
- Elert, K.; Rodriguez-Navarro, C.; Pardo, E.S.; Hansen, E.; Cazalla, O. Lime mortars for the conservation of historic buildings. Stud. Conserv. 2002, 47, 62–75. [Google Scholar] [CrossRef]
- Boynton, R.S. Chemistry and Technology of Lime and Limestone; John Wiley & Sons: Hoboken, NJ, USA, 1966. [Google Scholar]
- Kemperl, J.; Maček, J. Precipitation of calcium carbonate from hydrated lime of variable reactivity, granulation and optical properties. Int. J. Miner. Process. 2009, 93, 84–88. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Ortega-Huertas, M.; Hansen, E. Nanostructure and irreversible colloidal behavior of Ca(OH)2: Implications in cultural heritage conservation. Langmuir 2005, 21, 10948–10957. [Google Scholar] [CrossRef]
- Whitman, W.G.; Davis, G.H.B. The hydration of lime. Ind. Eng. Chem. 1926, 18, 118–120. [Google Scholar] [CrossRef]
- Adams, F.W. Effect of particle size on the hydration of lime. Ind. Eng. Chem. 1927, 19, 589–591. [Google Scholar] [CrossRef]
- Navrátilová, E.; Tihlaříková, E.; Neděla, V.; Rovnaníková, P.; Pavlík, J. Effect of the preparation of lime putties on their properties. Sci. Rep. 2017, 7, 17260. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Rodriguez-Navarro, C. Microstructure and rheology of lime putty. Langmuir 2010, 26, 3868–3877. [Google Scholar] [CrossRef] [PubMed]
- Ghiasi, M.; Abdollahy, M.; Khalesi, M. Removal of iron from milk of lime to produce pure precipitated calcium carbonate. Sep. Sci. Technol. 2020, 55, 1425–1435. [Google Scholar] [CrossRef]
- Schweigert, K. The Effects of Impurities on Lime Quality. Available online: http://www.penta.net/wp-content/uploads/2018/10/Effects-of-Impurities-on-Lime-Quality.pdf (accessed on 10 September 2021).
- Kang, S.-H.; Lee, S.-O.; Hong, S.-G.; Kwon, Y.-H. Historical and scientific investigations into the use of hydraulic lime in korea and preventive conservation of historic masonry structures. Sustainability 2019, 11, 5169. [Google Scholar] [CrossRef] [Green Version]
- Eckel, E.C. Cements, Limes and Plasters: Their Materials, Manufacture, and Properties, 1st ed.; John Wiley & Sons: New York, NY, USA, 1905. [Google Scholar]
- Holmes, S.; Wingate, M. Building with Lime: A Practical Introduction, 2nd ed.; Practical Action Publishing: Rugby, UK, 2002. [Google Scholar]
- Mendoza, E.; Alonso-Guzman, E.; Ruvalcaba-Sil, J.; Sánchez Calvillo, A.; Martinez Molina, W.; García, H.; Bedolla-Arroyo, J.; Becerra-Santacruz, H.; Borrego, J. Compressive strength and ultrasonic pulse velocity of mortars and pastes, elaborated with slaked lime and high purity hydrated lime, for restoration works in México. Key Eng. Mater. 2020, 862, 51–55. [Google Scholar] [CrossRef]
- Faria, P.; Henriques, F.; Rato, V. Comparative evaluation of lime mortars for architectural conservation. J. Cult. Herit. 2008, 9, 338–346. [Google Scholar] [CrossRef]
- Klein, D.; Haas, S.; Schmidt, S.-O.; Middendorf, B. Morphological and chemical influence of calcium hydroxide on the plasticity of lime based mortars. In Historic Mortars; Válek, J., Hughes, J.J., Groot, C.J.W.P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 319–328. [Google Scholar]
- Paiva, H.; Velosa, A.; Veiga, R.; Ferreira, V.M. Effect of maturation time on the fresh and hardened properties of an air lime mortar. Cem. Concr. Res. 2010, 40, 447–451. [Google Scholar] [CrossRef]
- Azeiteiro, L.C.; Velosa, A.; Paiva, H.; Mantas, P.Q.; Ferreira, V.M.; Veiga, R. Development of grouts for consolidation of old renders. Constr. Build. Mater. 2014, 50, 352–360. [Google Scholar] [CrossRef]
- Pachta, V.; Papayianni, I.; Spyriliotis, T. Assessment of laboratory and field testing methods in lime-based grouts for the consolidation of architectural surfaces. Int. J. Archit. Herit. 2020, 14, 1098–1105. [Google Scholar] [CrossRef]
- Pasian, C.; Piqué, F.; Jornet, A.; Cather, S. A ‘Sandwich’ specimen preparation and testing procedure for the evaluation of non-structural injection grouts for the re-adhesion of historic plasters. Int. J. Archit. Herit. 2021, 15, 455–466. [Google Scholar] [CrossRef]
- Luso, E.; Lourenço, P.B. Experimental laboratory design of lime based grouts for masonry consolidation. Int. J. Archit. Herit. 2017, 11, 1143–1152. [Google Scholar] [CrossRef] [Green Version]
- Padovnik, A.; Bokan-Bosiljkov, V. Effect of ultralight filler on the properties of hydrated lime injection grout for the consolidation of detached historic decorative plasters. Materials 2020, 13, 3360. [Google Scholar] [CrossRef]
- Pachta, V. The role of glass additives in the properties of lime-based grouts. Heritage 2021, 4, 906–916. [Google Scholar] [CrossRef]
- UNI EN 459-1. Building Lime—Part 1: Definitions, Specifications and Conformity Criteria; CEN: Brussels, Belgium, 2010. [Google Scholar]
- EN 196-2. Method of Testing Cement—Part 2: Chemical Analysis of Cement; CEN: Brussels, Belgium, 2013. [Google Scholar]
- Cizer, Ö.; Van Balen, K.; Elsen, J.; Van Gemert, D. Real-time investigation of reaction rate and mineral phase modifications of lime carbonation. Constr. Build. Mater. 2012, 35, 741–751. [Google Scholar] [CrossRef] [Green Version]
- EN 196-6. Methods of Testing Cement—Part 6: Determination of Fineness; CEN: Brussels, Belgium, 2018. [Google Scholar]
- Fact Sheet, Properties of Typical Commercial Lime Products. Available online: https://www.lime.org/documents/lime_basics/lime-physical-chemical.pdf (accessed on 25 June 2021).
- Papayianni, I.; Stefanidou, M. Strength-porosity relationships in lime-pozzolan mortars. Constr. Build. Mater. 2006, 20, 700–705. [Google Scholar] [CrossRef]
- Arandigoyen, M.; Bicer-Simsir, B.; Alvarez, J.I.; Lange, D.A. Variation of microstructure with carbonation in lime and blended pastes. Appl. Surf. Sci. 2006, 252, 7562–7571. [Google Scholar] [CrossRef] [Green Version]
- Pachta, V.; Goulas, D. Fresh and hardened state properties of fiber reinforced lime-based grouts. Constr. Build. Mater. 2020, 261, 119818. [Google Scholar] [CrossRef]
- Beruto, D.T.; Botter, R. Liquid-like H2O adsorption layers to catalyse the Ca(OH)2/CO2 solid–gas reaction and to form a non-protective solid product layer at 20 °C. J. Eur. Ceram. Soc. 2000, 20, 497–503. [Google Scholar] [CrossRef]
- Domone, P.; Hsi-Wen, C. Testing of binders for high performance concrete. Cem. Concr. Res. 1997, 27, 1141–1147. [Google Scholar] [CrossRef]
- ASTM C940-16. Standard Test Method for Expansion and Bleeding of Freshly Mixed Grouts for Preplaced-Aggregate Concrete in the Laboratory; ASTM International: Harrisburg, PA, USA, 2016. [Google Scholar]
- EN 1015-6. Methods of Test for Mortar for Masonry—Part 6: Determination of Bulk Density of Fresh Mortar; CEN: Brussels, Belgium, 1999. [Google Scholar]
- Biçer-Şimşir, B.; Rainer, H.L. Evaluation of Lime-Based Hydraulic Injection Grouts for the Conservation of Architectural Surfaces: A Manual of Laboratory and Field Test Methods; Getty Conservation Institute: Los Angeles, CA, USA, 2013; ISBN 978-1-937433-15-4. [Google Scholar]
- PrEN 1015-8. Methods of Test for Mortar for Masonry—Part 8: Determination of Water Retentivity of Fresh Mortar; CEN: Brussels, Belgium, 1999. [Google Scholar]
- EN 1015-10. Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar; CEN: Brussels, Belgium, 1999. [Google Scholar]
- SIST EN 1015-18. Methods of Test for Mortar for Masonry—Part 18: Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar; CEN: Brussels, Belgium, 2004. [Google Scholar]
- SIA 262/1. Construction En Béton-Spécifications Complémentaires, Appendix A; SIA: Zurich, Switzerland, 2003. [Google Scholar]
- EN 1015-11. Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar; CEN: Brussels, Belgium, 1999. [Google Scholar]
- ASTM C496/C496M-1. Standard Test Method for Splitting Tensile Strength of Cylindrical Concrete Specimens; ASTM International: Harrisburg, PA, USA, 2004. [Google Scholar]
- Ince, C.; Ozturk, Y.; Carter, M.A.; Wilson, M.A. The influence of supplementary cementing materials on water retaining characteristics of hydrated lime and cement mortars in masonry construction. Mater. Struct. 2014, 47, 493–501. [Google Scholar] [CrossRef]
- Ince, C.; Carter, M.A.; Wilson, M.A.; Collier, N.C.; El-Turki, A.; Ball, R.J.; Allen, G.C. Factors affecting the water retaining characteristics of lime and cement mortars in the freshly-mixed state. Mater. Struct. 2011, 44, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Jornet, A.; Mosca, C.; Cavallo, G.; Corredig, G. Comparison between traditional, lime based, and industrial, dry mortars. In Historic Mortars; Válek, J., Hughes, J.J., Groot, C.J.W.P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 227–237. [Google Scholar]
- Swenson, E.G.; Sereda, P.J. Mechanism of the carbonatation shrinkage of lime and hydrated cement. J. Appl. Chem. 1968, 18, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Sahu, S.; Badger, S.; Thaulow, N.; Lee, R.J. Determination of water–cement ratio of hardened concrete by scanning electron microscopy. Scanning Electron Microsc. Cem. Concr. 2004, 26, 987–992. [Google Scholar] [CrossRef]
- Arandigoyen, M.; Bernal, J.L.P.; López, M.A.B.; Alvarez, J.I. Lime-pastes with different kneading water: Pore structure and capillary porosity. Appl. Surf. Sci. 2005, 252, 1449–1459. [Google Scholar] [CrossRef] [Green Version]
- Martys, N.S.; Ferraris, C.F. Capillary transport in mortars and concrete. Cem. Concr. Res. 1997, 27, 747–760. [Google Scholar] [CrossRef]
- Pavía, S.; Aly, M. Influence of aggregate and supplementary cementitious materials on the properties of hydrated lime (CL90s) mortars. Mater. Constr. 2016, 66, e104. [Google Scholar] [CrossRef] [Green Version]
- Veiga, R. Air lime mortars: What else do we need to know to apply them in conservation and rehabilitation interventions? A review. Constr. Buld. Mater. 2017, 157, 132–140. [Google Scholar] [CrossRef]
- Papayianni, I.; Pachta, V. Experimental study on the performance of lime-based grouts used in consolidating historic masonries. Mater. Struct. 2015, 48, 2111–2121. [Google Scholar] [CrossRef]
- Vavričuk, A.; Bokan-Bosiljkov, V.; Kramar, S. The influence of metakaolin on the properties of natural hydraulic lime-based grouts for historic masonry repair. Constr. Build. Mater. 2018, 172, 706–716. [Google Scholar] [CrossRef]
- Ferragni, D.; Forti, M.; Malliet, J.; Mora, P.; Teutonico, J.M.; Torraca, G. Injection Grouting of mural paintings and mosaics. Stud. Conserv. 1984, 29, 110–116. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage, A Compendium of Materials and Techniques; Springer Science and Business Media: New York, NY, USA, 2015; pp. 1–144. ISBN 978-94-017-9302-5. [Google Scholar]
- Fernández, J.M.; Duran, A.; Navarro-Blasco, I.; Lanas, J.; Sirera, R.; Alvarez, J.I. Influence of nanosilica and a polycarboxylate ether superplasticizer on the performance of lime mortars. Cem. Concr. Res. 2013, 43, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Silva, B.; Ferreira Pinto, A.P.; Gomes, A.; Candeias, A. Fresh and hardened state behaviour of aerial lime mortars with superplasticizer. Constr. Build. Mater. 2019, 225, 1127–1139. [Google Scholar] [CrossRef]
- Lanas, J.; Alvarez, J.I. Masonry repair lime-based mortars: Factors affecting the mechanical behavior. Cem. Concr. Res. 2003, 33, 1867–1876. [Google Scholar] [CrossRef] [Green Version]
- Zacharopoulou, G. The Effect of wet slaked lime putty and putty prepared from dry hydrate on the strength of lime mortars. In 2nd Conference on Historic Mortars–HMC 2010 and RILEM TC 203-RHM Final Workshop; Válek, J., Groot, C., Hughes, J.J., Eds.; RILEM Publications SARL Publication: Prague, Czech Republic, 2010; pp. 1283–1291. [Google Scholar]
- Van Balen, K. Carbonation reaction of lime, kinetics at ambient temperature. Cem. Concr. Res. 2005, 35, 647–657. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, H.; Yan, Z.; Ju, J.W.; Zhang, L. A Micromechanical study of the breakage mechanism of microcapsules in concrete using PFC2D. Constr. Build. Mater. 2016, 115, 452–463. [Google Scholar] [CrossRef]





| Sample | CaO (%) | MgO (%) | Al2O3 (%) | Fe2O3 (%) | SO3 (%) | SiO2 (%) | I.L. (%) |
|---|---|---|---|---|---|---|---|
| SI-CL70 lime | 71.25 | 2.09 | 0.60 | 0.19 | 0.06 | 0.79 | 25.69 |
| SI-CL90 lime | 71.01 | 3.05 | 0.58 | 0.20 | 0.14 | 2.14 | 23.38 |
| CH-CL90 lime | 74.90 | 0.40 | 0.02 | 0.01 | 0.02 | 0.05 | 25.00 |
| Limestone filler | 55.38 | 0.76 | 0.15 | 0.01 | 0.01 | <0.01 | 44.02 |
| Sample | Portlandite (Ca(OH)2) | Calcite (CaCO3) | Periclase (MgO) | Quartz (SiO2) | Lime (CaO) | Magnesite MgCO4 | Larnite (Ca2SiO4) | Dolomite (CaMg(CO3)2) |
|---|---|---|---|---|---|---|---|---|
| SI-CL70 lime | 95.8 | 2.9 | 0.2 | 0.3 | 0.8 | |||
| SI-CL90 lime | 92.5 | 1.2 | 2.3 | 0.2 | 0.1 | 0.4 | 3.5 | |
| CH-CL90 lime | 97.0 | 3.0 | ||||||
| Limestone filler | 95.3 | 4.7 |
| Sample | Particle Density (g/cm3) | Blaine Fineness (cm2/g) |
|---|---|---|
| SI-CL70 lime | 2.237 | 9623 |
| SI-CL90 lime | 2.217 | 8767 |
| CH-CL90 lime | 2.343 | 16,198 |
| Limestone filler | 2.764 | 3194 |
| Mixture | Wet Density (g/cm3) | Mini Slump Flow (mm) | Bleeding after 3 h (%) | Water Retention Capacity (%) |
|---|---|---|---|---|
| SI-70 | 1.74 ± 0.02 | 259 ± 16 | 1.0 ± 0.3 | 83 ± 1 |
| SI-90 | 1.74 ± 0.00 | 300 ± 15 | 1.5 ± 0.3 | 82 ± 2 |
| CH-90 | 1.76 ± 0.02 | 254 ± 10 | 0.9 ± 0.4 | 85 ± 2 |
| Mixture | Crushed Lime Mortar | |
|---|---|---|
| Dry | Wet | |
| SI-70 | E | E |
| SI-90 | E | E |
| CH-90 | D25 | F |
| Mixture | Dry Mortar Cup | Pre-Wetted Mortar Cup | ||
|---|---|---|---|---|
| Separation Size (mm) | Crack Size (mm) | Separation Size (mm) | Crack Size (mm) | |
| SI-70 | 0.2 | 0 | 0.2 | 0.1 |
| SI-90 | 0.2 | 0.1 | 0.2 | 0.2 |
| CH-90 | 0.4 | 0 | 0.4 | 0.3 |
| Mixture | Dry Density (g/cm3) | Total Porosity (%) | Capillary Porosity (%) | Content of Air Pores (%) | W24 (kg/(m2√min)) | W10 (kg/(m2√min)) |
|---|---|---|---|---|---|---|
| SI-70 | 1.51 ± 0.01 | 43 ± 0 | 37 ± 0 | 6 | 0.43 ± 0.02 | 1.01 |
| SI-90 | 1.45 ± 0.01 | 40 ± 1 | 38 ± 1 | 2 | 0.46 ± 0.01 | 2.59 |
| CH-90 | 1.50 ± 0.02 | 44 ± 0 | 38 ± 0 | 6 | 0.43 ± 0.03 | 3.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padovnik, A.; Bokan-Bosiljkov, V. The Influence of Dry Hydrated Limes on the Fresh and Hardened Properties of Architectural Injection Grout. Materials 2021, 14, 5585. https://doi.org/10.3390/ma14195585
Padovnik A, Bokan-Bosiljkov V. The Influence of Dry Hydrated Limes on the Fresh and Hardened Properties of Architectural Injection Grout. Materials. 2021; 14(19):5585. https://doi.org/10.3390/ma14195585
Chicago/Turabian StylePadovnik, Andreja, and Violeta Bokan-Bosiljkov. 2021. "The Influence of Dry Hydrated Limes on the Fresh and Hardened Properties of Architectural Injection Grout" Materials 14, no. 19: 5585. https://doi.org/10.3390/ma14195585