Synergistic Anti-Candida Activity of Bengazole A in the Presence of Bengamide A †
Abstract
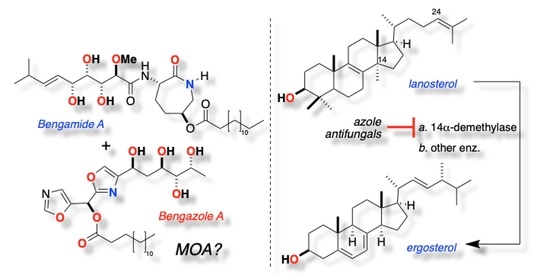
:1. Introduction
2. Results
2.1. Extraction–Isolation of Bengamides–Bengazoles
2.2. Sterol Composition in C. albicans Co-Cultured with Azoles
2.3. Synergistic Antifungal Activity of Bengazole–Bengamide Mixtures
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Extraction and Purification of Bengazole A
4.3. Quantification of Ergosterol from C. albicans ATCC 14503
4.4. Antifungal Disk Diffusion Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quiñoá, E.; Adamczeski, M.; Crews, P.J. Bengamides, Heterocyclic Anthelmintics from a Jaspidae Marine Sponge. J. Org. Chem. 1986, 51, 4494–4497. [Google Scholar] [CrossRef]
- Adamczeski, M.; Quiñoá, E.; Crews, P.J. Novel Sponge-derived Amino Acids. 11. The Entire Absolute Stereochemistry of the Bengamides. J. Org. Chem. 1990, 55, 240–242. [Google Scholar] [CrossRef]
- Thale, Z.; Kinder, F.R.; Bair, K.W.; Bontempo, J.; Czuchta, A.M.; Versace, R.W.; Phillips, P.E.; Sanders, M.L.; Wattanasin, S.; Crews, P. Bengamides Revisited: New Structures and Antitumor Studies. J. Org. Chem. 2001, 66, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Kinder, F.R., Jr.; Versace, R.W.; Bair, K.W.; Bontempo, J.M.; Cesarz, D.; Chen, S.; Crews, P.; Czuchta, A.M.; Jagoe, C.T.; Yin, M.; et al. Synthesis and Antitumor Activity of Ester-Modified Analogues of Bengamide B. J. Med. Chem. 2001, 44, 3692–3699. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Bair, K.W.; DeCaprio, J.A.; Eck, M.J.; Kim, S.; Kinder, F.R.; Morollo, A.; Mueller, D.R.; Schindler, P.; Song, H.K.; et al. Proteomics-based Target Identification: Bengamides as a New Class of Methionine Aminopeptidase Inhibitors. J. Biol. Chem. 2003, 278, 52964–52971. [Google Scholar] [CrossRef]
- Hu, X.; Dang, Y.; Tenney, K.; Crews, P.; Tsai, C.W.; Sixt, K.M.; Cole, P.A.; Liu, J.O. Regulation of c-Src Nonreceptor Tyrosine Kinase Activity by Bengamide A through Inhibition of Methionine Aminopeptidases. Chem. Biol. 2007, 14, 764–774. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.D.; Waykole, L.; Calienni, J.V.; Ciszewski, L.; Lee, G.T.; Liu, W.; Szewczyk, J.; Vargas, K.; Prasad, K.; Repič, O.; et al. An Expedient Synthesis of LAF389, a Bengamide B Analogue. Org. Proc. Res. Dev. 2003, 7, 856–865. [Google Scholar] [CrossRef]
- Dumez, H.; Gall, H.; Capdeville, R.; Dutreix, C.; van Oosterom, A.T.; Giaccone, G. A Phase I and Pharmacokinetic Study of LAF389 Administered to Patients with Advanced Cancer. Anticancer Drugs 2007, 18, 219–225. [Google Scholar] [CrossRef]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging Opportunistic Yeast Infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Adamczeski, M.; Quiñoa, E.; Crews, P. Unusual Anthelminthic Oxazoles from a Marine Sponge. J. Am. Chem. Soc. 1988, 110, 1598–1602. [Google Scholar] [CrossRef]
- Rodríguez, J.; Nieto, R.M.; Crews, P. New Structures and Bioactivity Patterns of Bengazole Alkaloids from a Choristid Marine Sponge. J. Nat. Prod. 1993, 56, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Searle, P.A.; Richter, R.K.; Molinski, T.F. Bengazoles C−G from the Sponge Jaspis sp. Synthesis of the Side Chain and Determination of Absolute Configuration. J. Org. Chem. 1996, 61, 4073. [Google Scholar] [CrossRef] [PubMed]
- Chida, N.; Tobe, T.; Okada, S.; Ogawa, S. Total Synthesis and Absolute Configuration of Bengamide A. J.C.S. Chem. Comm. 1992, 1064–1066. [Google Scholar] [CrossRef]
- Mulder, R.J.; Shafer, C.M.; Molinski, T.F. First Total Synthesis of Bengazole A. J. Org. Chem. 1999, 64, 4995–4998. [Google Scholar] [CrossRef] [PubMed]
- Shafer, C.M.; Molinski, T.F. Synthesis of the C1-C9 Core of Bengazole A. Harnessing the Ambident Nucleophilicity of 2-Lithiooxazole. Tetrahedron Lett. 1998, 39, 2903–2906. [Google Scholar] [CrossRef]
- Bull, J.A.; Balskus, E.P.; Horan, R.A.; Langner, M.; Ley, S.V. Stereocontrolled Total Synthesis of Bengazole A: A Marine Bisoxazole Natural Product Displaying Potent Antifungal Properties. Angew. Chem. 2006, 13, 6714–6718. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Sudhakar, A. Total Synthesis of Bengazole A. Org. Lett. 2010, 12, 236–238. [Google Scholar] [CrossRef]
- García-Ruiz, C.; Sarabia, F. Chemistry and Biology of Bengamides and Bengazoles, Bioactive Natural Products from Jaspis Sponges. Mar. Drugs 2014, 12, 1580–1622. [Google Scholar] [CrossRef] [PubMed]
- Mulder, R.J.; Shafer, C.M.; Dalisay, D.S.; Molinski, T.F. Synthesis and Structure–activity Relationships of Bengazole A Analogs. Bioorg. Med. Chem. Lett. 2009, 19, 2928–2930. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Molinski, T.F. Screening of Marine Invertebrates for the Presence of Ergosterol-Sensitive Antifungal Compounds. J. Nat. Prod. 1993, 56, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Goodman Gilman, A.; Rall, T.W.; Nies, A.S.; Taylor, P. Goodman and Gilman’s the Pharmacological Basis of Therapeutics, 8th ed.; Pergamon: New York, NY, USA, 1990. [Google Scholar]
- Molinski, T.F. Developments in Marine Natural Products. Receptor-Specific Bioactive Compounds. J. Nat. Prod. 1993, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A. History of the Development of Azole Derivatives. Clin. Microb. Infect. 2004, 10, 1–10. [Google Scholar] [CrossRef]
- Sagatova, A.A.; Keniya, M.V.; Wilson, R.K.; Monk, B.C.; Tyndall, J.D.A. Structural Insights into Binding of the Antifungal Drug Fluconazole to Saccharomyces cerevisiae Lanosterol 14α-Demethylase. Antimicrob. Agents Chemother. 2015, 59, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, H.H.; Vinick, F.J.; Chang, Y.C. Reaction of Oxazoles with Singlet Oxygen. Mechanism of the Rearrangement of Triamides. J. Am. Chem. Soc. 1972, 94, 7180–7182. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Druckrey, E. The Reaction of Oxazoles with Singlet Oxygen. II. A Novel Method for the Preparation of ω-Cyano acids. J. Am. Chem. Soc. 1968, 90, 2440–2441. [Google Scholar]
- Molinski, T.F. Nanomole-scale Natural Products Discovery. Curr. Opin. Drug Discov. Dev. 2009, 12, 197–206. [Google Scholar]
- Dalisay, D.S.; Molinski, T.F. NMR Quantitation of Natural Products at the Nanomole Scale. J. Nat. Prod. 2009, 72, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Bolard, J. How Do the Polyene Macrolide Antibiotics Affect the Cellular Membrane Properties? Biochim. Biophys. Acta 1986, 864, 257–304. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The Biology and Chemistry of Antifungal Agents: A Review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef]
- Chaudhary, P.M.; Tupe, S.G.; Deshpande, M.V. Chitin Synthase Inhibitors as Antifungal Agents. Mini Rev. Med. Chem. 2013, 13, 222–236. [Google Scholar]
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in Synthetic Approach to and Antifungal Activity of Triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.H.R.; Harrison, D.M.; Moss, G.P. 24-Methylenedihydrolanosterol as a Precursor of Steroids and Triterpenoids. Chem. Commun. 1966, 17, 595–596. [Google Scholar] [CrossRef]
- Lahey, F.N.; Strasser, P.H.A. Erburicoic Acid. J. Chem. Soc. 1951, 0, 873–877. [Google Scholar] [CrossRef]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy Tests by E Test and Checkerboard Methods of Antimicrobial Combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, V.W.-H.; Romeiro, N.C.; Abreu, P.A. Design Strategies of Oxidosqualene Cyclase Inhibitors: Targeting the Sterol Biosynthetic Pathway. J. Steroid Biochem. Mol. Biol. 2017, 171, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Jamison, M.T.; Macho, J.; Molinski, T.F. Structure–activity of Antifungal Compounds Inspired by Aminobisabolenes from the Sponge Halichondria sp. Bioorg. Med. Chem. Lett. 2016, 26, 5244–5246. [Google Scholar] [CrossRef] [PubMed]
- Acar, J. F Antibiotic Synergism and Antagonism. Med. Clin. N. Am. 2000, 84, 1391–1406. [Google Scholar] [CrossRef]
- Wind, C.M.; de Vries, H.J.C.; van Dam, A.P. Determination of In Vitro Synergy for Dual Antimicrobial Therapy Against Resistant Neisseria gonorrhoeae Using Etest and agar dilution. Int. J. Antimicrob. Agents 2015, 45, 305–308. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Wang, D.; Yang, Y.; Sun, W.; Liu, J.; Sun, S. Synergistic Antifungal Effect of Fluconazole Combined with Licofelone against Resistant Candida albicans. Front. Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Ernst-Russell, M.A.; de Ropp, J.S.; Molinski, T.F. Lobocyclamides A-C, Lipopeptides from a Cryptic Cyanobacterial Mat Containing Lyngbya confervoides. J. Org. Chem. 2002, 67, 8210–8215. [Google Scholar] [CrossRef]
- Frankmölle, W.P.; Knübel, G.; Moore, R.E.; Patterson, G.M.L. Antifungal Cyclic Peptides from the Terrestrial Blue-Green Alga Anabaena laxa. J. Antibiot. 1992, 45, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Frankmölle, W.P.; Larsen, L.K.; Caplan, F.R.; Patterson, G.M.L.; Knübel, G.; Levine, I.A.; Moore, R.E. Antifungal Cyclic Peptides from the Terrestrial Blue-Green Alga Anabaena laxa. J. Antibiot. 1992, 45, 1451–1457. [Google Scholar] [CrossRef]
- Cai, W.; Matthew, S.; Chen, Q.-Y.; Paul, V.J.; Luesch, H.P. Discovery of new A- and B-type Laxaphycins with Synergistic Anticancer Activity. Bioorg. Med. Chem. 2018, 26, 2310–2319. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F. Antifungal Compounds from Marine Organisms. Curr. Med. Chem. Anti-Infect. Agents 2004, 3, 197–220. [Google Scholar] [CrossRef]
- Nicholas, G.M.; Molinski, T.F. Enantiodivergent Biosynthesis of the Dimeric Sphingolipid Oceanapiside from the Marine Sponge Oceanapia phillipensis. Determination of Remote Stereochemistry. J. Am. Chem. Soc 2000, 122, 4011–4019. [Google Scholar] [CrossRef]
- Pruett, S.T.; Bushnev, A.; Hagedorn, K.; Adiga, M.; Haynes, C.A.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H. Thematic Review Series: Sphingolipids. Biodiversity of Sphingoid Bases (“Sphingosines”) and Related Amino Alcohols. J. Lipid Res. 2008, 49, 1621–1639. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, G.N.; Li, R.; MacMillan, J.B.; Molinski, T.F. Antifungal Activity of Bifunctional Sphingolipids. Intramolecular Synergism Within Long-Chain, α,ω-bis-Aminoalcohols. Bioorg. Med. Chem. Lett. 2002, 12, 2159–2162. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Rogers, E.W.; Molinski, T.F. Oceanapiside Targets the Sphingolipid Pathway of Fluconazole-Resistant Candida glabrata. in preparation.
- Salib, M.N.; Molinski, T.F. Six Trikentrin-like Cyclopentanoindoles from Trikentrion flabbeliforme. Absolute Structural Assignment by NMR and ECD. J. Org. Chem. 2018, 83, 1278–1286. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamison, M.T.; Wang, X.; Cheng, T.; Molinski, T.F. Synergistic Anti-Candida Activity of Bengazole A in the Presence of Bengamide A. Mar. Drugs 2019, 17, 102. https://doi.org/10.3390/md17020102
Jamison MT, Wang X, Cheng T, Molinski TF. Synergistic Anti-Candida Activity of Bengazole A in the Presence of Bengamide A. Marine Drugs. 2019; 17(2):102. https://doi.org/10.3390/md17020102
Chicago/Turabian StyleJamison, Matthew T., Xiao Wang, Tina Cheng, and Tadeusz F. Molinski. 2019. "Synergistic Anti-Candida Activity of Bengazole A in the Presence of Bengamide A" Marine Drugs 17, no. 2: 102. https://doi.org/10.3390/md17020102