The Form of N Supply Determines Plant Growth Promotion by P-Solubilizing Microorganisms in Maize
Abstract
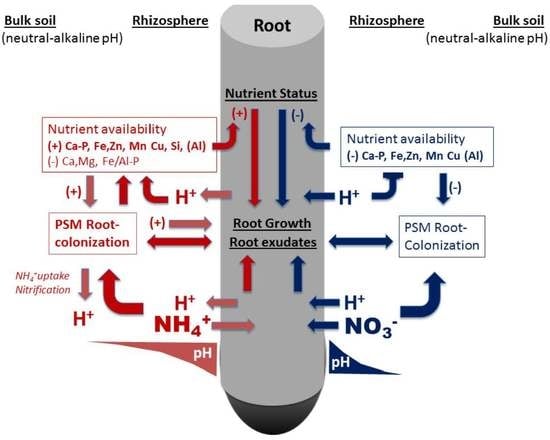
:1. Introduction
2. Materials and Methods
2.1. Pot Experiments on Artificial Sand Sub-soil Substrates
2.1.1. Substrate Characteristics and Fertilization
2.1.2. PSM Inoculation and Plant Culture
2.2. Pot Experiment on Field Soil
2.2.1. Substrate Characteristics and Fertilization
2.2.2. PSM Inoculation and Plant Culture
2.3. Field Experiment
2.3.1. Soil Characteristics and Fertilization
2.3.2. PSM Inoculation and Plant Culture
2.4. Plant Biomass and Root Length Determination
2.5. Shoot Mineral Analysis
3. Results
3.1. Experiments on Artificial Growth Substrates (Sub-Soil-Sand Mixtures)
3.2. Pot Experiment on Field Soil
3.2.1. Shoot Growth and Root Development
3.2.2. Mineral Nutrient Status
3.3. Field Experiment
4. Discussion
4.1. PGPM Effects on Artificial Sand/sub-soil Substrates
4.2. PGPM Effects on Field Soil
4.3. PGPM Effects under Field Conditions
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, K.; Binkley, D.; Doxtader, K.G. A new method for estimating gross phosphorus mineralization and immobilization rates in soils. Plant Soil 1992, 147, 243–250. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef]
- Marschner, H. Rhizosphere pH effects on phosphorus nutrition. In Genetic Manipulation of Crop Plants to Enhance Integrated Nutrient Management in Cropping Systems. 1. Phosphorus; Johansen, C., Lee, K.K., Sharma, K.K., Subbaro, G.V., Kueneman, E.A., Eds.; Proceedings of an FAO/ICRISAT Expert Consultary Workshop, ICRISAT Asia Center, India; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, Andhra Pradesh, India, 1995; pp. 107–115. [Google Scholar]
- Neumann, G.; Römheld, V. Root-induced changes in the availability of nutrients in the rhizosphere. In Plant Roots the Hidden Half, 3rd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 617–649. [Google Scholar]
- Neumann, G.; Römheld, V. The release of root exudates as affected by the plant physiological status. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface, 2nd ed.; Pinton, R., Varanini, Z., Nannipieri, Z., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 23–72. [Google Scholar]
- Sharma, B.S.; Riyaz, Z.; Sayed, M.H.T.; Thivakaran, A.G. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jongmans, A.G.; van Breemen, N.; Lundström, U.; van Hees, P.A.W.; Finlay, R.D.; Srinivasan, M.; Unestam, T.; Giesler, R.; Melkerud, P.; Olsson, M. Rock-eating fungi. Nature 1997, 389, 682–683. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef]
- Menzies, N.; Harbison, D.; Dart, P. Soil chemistry-facts and fiction and their influence on the fertilizer decision making process. In Proceedings of the 26th Annual Conference of the Grassland Society of NSW, Bathurst, Australia, 26–28 July 2011; pp. 49–63. [Google Scholar]
- van Veen, J.A.; van Overbeek, L.S.; van Elsas, J.D. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 1997, 611, 21–35. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef]
- Schütz, L.; Gattinger, A.; Meier, M.; Muller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization—A global meta-analysis. Front. Plant Sci. 2017, 8, 2204. [Google Scholar] [CrossRef]
- Abbasi, M.K.; Musa, N.; Manzoor, M. Mineralization of soluble P fertilizers and insoluble rock phosphate in response to phosphate-solubilizing bacteria and poultry manure and their effect on the growth and P utilization efficiency of chilli (Capsicum annuum L.). Biogeosciences 2015, 12, 4607–4619. [Google Scholar] [CrossRef]
- Thonar, C.; Lekfeldt, J.D.S.; Cozzolino, V.; Kundel, D.; Kulhánek, M.; Mosimann, C.; Neumann, G.; Piccolo, A.; Rex, M.; Symanczik, S.; et al. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4, 7. [Google Scholar] [CrossRef]
- Mpanga, I.K.; Dapaah, H.K.; Geistlinger, J.; Ludewig, U.; Neumann, G. Soil type-dependent interactions of P-solubilizing microorganisms with organic and inorganic fertilizers mediate plant growth promotion in tomato. Agronomy 2018, 8, 213. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Müller, T. Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: From lab to field. Chem. Biol. Technol. Agric. 2016, 3, 15. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Neumann, G.; Müller, T. Densely rooted rhizosphere hotspots induced around subsurface NH4+-fertilizer depots: A home for soil PGPMs? Chem. Biol. Technol. Agric. 2017, 4, 29. [Google Scholar] [CrossRef]
- Liu, H.; White, P.J.; Li, C. Biomass partitioning and rhizosphere responses of maize and faba bean to phosphorus deficiency. Crop Pasture Sci. 2016, 67, 847–856. [Google Scholar] [CrossRef]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten e.V. Speyer, Germany). Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik Methodenbuch Band I Die Untersuchung von Böden, 4th ed.; VDLUFA Verlag: Darmsatdt, Germany, 1991. [Google Scholar]
- Gericke, S.; Kurmis, B. Die kolorimetrische Phophorsäurebestimmung mit Ammonium-Vanadat-Molybdat und ihre Anwendung in der Pflanzenanalyse. Z. Pflanzenernaehr. Bodenkd. 1952, 59, 235–247. [Google Scholar]
- Campbell, R.C. Reference Sufficiency Ranges for Plant Analysis in the Southern Region of the United States; Southern Cooperative Series Bulletin #394; North Carolina Department of Agriculture and Consumer Services Agronomic Division: Raleigh, NC, USA, 2009; p. 11.
- Cottenie, A. FAO Soils Bulletin 38/1, Soil and Plant Testing and Analysis; Food and Agriculture Organisation of the United Nations: Rome, Italy, 1980. [Google Scholar]
- Gulden, R.H.; Vessey, J.K. Penicillium bilaii inoculation increases root-hair production in field pea. Can. J. Plant Sci. 2000, 80, 801–804. [Google Scholar] [CrossRef]
- Fröhlich, A.; Buddrus-Schiemann, K.; Durner, J.; Hartmann, A.; Rad, U. Von Response of barley to root colonization by Pseudomonas sp. DSMZ 13134 under laboratory, greenhouse, and field conditions. J. Plant Interact. 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Lekfeldt, J.D.S.; Rex, M.; Mercy, F.; Magid, K.; Tlustoš, P.; Magid, J. Effect of bioeffectors and recycled P-fertiliser products on the growth of spring wheat. Chem. Biol. Technol. Agric. 2016, 3, 22. [Google Scholar] [CrossRef]
- Jing, J.; Ruia, Y.; Zhanga, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Bittman, S.; Kowalenko, C.G.; Hunt, D.E.; Forge, T.A.; Wu, X. Starter phosphorus and broadcast nutrients on corn with contrasting colonization by mycorrhizae. Agron. J. 2006, 98, 394–401. [Google Scholar] [CrossRef]
- Chekanaia, V.; Chikowoa, R.; Vanlauwe, B. Response of common bean (Phaseolus vulgaris L.) to nitrogen, phosphorus and rhizobia inoculation across variable soils in Zimbabwe. Agric. Ecosyst. Environ. 2018, 266, 167–173. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, K.; Teng, W.; Zhan, A.; Tong, Y.; Feng, G.; Cui, Z.; Zhang, F.; Chenet, X. Is the Inherent Potential of Maize Roots Efficient for Soil Phosphorus Acquisition? PLoS ONE 2014, 9, e90287. [Google Scholar] [CrossRef]
- Hajabbasi, M.A.; Schumacher, T.E. Phosphorus effects on root growth and development in two maize genotypes. Plant Soil 1994, 158, 39–46. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, J.; Han, Z.; Yang, J.; Ge, C.; Wu, Q. Revealing new insights into different phosphorus-starving responses between two maize (Zea mays) inbred lines by transcriptomic and proteomic studies. Sci. Rep. 2017, 7, 44294. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, P.; Lynch, J.P. Greater lateral root branching density in maize improves phosphorus acquisition from low phosphorus soil. J. Exp. Bot. 2018, 69, 4961–4970. [Google Scholar] [CrossRef] [PubMed]
- Nkebiwe, P.M.; Weinmann, M.; Bar-Tal, A.; Müller, T. Fertilizer placement to improve crop nutrient acquisition and yield: A review and meta-analysis. Field Crops Res. 2016, 196, 389–401. [Google Scholar] [CrossRef]
- Benckiser, G.; Christ, E.; Herbert, T.; Weiske, A.; Blome, J.; Hardt, M. The nitrification inhibitor 3,4-dimethylpyrazole-phosphat (DMPP)—Quantification and effects on soil metabolism. Plant Soil 2013. [Google Scholar] [CrossRef]
- Bharucha, U.; Patel, K.; Trivedi, U.B. Optimization of indole acetic acid production by Pseudomonas putida UB1 and its effect as plant growth-promoting rhizobacteria on mustard (Brassica nigra). Agric. Res. 2013, 2, 215–221. [Google Scholar] [CrossRef]
- Ögüt, M.; Er, F.; Neumann, G. Increased proton extrusion of wheat roots by inoculation with phosphorus solubilising microorganisms. Plant Soil 2011, 339, 285–297. [Google Scholar] [CrossRef]
- Brown, M.E.; Hornby, D. Effects of nitrate and ammonium on wheat roots in gnotobiotic culture: Amino acids, cortical cell death and take-all (caused by Gaeumannomyces graminis var. Tritici). Soil Biol. Biochem. 1987, 19, 567–573. [Google Scholar] [CrossRef]
- Imas, P.; Bar-Yosef, B.; Kafkafi, U.; Ganmore-Neumann, R. Release of carboxylic anions and protons by tomato roots in response to ammonium nitrate ratio and pH in nutrient solution. Plant Soil 1997, 191, 27–34. [Google Scholar] [CrossRef]
- Yang, H.; Menz, J.; Haussermann, I.; Benz, M.; Fujiwara, T.; Ludewig, U. 2015. High and Low Affinity Urea Root Uptake: Involvement of NIP5;1. Plant Cell Physiol. 2015, 56, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Menz, J.; Li, Z.; Schulze, W.X.; Ludewig, U. Early nitrogen-deprivation responses in Arabidopsis roots reveal distinct differences on transcriptome and (phospho-) proteome levels between nitrate and ammonium nutrition. Plant J. 2016, 88, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Sommermann, L.; Geistlinger, J.; Wibberg, D.; Deubel, A.; Zwanzig, J.; Babin, D.; Schluter, A.; Schellenberg, I. Fungal community profiles in agricultural soils of a long-term field trial under different tillage, fertilization and crop rotation conditions analyzed by high-throughput ITS-amplicon sequencing. PLoS ONE 2018, 13, e0195345. [Google Scholar] [CrossRef]
- Babin, D.; Deubel, A.; Jacquiod, S.; Sørensen, S.J.; Geistlinger, J.; Grosch, R.; Smalla, K. Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biol. Biochem. 2018, in press. [Google Scholar] [CrossRef]
- Yua, H.; Ning Ling, N.; Wang, T.; Zhub, C.; Wanga, Y.; Wanga, S.; Ga, Q. Responses of soil biological traits and bacterial communities to nitrogen fertilization mediate maize yields across three soil types. Soil Tillage Res. 2019, 185, 61–69. [Google Scholar] [CrossRef]
- Mahmood, T.; Kaiser, W.M.; Ali, R.; Ashraf, M.; Gulnaz, G.; Iqbal, Z. Ammonium versus nitrate nutrition of plants stimulates microbial activity in the rhizosphere. Plant Soil 2005, 277, 233–243. [Google Scholar] [CrossRef]



| N | P | K | Mn | |
|---|---|---|---|---|
| Shoot Mineral Concentration (mg g−1) | ||||
| No P | 12.4 d | 2.0 d | 41.3 ab | 0.02 b |
| NH4_Rock-P | 25.6 ab | 2.5 ab | 45.9 ab | 0.02 b |
| NH4_ Rock-P _Trianum P | 35.2 ab * | 2.4 abc | 45.0 ab | 0.03 a * |
| NH4_ Rock-P _Proradix | 33.9 ab * | 2.3 abcd | 45.9 ab | 0.03 a * |
| NO3_ Rock-P _Proradix | 36.4 ab * | 2.6 a | 46.6 a | 0.03 a * |
| NH4_ Rock-P _Rhizovital | 34.6 ab * | 2.2 bcd | 44.0 ab | 0.03 a * |
| NH4_ Rock-P _Paenibacillus | 31.9 b * | 2.2 bcd | 42.9 ab | 0.03 a * |
| NH4_ Rock-P _BFOD | 37.4 a * | 2.3 abcd | 45.5 ab | 0.03 a * |
| NH4_ Rock-P _Vit SP11 | 34.2 ab * | 2.2 bcd | 40.8 ab | 0.03 a * |
| NH4_ Rock-P _CombifectorA | 34.6 ab * | 2.1 cd | 41.2 ab | 0.03 a * |
| NO3_TSP | 35.0 ab * | 2.2 bcd | 39.5 b | 0.03 a * |
| Shoot Mineral Content (mg Plant−1) | ||||
| No P | 45.7 d | 7.4 d | 151.6 d | 0.07 d |
| NH4_Rock-P | 271.5 bc | 19.5 bc | 356.6 bc | 0.24 bc |
| NH4_ Rock-P _Trianum P | 311.7 ab | 21.3 abc * | 398.8 ab * | 0.27 abc |
| NH4_ Rock-P _Proradix | 330.9 a * | 22.2 ab * | 448.9 a * | 0.32 a * |
| NO3_ Rock-P _Proradix | 250.2 c | 17.7 c | 317.0 c | 0.22 c |
| NH4_ Rock-P _Rhizovital | 328.1 a | 20.9 abc | 415.1 ab * | 0.28 abc * |
| NH4_ Rock-P _Paenibacillus | 302.6 abc | 21.4 abc | 409.9 ab * | 0.27 abc |
| NH4_ Rock-P _BFOD | 318.3 ab | 19.6 bc | 386.2 abc | 0.27 abc |
| NH4_ Rock-P _Vit SP11 | 339.9 a * | 22.0 abc | 404.7 ab * | 0.29 abc * |
| NH4_ Rock-P _CombifectorA | 341.7 a * | 20.4 bc | 405.0 ab * | 0.30 ab * |
| NO3_TSP | 289.0 abc | 25.0 a | 443.1 a | 0.26 abc |
| Treatment | Shoot DM(42 DAS) (g) | Grain Yield (t ha−1) | Shoot-P(42 DAS) % (mg plant−1) | Shoot-N(42 DAS) % (mg plant−1) |
|---|---|---|---|---|
| Stabilized NH4+ no P | 33.3 c | 15.3 d | 0.45 a (0.15 b) | 3.3 a (1.08 c) |
| Stabilized NH4+ + TSP | 41.2 ab (+ 24%) | 16.1 ab (+ 5.2%) | 0.48 a (0.20 ab) | 3.2 a (1.35 ab) |
| Stabilized NH4+ + Combi-A | 42.4 ab (+ 27%) | 15.9 ab (+ 3.9%) | 0.47 a (0.20 ab) | 3.3 a (1.42 ab) |
| Stabilized NH4+ + Combi-B | 46.7 a (+ 40%) | 16.0 ab (+ 4.0%) | 0.48 a (0.22 a) | 3.4 a (1.58 a) |
| Stabilized NH4+ + FZB42+HA | 44.4 a (+ 33%) | 16.3 a (+ 6.5%) | 0.47 a (0.21 a) | 3.3 a (1.48 a) |
| Stabilized NH4+ + B. amylolique-faciens + seaweed extract | 45.6 a (+ 37%) | 15.6 bcd (+ 1.9%) | 0.44 a (0.20 ab) | 3.3 a (1.50 a) |
| Urea+DAP (farmers practice) | 36.6 c (+ 10%) | 15.8 abc (+ 3.2%) | 0.48 a (0.18 ab) | 3.2 a (1.35 ab) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpanga, I.K.; Nkebiwe, P.M.; Kuhlmann, M.; Cozzolino, V.; Piccolo, A.; Geistlinger, J.; Berger, N.; Ludewig, U.; Neumann, G. The Form of N Supply Determines Plant Growth Promotion by P-Solubilizing Microorganisms in Maize. Microorganisms 2019, 7, 38. https://doi.org/10.3390/microorganisms7020038
Mpanga IK, Nkebiwe PM, Kuhlmann M, Cozzolino V, Piccolo A, Geistlinger J, Berger N, Ludewig U, Neumann G. The Form of N Supply Determines Plant Growth Promotion by P-Solubilizing Microorganisms in Maize. Microorganisms. 2019; 7(2):38. https://doi.org/10.3390/microorganisms7020038
Chicago/Turabian StyleMpanga, Isaac Kwadwo, Peteh Mehdi Nkebiwe, Mira Kuhlmann, Vincenza Cozzolino, Alessandro Piccolo, Jörg Geistlinger, Nils Berger, Uwe Ludewig, and Günter Neumann. 2019. "The Form of N Supply Determines Plant Growth Promotion by P-Solubilizing Microorganisms in Maize" Microorganisms 7, no. 2: 38. https://doi.org/10.3390/microorganisms7020038