Structure-Guided Identification of Black Cohosh (Actaea racemosa) Triterpenoids with In Vitro Activity against Multiple Myeloma
Abstract
:1. Introduction
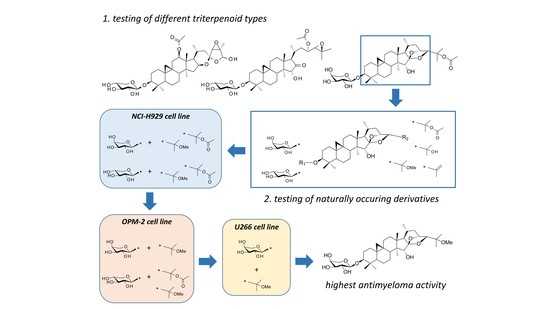
2. Results
2.1. Investigation of Different Triterpenoid Types
2.2. Investigation of Cimigenol-Type Triterpenoids
3. Discussion
4. Materials and Methods
4.1. Extraction and Isolation
4.1.1. Plant Material, Reagents, and Experimental Procedures
4.1.2. Isolation and Identification of Investigated Compounds
4.2. Cytotoxicity Assays
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hardy, M.L. Herbs of special interest to women. J. Am. Pharm. Assoc. 2000, 40, 234–242. [Google Scholar] [CrossRef]
- McKenna, D.J.; Jones, K.; Humphrey, S.; Hughes, K. Black cohosh: Efficacy, safety, and use in clinical and preclinical applications. Altern. Ther. Health Med. 2001, 7, 93–100. [Google Scholar] [PubMed]
- Beer, A.M.; Osmers, R.; Schnitker, J.; Bai, W.; Mueck, A.O.; Meden, H. Efficacy of black cohosh (Cimicifuga racemosa) medicines for treatment of menopausal symptoms – comments on major statements of the Cochrane Collaboration report 2012 “black cohosh (Cimicifuga spp.) for menopausal symptoms (review)”. Gynecol. Endocrinol. 2013, 29, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Henneicke von Zepelin, H.-H. 60 years of Cimicifuga racemosa medicinal products: Clinical research milestones, current study findings and current development. Wien. Med. Wochenschr. 2017, 167, 147–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, W.P.; Henneicke von Zepelin, H.H.; Wang, S.Y.; Zheng, S.R.; Liu, J.L.; Zhang, Z.L.; Geng, L.; Hu, L.N.; Jiao, C.F.; Liske, E. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: A randomized, double blind, parallel-controlled study versus tibolone. Maturitas 2007, 58, 31–41. [Google Scholar] [CrossRef]
- Black Cohosh Herbal Summary—ema.europe.eu. Available online: https://www.ema.europa.eu/en/documents/herbal-summary/black-cohosh-summary-public_en.pdf (accessed on 18 December 2019).
- Cicek, S.S.; Girreser, U.; Zidorn, C. Quantification of the total amount of black cohosh cycloartanoids by integration of one specific 1H NMR signal. J. Pharm. Biomed. 2018, 155, 109–115. [Google Scholar] [CrossRef]
- Qiu, F.; McAlpine, J.B.; Krause, E.C.; Chen, S.N.; Pauli, G.F. Pharmacognosy of Black Cohosh: The Phytochemical and Biological Profile of a Major Botanical Dietary Supplement. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Kobayashi, J., Eds.; Springer Nature: Berlin, Germany, 2014; Volume 9, pp. 1–68. [Google Scholar]
- Cicek, S.S.; Khom, S.; Taferner, B.; Hering, S.; Stuppner, H. Bioactivity-guided isolation of GABAA receptor modulating constituents from the rhizomes of Actaea racemosa. J. Nat. Prod. 2010, 73, 2024–2028. [Google Scholar] [CrossRef]
- Strommer, B.; Khom, S.; Kastenberger, I.; Cicek, S.S.; Stuppner, H.; Schwarzer, C.; Hering, S. A Cycloartane glycoside derived from Actaea racemosa L. modulates GABAA receptors and induces pronounced sedation in mice. J. Pharmacol. Exp. Ther. 2014, 351, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.M. Deoxyactein stimulates osteoblast function and inhibits boneresorbing mediators in MC3T3-E1 cells. J. Appl. Toxicol. 2013, 33, 190–195. [Google Scholar] [CrossRef]
- Choi, E.M. Deoxyactein Isolated from Cimicifuga racemosa protects osteoblastic MC3T3-E1 cells against antimycin A-induced cytotoxicity. J. Appl. Toxicol. 2013, 33, 488–494. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, E.M. Actein isolated from black cohosh promotes the function ofosteoblastic MC3T3-E1 cells. J. Med. Food 2014, 17, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yin, T.; Wang, X.; Zhang, F.; Pan, G.; Lv, H.; Wang, X.; Orgah, J.O.; Zhua, Y.; Wu, H. Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review. J. Ethnopharmacol. 2017, 209, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Shimizu, M.; Xiao, D.H.; Nuntanakorn, P.; Lim, J.T.E.; Suzui, M.; Seter, C.; Pertel, T.; Kennelly, E.J.; Kronenberg, F.; et al. Growth inhibitory activity of extracts and purified components of black cohosh on human breast cancer cells. Breast Cancer Res. Treat. 2004, 83, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Su, T.; Wu, H.A.; Friedman, R.; Wang, X.; Ramirez, A.; Kronenberg, F.; Weinstein, I.B. The growth inhibitory effect of actein on human breast cancer cells is associated with activation of stress response pathways. Int. J. Cancer 2007, 121, 2073–2083. [Google Scholar] [CrossRef]
- Einbond, L.S.; Wen-Cai, Y.; He, K.; Wu, H.; Cruz, E.; Roller, M.; Kronenberg, F. Growth inhibitory activity of extracts and compounds from Cimicifuga species on human breast cancer cells. Phytomedicine 2008, 15, 504–511. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.Z.; Nian, Y.; Li, W.; Wu, J.J.; Ge, G.B.; Dong, P.P.; Zhang, Y.Y.; Qiu, M.H.; Liu, L.; Yang, L. Cycloartane triterpenoids from Cimicifuga yunnanensis induce apoptosis of breast cancer cells (MCF7) via p53-dependent mitochondrial signaling pathway. Phytother. Res. 2011, 25, 17–24. [Google Scholar] [CrossRef]
- Yue, G.G.L.; Xie, S.; Lee, J.K.M.; Kwok, H.F.; Gao, S.; Nian, Y.; Wu, X.X.; Wong, C.K.; Qiu, M.H.; Lau, C.B.S. New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci. Rep. 2016, 6, 35263. [Google Scholar] [CrossRef]
- Rölling, C.; Knop, S.; Bornhäuser, M. Multiple Myeloma. Lancet 2015, 385, 2197–2208. [Google Scholar] [CrossRef]
- Chim, C.S.; Kumar, S.K.; Orlowski, R.Z.; Cook, G.; Richardson, P.G.; Gertz, M.A.; Giralt, S.; Mateos, M.V.; Leleu, X.; Anderson, K.C. Management of relapsed and refractory multiple myeloma: Novel agents, antibodies, immunotherapies and beyond. Leukemia 2018, 32, 252–2262. [Google Scholar] [CrossRef] [PubMed]
- Cicek, S.S.; Aberham, A.; Ganzera, M.; Stuppner, H. Quantitative analysis of cycloartane glycosides in black cohosh rhizomes and dietary supplements by RRLC-ELSD and RRLC-qTOF-MS. Anal. Bioanal. Chem. 2011, 400, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; McAlpine, J.B.; Lankin, D.C.; Burton, I.; Karakach, T.; Farnsworth, N.R.; Chen, S.N.; Pauli, G.F. Dereplication, residual complexity, and rational naming: The case of the Actaea triterpenes. J. Nat. Prod. 2011, 75, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Mimaki, Y.; Sakagami, H.; Sashida, Y. Cycloartane glycosides from the rhizomes of Cimicifuga racemosa and their cytotoxic activities. Chem. Pharm. Bull. 2002, 50, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeifer Barbosa, A.L.; Wenzel-Storjohann, A.; Barbosa, J.D.; Zidorn, C.; Peifer, C.; Tasdemir, D.; Cicek, S.S. Antimicrobial and cytotoxic effects of the Copaifera reticulata oleoresin and its main diterpene acids. J. Ethnopharmacol. 2019, 233, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Koopman, G.; Reutelingsperger, C.P.M.; Kuijten, G.A.M.; Keehnen, R.M.J.; Pals, S.T.; van Oers, M.H.J. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |





| MM Cells % Alive | PBMC % Alive | ||||
|---|---|---|---|---|---|
| cpd. | conc. | NCI-H929 | OPM-2 | U266 | |
| 1 | 50 µM | 48.2 ± 10.9 | 81.1 ± 4.7 | 75.1 ± 6.4 | 85.6 ± 1.2 |
| 25 µM | 80.5 ± 5.9 | 93.2 ± 3.7 | 90.2 ± 4.1 | 96.4 ± 0.1 | |
| 2 | 50 µM | 27.9 ± 12.2 | 71.0 ± 11.2 | 56.3 ± 21.4 | 84.0 ± 3.0 |
| 25 µM | 87.7 ± 4.9 | 86.2 ± 8.6 | 79.4 ± 20.2 | 94.7 ± 1.8 | |
| 3 | 50 µM | 10.3 ± 2.8 | 19.7 ± 5.7 | 12.9 ± 3.5 | 6.1 ± 1.5 |
| 25 µM | 52.9 ± 7.1 | 63.8 ± 12 | 89.3 ± 3.2 | 86.5 ± 2.7 | |
| MM Cells % Alive | PBMC % Alive | ||||
|---|---|---|---|---|---|
| cpd. | conc. | NCI-H929 | OPM-2 | U266 | |
| 1 | 50 µM | 13.2 ± 2.4 | 67.3 ± 6.7 | 35.5 ± 6.7 | 74.9 ± 1.2 |
| 25 µM | 70.2 ± 4.3 | 93.1 ± 2.4 | 81.0 ± 5.9 | 94.6 ± 0.2 | |
| 2 | 50 µM | 17.4 ± 7.3 | 51.7 ± 8.9 | 62.1 ± 0.4 | 76.1 ± 2.1 |
| 25 µM | 63.9 ± 10.6 | 84.6 ± 1.0 | 90.2 ± 1.7 | 92.7 ± 1.7 | |
| 3 | 50 µM | 6.6 ± 2.2 | 9.6 ± 2.8 | 9.1 ± 3.8 | 4.6 ± 0.1 |
| 25 µM | 18.9 ± 3.3 | 43.4 ± 9.3 | 66.8 ± 9.5 | 76.2 ± 1.8 | |
| MM Cells % Alive | PBMC % Alive | ||||
|---|---|---|---|---|---|
| cpd. | conc. | NCI-H929 | OPM-2 | U266 | |
| 3 | 37.5 µM | 11.8 ± 4.3 | 70.8 ± 11.5 | 48.0 ± 7.4 | 58.7 ± 12.7 |
| 25 µM | 59.5 ± 7.8 | 88.8 ± 1.8 | 77.8 ± 4.6 | 98.3 ± 2.0 | |
| 12.5 µM | 93.3 ± 1.8 | 99.5 ± 2.0 | 95.0 ± 2.4 | 101.7 ± 0.3 | |
| 4 | 37.5 µM | 27.8 ± 6.6 | 56.0 ± 13.0 | 88.0 ± 1.2 | 28.7 ± 5.9 |
| 25 µM | 59.5 ± 8.7 | 90.3 ± 3.7 | 87.8 ± 2.0 | 72.7 ± 5.4 | |
| 12.5 µM | 84.3 ± 8.6 | 94.8 ± 5.3 | 91.8 ± 4.2 | 96.7 ± 2.9 | |
| 5 | 37.5 µM | 96.3 ± 1.3 | 103.5 ± 1.3 | 93.5 ± 3.5 | |
| 25 µM | 97.0 ± 1.2 | 103.3 ± 1.4 | 95.8 ± 2.8 | ||
| 12.5 µM | 98.8 ± 0.9 | 99.8 ± 2.0 | 95.0 ± 3.1 | ||
| 6 | 37.5 µM | 88.5 ± 0.3 | 100.5 ± 2.4 | 93.5 ± 3.2 | |
| 25 µM | 91.8 ± 3.4 | 99.5 ± 1.8 | 94.5 ± 3.5 | ||
| 12.5 µM | 98.5 ± 0.8 | 101.7 ± 0.9 | 94.8 ± 3.0 | ||
| 7 | 37.5 µM | 2.5 ± 1.3 | 31.3 ± 8.6 | 5.3 ± 2.8 | 25.0 ± 6.3 |
| 25 µM | 34.3 ± 8.0 | 88.0 ± 4.5 | 51.3 ± 11.8 | 81.0 ± 8.5 | |
| 12.5 µM | 95.5 ± 1.8 | 99.8 ± 1.7 | 97.0 ± 3.5 | 105.3 ± 0.6 | |
| 8 | 37.5 µM | 5.0 ± 1.7 | 27.0 ± 9.4 | 81.0 ± 11.9 | 64.3 ± 8.0 |
| 25 µM | 31.3 ± 6.6 | 84.3 ± 4.5 | 97.5 ± 3.8 | 96.3 ± 1.7 | |
| 12.5 µM | 84.8 ± 5.0 | 97.8 ± 2.1 | 102.0 ± 3.0 | 104.3 ± 0.3 | |
| 9 | 37.5 µM | 92.0 ± 1.5 | 104.0 ± 4.7 | 101.3 ± 3.3 | |
| 25 µM | 92.8 ± 1.7 | 100.0 ± 1.9 | 101.8 ± 4.3 | ||
| 12.5 µM | 96.0 ±1.0 | 95.8 ± 2.4 | 101.5 ± 5.1 | ||
| BTZ | 10 nM | 6.3 ± 5.4 | 66.0 ± 9.4 | 25.0 ± 8.3 | |
| 5 nM | 3.0 ± 1.0 | 85.3 ± 7.6 | 80.7 ± 14.1 | ||
| cpd. | NCI-H929 | OPM-2 | U266 | PBMC |
|---|---|---|---|---|
| 3 | 18.1 | >37.5 | 24.7 | 25.9 |
| 4 | 20.3 | 26.3 | >37.5 | 20.7 |
| 7 | 14.7 | 21.2 | 16.1 | 19.9 |
| 8 | 14.2 | 20.7 | 31.1 | 26.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jöhrer, K.; Stuppner, H.; Greil, R.; Çiçek, S.S. Structure-Guided Identification of Black Cohosh (Actaea racemosa) Triterpenoids with In Vitro Activity against Multiple Myeloma. Molecules 2020, 25, 766. https://doi.org/10.3390/molecules25040766
Jöhrer K, Stuppner H, Greil R, Çiçek SS. Structure-Guided Identification of Black Cohosh (Actaea racemosa) Triterpenoids with In Vitro Activity against Multiple Myeloma. Molecules. 2020; 25(4):766. https://doi.org/10.3390/molecules25040766
Chicago/Turabian StyleJöhrer, Karin, Hermann Stuppner, Richard Greil, and Serhat Sezai Çiçek. 2020. "Structure-Guided Identification of Black Cohosh (Actaea racemosa) Triterpenoids with In Vitro Activity against Multiple Myeloma" Molecules 25, no. 4: 766. https://doi.org/10.3390/molecules25040766