Cornus mas L. Stones: A Valuable by-Product as an Ellagitannin Source with High Antioxidant Potential
Abstract
:1. Introduction
2. Results
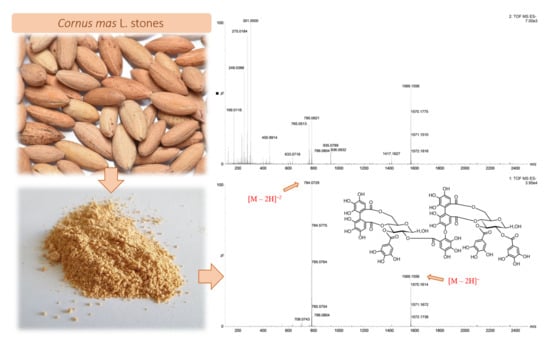
2.1. Qualitative Identification by Means of UPLC-ESI-qTOF-MS/MS
2.2. Quantitative Identification of Compounds
2.3. Antioxidant Properties and Total Phenolic Content (TPC)
3. Discussion
4. Materials and Methods
4.1. Reagents and Standards
4.2. Raw Material
4.3. Sample Preparation
4.4. Qualitative Identification by UPLC-ESI-qTOF-MS/MS
4.5. Quantitative Determination of Phenolic Compounds by HPLC-DAD
4.6. Total Phenolic Content and Antioxidant Activity
4.6.1. Total Phenolic Content
4.6.2. ABTS, FRAP, and DPPH Assays
4.7. Chemical Structures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- West, B.J.; Deng, S.; Jensen, C.J.; Palu, A.K.; Berrio, L.F. Antioxidant, toxicity, and iridoid tests of processed Cornelian cherry fruits. Int. J. Food Sci. Technol. 2012, 47, 1392–1397. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef] [Green Version]
- Tural, S.; Koca, I. Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci. Hortic. 2008, 116, 362–366. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Piórecki, N. Morphological, physical and chemical, and antioxidant profiles of polish varieties of cornelian cherry fruit (Cornus mas L.). Zywnosc Nauka Technol. Jakosc (Poland) 2011, 3, 78–89. [Google Scholar] [CrossRef]
- Szumny, D.; Sozański, T.; Kucharska, A.Z.; Dziewiszek, W.; Piórecki, N.; Magdalan, J.; Chlebda-Sieragowska, E.; Kupczyński, R.; Szelag, A.; Szumny, A. Application of Cornelian Cherry Iridoid-Polyphenolic Fraction and Loganic Acid to Reduce Intraocular Pressure. Evid. Based Complement. Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus Officinalis—Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid–loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Wiśniewski, J.; Fleszar, M.G.; Rapak, A.; Gomulkiewicz, A.; Dzięgiel, P.; Magdalan, J.; Nowak, B.; Szumny, D.; et al. The iridoid loganic acid and anthocyanins from the cornelian cherry (Cornus mas L.) fruit increase the plasma l-arginine/ADMA ratio and decrease levels of ADMA in rabbits fed a high-cholesterol diet. Phytomedicine 2019, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The Effects of Natural Iridoids and Anthocyanins on Selected Parameters of Liver and Cardiovascular System Functions. Oxidative Med. Cell. Longev. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Fitsiou, E.; Spyridopoulou, K.; Vasileiadis, S.; Iliopoulos, C.; Galanis, A.; Vekiari, S.; Pappa, A.; Chlichlia, K.; Kourpeti, T. Evaluation of Antioxidant and Antiproliferative Properties of Cornus mas L. Fruit Juice. Antioxidants 2019, 8, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Zając, K. Fatty Acid compositions of seed oils of cornelian cherry (Cornus mas L.). Acta Biochim. Pol. 2009, 56 (Suppl. 2), 21–22. [Google Scholar]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Akalın, M.K.; Tekin, K.; Karagöz, S. Hydrothermal liquefaction of cornelian cherry stones for bio-oil production. Bioresour. Technol. 2012, 110, 682–687. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Piwnicki, K. Pestki owoców jako cenny surowiec wtórny przemysłu spożywczego. Postępy Techniki Przetwórstwa Spożywczego 2007, 2, 62–66. [Google Scholar]
- Mendu, V.; Harman-Ware, A.E.; Crocker, M.; Jae, J.; Stork, J.; Morton, S.; Placido, A.; Huber, G.; DeBolt, S. Identification and thermochemical analysis of high-lignin feedstocks for biofuel and biochemical production. Biotechnol. Biofuels 2011, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Kostić, M.D.; Veličković, A.V.; Jokovic, N.; Stamenković, O.S.; Veljković, V.B. Optimization and kinetic modeling of esterification of the oil obtained from waste plum stones as a pretreatment step in biodiesel production. Waste Manag. 2016, 48, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Rasul, M.; Ashwath, N. Optimization of biodiesel production from stone fruit kernel oil. Energy Procedia 2019, 160, 268–276. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. Eur. Food Res. Technol. 2018, 245, 1123–1136. [Google Scholar] [CrossRef] [Green Version]
- Home|Department of Economic and Social Affairs. Available online: https://sdgs.un.org/ (accessed on 30 September 2020).
- Vidrih, R.; Čejić, Ž.; Hribar, J. Content of certain food components in flesh and stones of the cornelian cherry (Cornus mas L.) genotypes. Croat. J. Food Sci. Technol. 2012, 4, 64–70. [Google Scholar]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Correlation of oxidative transformations of hydrolyzable tannins and plant evolution. Phytochemistry 2000, 55, 513–529. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S310–S329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuda, T.; Hatano, T.; Ogawa, N.; Kira, R.; Matsuda, M. Cornusiin A, a dimeric ellagitannin forming four tautomers, and accompanying new tannins in Cornus officinalis. Chem. Pharm. Bull. 1984, 32, 4662–4665. [Google Scholar] [CrossRef]
- Hatano, T.; Ikegami, Y.; Shingu, T.; Okuda, T. Camptothins A and B, new dimeric hydrolyzable tannins from Camptotheca acuminata DECNE. Chem. Pharm. Bull. 1988, 36, 2017–2022. [Google Scholar] [CrossRef] [Green Version]
- Hatano, T.; Ogawa, N.; Kira, R.; Yasuhara, T.; Okuda, T. Tannins of cornaceous plants. I. Cornusiins A, B and C, dimeric monomeric and trimeric hydrolyzable tannins from Cornus officinalis, and orientation of valoneoyl group in related tannins. Chem. Pharm. Bull. 1989, 37, 2083–2090. [Google Scholar] [CrossRef] [Green Version]
- Hatano, T.; Yasuhara, T.; Okuda, T. Tannins of cornaceous plants. II. Cornusiins D, E and F, new dimeric and trimeric hydrolyzable tannins from Cornus officinalis. Chem. Pharm. Bull. 1989, 37, 2665–2669. [Google Scholar] [CrossRef] [Green Version]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A.; Kucharska, A.Z.; Fecka, I. Composition and Antibacterial Activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. Leaf Extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef] [PubMed]
- Del Bubba, M.; Checchini, L.; Chiuminatto, U.; Doumett, S.; Fibbi, D.; Giordani, E. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of polyphenolic composition of four cultivars ofFragaria vescaL. berries and their comparative evaluation. J. Mass Spectrom. 2012, 47, 1207–1220. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Kokko, H.; Mintz-Oron, S.; Venger, I.; Kärenlampi, S.; Aharoni, A. Non-targeted analysis of spatial metabolite composition in strawberry (Fragaria×ananassa) flowers. Phytochemistry 2008, 69, 2463–2481. [Google Scholar] [CrossRef]
- Barry, K.M.; Davies, N.W.; Mohammed, C.L. Identification of hydrolysable tannins in the reaction zone of Eucalyptus nitens wood by high performance liquid chromatography-electrospray ionisation mass spectrometry. Phytochem. Anal. 2001, 12, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, C.K.; Bohm, B.A. Ellagitannins from Tellima grandiflora. Phytochemistry 1976, 15, 211–214. [Google Scholar] [CrossRef]
- Hatano, T.; Okonogi, A.; Yazaki, K.; Okuda, T. Trapanins A and B, oligomeric hydrolyzable tannins from Trapa japonica Flerov. Chem. Pharm. Bull. 1990, 38, 2707–2711. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Feng, Z.-L.; Chen, H.-B.; Wang, F.-S.; Lu, J.-H. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatano, T.; Yasuhara, T.; Abe, R.; Okuda, T. A galloylated monoterpene glucoside and a dimeric hydrolysable tannin from Cornus officinalis. Phytochemistry 1990, 29, 2975–2978. [Google Scholar] [CrossRef]
- De Ancos, B.; Gonzalez, E.M.; Cano, M.P. Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch. Pharmacal Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef]
- Chen, Y.; Al-Ghamdi, A.A.; Elshikh, M.S.; Shah, M.H.; Al-Dosary, M.A.; Abbasi, A.M. Phytochemical profiling, antioxidant and HepG2 cancer cells’ antiproliferation potential in the kernels of apricot cultivars. Saudi J. Biol. Sci. 2020, 27, 163–172. [Google Scholar] [CrossRef]
- Szajdek, A.; Borkowska, J. Antioxidant properties of a plant-based food products. Food. Sci. Technol. Qual. 2004, 4S, 5–28. [Google Scholar]
- Onyeneho, S.; Hettiarachchy, N. Effect of Navy Bean Hull Extract on the Oxidative Stability of Soy and Sunflower Oils. J. Agric. Food Chem. 1991, 39, 1701–1704. [Google Scholar] [CrossRef]
- Lavoie, S.; Côté, I.; Pichette, A.; Gauthier, C.; Ouellet, M.; Nagau-Lavoie, F.; Mshvildadze, V.; Legault, J. Chemical composition and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from Cornus canadensis. BMC Complement. Altern. Med. 2017, 17, 123. [Google Scholar] [CrossRef]
- Okuda, T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef]
- Nakashima, H.; Murakami, T.; Yamamoto, N.; Sakagami, H.; Tanuma, S.-I.; Hatano, T.; Yoshida, T.; Okuda, T. Inhibition of human immunodeficiency viral replication by tannins and related compounds. Antivir. Res. 1992, 18, 91–103. [Google Scholar] [CrossRef]
- Sakagami, H.; Satoh, K.; Ida, Y.; Koyama, N.; Premanathan, M.; Arakaki, R.; Nakashima, H.; Hatano, T.; Okuda, T.; Yoshida, T. Induction of Apoptosis and Anti-HIV Activity by Tannin- and Lignin-Related Substances. In Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology; Gross, G.G., Hemingway, R.W., Yoshida, T., Branham, S.J., Eds.; Basic Life Sciences; Springer US: Boston, MA, USA, 1999; pp. 595–611. [Google Scholar] [CrossRef]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Shimizu, M.; Mizusima, T.; Ito, H.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Restoration of effectiveness of β-lactams on methicillin-resistantStaphylococcus aureusby tellimagrandin I from rose red. FEMS Microbiol. Lett. 2000, 185, 135–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiota, S.; Shimizu, M.; Sugiyama, J.; Morita, Y.; Mizushima, T.; Tsuchiya, T. Mechanisms of Action of Corilagin and Tellimagrandin I That Remarkably Potentiate the Activity of β-Lactams against Methicillin-ResistantStaphylococcus aureus. Microbiol. Immunol. 2004, 48, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.U.; Garcia, F.P.; Cortez, D.A.G.; Ueda-Nakamura, T.; Filho, B.P.D.; Nakamura, C.V. Antifungal effects of Ellagitannin isolated from leaves of Ocotea odorifera (Lauraceae). Antonie Van Leeuwenhoek 2010, 99, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, D.S.; Kim, N.H.; Lee, Y.M.; Kim, J.; Kim, J.S. Galloyl glucoses from the seeds of Cornus officinalis with inhibitory activity against protein glycation, aldose reductase, and cataractogenesis ex vivo. Biol. Pharm. Bull. 2011, 34, 443–446. [Google Scholar] [CrossRef] [Green Version]
- Berdowska, I.; Zieliński, B.; Saczko, J.; Sopel, M.; Gamian, A.; Fecka, I. Modulatory impact of selected ellagitannins on the viability of human breast cancer cells. J. Funct. Foods 2018, 42, 122–128. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Horiuchi, T.; Suganuma, M.; Nishiwaki, S.; Yatsunami, J.; Okabe, S.; Okuda, T.; Muto, Y.; Frenkel, K.; Troll, W.; et al. Penta-O-galloyl-β-d-glucose and (−)-epigallocatechin gallate. In Phenolic Compounds in Food and Their Effects on Health II; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Volume 507, pp. 316–325. [Google Scholar]
- Al-Sayed, E.; Korinek, M.; Esmat, A.; Chen, G.-Y.; Cheng, Y.-B.; Hsieh, P.-W.; Chen, B.-H.; Hwang, T.-L. Anti-inflammatory, hepatoprotective and antioxidant activity of ellagitannin isolated from Melaleuca styphelioides. Phytochemistry 2020, 177, 112429. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Pulvirenti, L.; Cornu, A.; Pouységu, L.; Deffieux, D.; Quideau, S.; Tringali, C. C-glucosidic ellagitannins and galloylated glucoses as potential functional food ingredients with anti-diabetic properties: A study of α-glucosidase and α-amylase inhibition. Food Chem. 2020, 313, 126099. [Google Scholar] [CrossRef]
- Kim, J.S. Chapter 45-Seeds of Cornus officinalis and diabetic cataracts. In Handbook of Nutrition, Diet and the Eye; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 451–458. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Ito, H.; Quideau, S. Ellagitannins renewed the concept of tannins. In Chemistry and Biology of Ellagitannins; Quideau, S., Ed.; World Scientific: Singapore, 2009; pp. 1–54. [Google Scholar] [CrossRef] [Green Version]
- Okuda, T.; Yoshida, T.; Hatano, T. Pharmacologically Active Tannins Isolated from Medicinal Plants. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Basic Life Sciences; Springer US: Boston, MA, USA, 1992; pp. 539–569. [Google Scholar] [CrossRef]
- Park, K.H.; Yin, J.; Yoon, K.H.; Hwang, Y.J.; Lee, M.W. Antiproliferative Effects of New Dimeric Ellagitannin from Cornus alba in Prostate Cancer Cells Including Apoptosis-Related S-Phase Arrest. Molecules 2016, 21, 137. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Chen, H. Characterization and functional properties of a pectin/tara gum based edible film with ellagitannins from the unripe fruits of Rubus chingii Hu. Food Chem. 2020, 325, 126964. [Google Scholar] [CrossRef]
- Wyspiańska, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J. Physico-chemical, antioxidant, and anti-inflammatory properties and stability of hawthorn (Crataegus monogyna Jacq.) procyanidins microcapsules with inulin and maltodextrin. J. Sci. Food Agric. 2016, 97, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in Antioxidant Effects and Their Relationship to Phytonutrients in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) during Maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, G.-C.; Chen, H.-Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]





| Peak No. | tR (min) | λmax (nm) | MW (Da) | MS1 [M − H]– (m/z) | Quantity (mg/100 g of Extract) | Compound Name (Isomer) |
|---|---|---|---|---|---|---|
| Mean ± SD | ||||||
| 1 | 1.57 | 215, 277 | 332.0743 | 331.0639 [M − H]− 663.1382 [2M – H]− | 256.29 ± 0.48 | Mono-O-galloyl-β-d-glucose (1) |
| 2 | 1.78 | 221, 270 | 170.0215 | 169.0143 [M − H]− | 430.16 ± 2.77 | Gallic acid |
| 3 | 1.86 | 215, 265 | 634.0806 | 633.0718 [M − H]− | 62.01 ± 6.45 | Gemin D (1) |
| 4 | 2.04 | 214, 272 | 484.0853 | 483.0763 [M – H]− | 86.23 ± 0.41 | Di-O-galloyl-β-d-glucose (1) |
| 5 | 2.25 | 215, 277 | 332.0743 | 331.0639 [M − H]− | 225.97 ± 8.22 | Mono-O-galloyl-β-d-glucose (2) |
| 6 | 2.45 | 215, 265 | 634.0806 | 633.0718 [M − H]− | 399.91 ± 11.42 | Gemin D (2) |
| 7 | 2.75 | 214, 272 | 484.0853 | 483.0763 [M – H]− | 33.49 ± 0.39 | Di-O-galloyl-β-d-glucose (2) |
| 8 | 3.22 | 222, 264 | 1418.1565 | 1417.1549 [M − H]− 708.0688 [M – 2H]−2 | 53.55 ± 0.28 | Camptothin A (1) |
| 9 | 3.31 | 214, 272 | 484.0853 | 483.0763 [M – H]− | 343.60 ± 1.38 | Di-O-galloyl-β-d-glucose (3) |
| 10 | 3.64 | 222, 264 | 1418.1565 | 1417.1549 [M − H]− 708.0688 [M – 2H]−2 | 52.92 ± 0.64 | Camptothin A (2) |
| 11 | 3.87 | 214, 271 | 954.0974 | 953.0919 [M − H]− | 591.55 ± 8.74 | Isorugosin B |
| 12 | 4.11 | 225, 266 | 2202.2325 | 2201.1279 [M – H]− 1100.6101 [M − 2H]−2 | 460.31 ± 5.98 | Cornusiin F (1) |
| 13 | 4.21 | 214, 272 | 484.0853 | 483.0763 [M – H]− | 61.05 ± 0.16 | Di-O-galloyl-β-d-glucose (4) |
| 14 | 4.27 | 218, 267 | 786.0916 | 785.0821 [M − H]− | 226.94 ± 0.11 | Tellimagrandin I (1) |
| 15 | 4.36 | 225, 266 | 2202.2325 | 2201.1279 [M – H]− 1100.6101 [M − 2H]−2 | 1186.56 ± 2.60 | Cornusiin F (2) |
| 16 | 4.49 | 215, 276 | 636.0963 | 1271.1876 [2M – H]− 635.0872 [M – H]− | 247.98 ± 32.96 | Tri-O-galloyl-β-d-glucose (1) |
| 17 | 4.57 | 232, 267 | 1570.1675 | 1569.1556 [M – 2H]− 784.0729 [M – 2H]–2 | 123.15 ± 0.83 | Cornusiin A (1) |
| 18 | 4.72 | 225, 266 | 2202.2325 | 2201.1184 [M − H]− 1100.6033 [M – 2H]−2 | 1336.99 ± 6.70 | Cornusiin F (3) |
| 19 | 4.92 | 215, 270 | 636.0963 | 1271.1949 [2M – H]– 635.0872 [M − H]− | 230.01 ± 0.01 | Tri-O-galloyl-β-d-glucose (2) |
| 20 | 5.08 | 218, 267 | 786.0916 | 785.0821 [M − H]− | 353.08 ± 4.58 | Tellimagrandin I (2) |
| 21 | 5.16 | 232, 262 | 2354.2434 | 2353.0769 [M − H]− 1176.1075 [M − 2H]−2 | 1354.73 ± 47.29 | Cornusiin C (1) |
| 22 | 5.22 | 219, 258 | 1086.0822 | 1085.0734 [M – H]− | 211.59 ± 18.72 | Cornusiin B |
| 23 | 5.32 | 216, 275 | 1570.1675 | 1569.1556 [M − 2H]− 784.0729 [M − 2H]−2 | 569.16 ± 19.64 | Cornusiin A (2) |
| 24 | 5.40 | 232, 262 | 1570.1675 | 1569.1556 [M – 2H]− 784.0729 [M – 2H]−2 | 1115.76 ± 99.63 | Cornusiin A (3) |
| 25 | 5.58 | 218, 270 | 1570.1675 | 1569.1719 [M − 2H]− 784.0729 [M − 2H]−2 | 181.21 ± 12.01 | Cornusiin A (4) |
| 26 | 5.69 | 221, 267 | 2354.2434 | 2353.0798 [M − H]− 1176.1145 [M − 2H]−2 | 155.74 ± 12.44 | Cornusiin C (2) |
| 27 | 5.87 | 230, 268 | 2354.2434 | 2353.0798 [M − H]− 1176.1145 [M − 2H]−2 | 304.03 ± 2.34 | Cornusiin C (3) |
| 28 | 6.04 | 221, 268 | 1570.1617 | 1569.1556 [M – H]− 784.0729 [M – 2H]−2 | 560.56 ± 3.44 | Cornusiin A (5) |
| 29 | 6.15 | 222, 272 | 1722.1785 | 1721.1445 [M – H]− 860.0745 [M – 2H]−2 | 391.86 ± 5.46 | Cornusiin D or Camptothin B (1) |
| 30 | 6.34 | 222, 271 | 1722.1785 | 1721.1445 [M – H]− 860.0745 [M – 2H]–2 | 441.18 ± 5.16 | Cornusiin D or Camptothin B (2) |
| 31 | 6.49 | 221, 271 | 938.1025 | 937.0892 [M – H]− | 168.79 ± 1.11 | Tellimagrandin II |
| 32 | 6.69 | 222, 275 | 788.1072 | 787.1022 [M – H]− | 166.31 ± 3.91 | Tetra-O-galloyl-β-d-glucose |
| 33 | 6.78 | 254, 360 | 302.0063 | 300.9964 [M – H]– | 342.65 ± 26.69 | Ellagic acid |
| 34 | 6.96 | 215, 272 | 2506.2544 | 1252.1165 [M – 2H]–2 | 84.77 ± 20.49 | Trapanin A (β or α) |
| 35 | 7.04 | 215, 271 | 1722.1785 | 1721.1700 [M – H]– 860.0745 [M –2H]–2 | 108.11 ± 24.18 | Cornusiin D or Camptothin B (3) |
| 36 | 7.42 | 216, 277 | 940.1182 | 939.1080 [M – H]– | 250.34 ± 8.59 | Penta-O-galloyl-β-d-glucose (1) |
| 37 | 7.77 | 216, 277 | 940.1182 | 939.1143 [M – H]– | 74.36 ± 0.21 | Penta-O-galloyl-β-d-glucose (2) |
| Antioxidant Activity | Total Phenolic Content | ||
|---|---|---|---|
| ABTS | FRAP | DPPH | |
| (mmol Tx/100 g) | (mmol Tx/100 g0 | (mmol Tx/100 g) | (mg GAE/100 g) |
| 255.99 ± 8.48 | 210.62 ± 5.45 | 191.00 ± 0.04 | 11,466.53 ± 1971.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. Stones: A Valuable by-Product as an Ellagitannin Source with High Antioxidant Potential. Molecules 2020, 25, 4646. https://doi.org/10.3390/molecules25204646
Przybylska D, Kucharska AZ, Cybulska I, Sozański T, Piórecki N, Fecka I. Cornus mas L. Stones: A Valuable by-Product as an Ellagitannin Source with High Antioxidant Potential. Molecules. 2020; 25(20):4646. https://doi.org/10.3390/molecules25204646
Chicago/Turabian StylePrzybylska, Dominika, Alicja Z. Kucharska, Iwona Cybulska, Tomasz Sozański, Narcyz Piórecki, and Izabela Fecka. 2020. "Cornus mas L. Stones: A Valuable by-Product as an Ellagitannin Source with High Antioxidant Potential" Molecules 25, no. 20: 4646. https://doi.org/10.3390/molecules25204646