New Fluorene Derivatives from Dendrobium gibsonii and Their α-Glucosidase Inhibitory Activity
Abstract
:1. Introduction
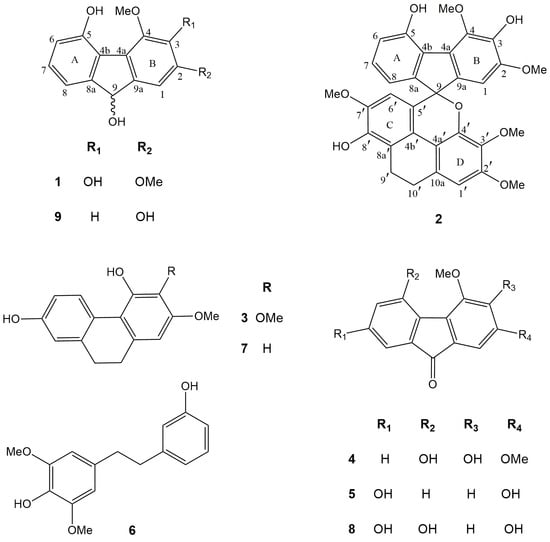
2. Results and Discussion
2.1. Structural Characterization
2.2. α-Glucosidase Inhibitory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Assay for α-Glucosidase Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tran, H.H.T.; Nguyen, M.C.; Le, H.T.; Nguyen, T.L.; Pham, T.B.; Chau, V.M.; Nguyen, T.D. Inhibitors of α-glucosidase and α-amylase from Cyperus rotundus. Pharm. Biol. 2014, 52, 74–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Standl, E.; Khunti, K.; Hansen, T.B.; Schnell, O. The global epidemics of diabetes in the 21st century: Current situation and perspectives. Eur. J. Prev. Cardiol. 2019, 26, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosak, C.; Mertes, G. Critical evaluation of the role of acarbose in the treatment of diabetes: Patient considerations. Diabetes Metab. Syndr. Obes. 2012, 5, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT J. Food Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Katzung, B.G.; Masters, S.B.; Trevor, A.J. Pancreatic Hormones and Antidiabetic drugs. In Basic and Clinical Pharmacology, 12th ed.; McGraw- Hill Companies: New York, NY, USA, 2012. [Google Scholar]
- Mooradian, A.D.; Thurman, J.E. Drug therapy of postprandial hyperglycaemia. Drugs. 1999, 57, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yang, X.W.; Wang, R.F. Bio-assay guided isolation and identification of α-glucosidase inhibitors from the leaves of Aquilaria sinensis. Phytochemistry 2011, 72, 242–247. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness. 2014, 3, 136–174. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. Biomed. Res. Int. 2013, 527570. [Google Scholar]
- Seidenfaden, G. Orchid genera in Thailand XII. Dendrobium Sw. Opera Bot. 1985, 83, 1–295. [Google Scholar]
- Hu, J.; Fan, W.; Dong, F.; Miao, Z.; Zhou, J. Chemical components of Dendrobium chrysotoxum. Chin. J. Chem. 2012, 30, 1327–1330. [Google Scholar] [CrossRef]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances-an overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef] [PubMed]
- Chuakul, W. Ethnomedical uses of Thai Orchidaceous plants. Mahidol J. Pharm. Sci. 2002, 29, 41–45. [Google Scholar]
- Chen, X.J.; Mei, W.L.; Cai, C.H.; Guo, Z.K.; Song, X.Q.; Dai, H.F. Four new bibenzyl derivatives from Dendrobium sinense. Phytochem. Lett. 2014, 9, 107–112. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, G.; Zhang, Y.; Chen, L.; Chen, Y. A new 9,10-dihydrophenanthrene from Dendrobium moniliforme. Nat. Prod. Res. 2016, 30, 174–179. [Google Scholar] [CrossRef]
- Vaddhanaphuti, N. A Field Guide to the Wild Orchids of Thailand, 4th ed.; Silkworm Books: Chiangmai, Thailand, 2005; p. 104. [Google Scholar]
- Paxton, J. Paxton’s Magazine of Botany, and Register of Flowering Plants; Orr and Smith: London, England, 1849; Volume 16. [Google Scholar]
- Talapatra, S.K.; Bose, S.; Mallik, A.K.; Talapatra, B. On the chemistry of indian orchidaceae plants−II: Dengibsin and dengibsinin, the first natural fluorenone derivatives from Dendrobium gibsonii Lindl. Tetrahedron 1985, 41, 2765–2769. [Google Scholar] [CrossRef]
- Talapatra, S.K.; Chakraborty, S.; Bose, S.; Talapatra, B. The chemistry of the Indian Orchidaceae plants. Part IV. Revised Structures of dengibsin and dengibsinin: Chemical shifts of chelated methoxyls. Indian J. Chem. Sec. B 1988, 27B, 250–252. [Google Scholar]
- Sarakulwattana, C.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. New bisbibenzyl and phenanthrene derivatives from Dendrobium scabrilingue and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2020, 34, 1694–1701. [Google Scholar] [CrossRef]
- San, H.T.; Boonsnongcheep, P.; Putalun, W.; Mekboonsonglarp, W.; Sritularak, B.; Likhitwitayawuid, K. α-Glucosidase inhibitory and glucose uptake stimulatory effects of phenolic compounds From Dendrobium christyanum. Nat. Prod. Commun. 2020, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tezuka, Y.; Hirano, H.; Kikuchi, T.; Xu, G.J. Constituents of Ephemerantha lonchophylla. Chem. Pharm. Bull. 1991, 39, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Klongkumnuankarn, P.; Busaranon, K.; Chanvorachote, P.; Sritularak, B.; Jongbunprasert, V.; Likhitwitayawuid, K. Cytotoxic and antimigratory activities of phenolic compounds from Dendrobium brymerianum. Evid. Based Complement. Alternat. Med. 2015, 350410. [Google Scholar]
- Juneja, R.K.; Sharma, S.C.; Tandon, J.S. Two substituted bibenzyls and dihydrophenanthrene from Cymbidium aloifolium. Phytochemistry 1987, 26, 1123–1125. [Google Scholar] [CrossRef]
- Guo, X.Y.; Wang, J.; Wang, N.L.; Kitanaka, S.; Yao, X.S. 9,10-Dihydrophenanthrene derivatives from Pholidota yunnanensis and scavenging activity on DPPH free radical. J. Asian Nat. Prod. Res. 2007, 9, 165–174. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Qing, C.; Zhang, Y.; Wang, L.; Liu, Y. 1,4,5-Trihydroxy-7-methoxy-9H-fluoren 9-one, a new cytotoxic compound from Dendrobium chrysotoxum. Food Chem. 2008, 108, 973–976. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chou, G.X.; Wang, Z.T.; Guo, Y.W.; Hu, Z.B.; Xu, L.S. Two new compounds from Dendrobium chrysotoxum. Helv. Chim. Acta. 2004, 87, 394–399. [Google Scholar] [CrossRef]
- Ye, Q.H.; Zhao, W.M.; Qin, G.W. New fluorenone and phenanthrene derivatives from Dendrobium chrysanthum. Nat. Prod. Res. 2003, 17, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev. Bras. Farmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Chatsumpun, N.; Sritularak, B.; Likhitwitayawuid, K. New biflavonoids with α-glucosidase and pancreatic lipase inhibitory activities from Boesenbergia rotunda. Molecules 2017, 22, 1862. [Google Scholar] [CrossRef] [Green Version]



| Position | 1 a | 2 b | ||||
|---|---|---|---|---|---|---|
| δH (Multiplicity, J in Hz) | δC | HMBC (Correlation with 1H) | δH (Multiplicity, J in Hz) | δC | HMBC (Correlation with 1H) | |
| 1 | 7.10 (1H, s) | 105.2 | 9 | 6.85 (1H, s) | 105.6 | - |
| 2 | - | 148.4 | 1 *, HO-3, MeO-2 | - | 148.6 | HO-3, MeO-2 |
| 3 | - | 139.0 | 1, HO-3 | - | 140.1 | 1, HO-3 * |
| 4 | - | 139.5 | MeO-4, HO-3 | - | 139.6 | MeO-4, HO-3 |
| 4a | - | 123.5 | 1, 9 | - | 124.2 | 1 |
| 4b | - | 123.6 | 6, 8, HO-5 | - | 122.5 | 6, 8, HO-5 |
| 5 | - | 151.1 | 7, HO-5 | - | 151.2 | 6 *,7, HO-5 * |
| 6 | 6.77 (1H, d, 7.5) | 116.1 | 8, HO-5 | 6.76 (1H, dd, 8.0, 1.0) | 117.3 | 7 *,8, HO-5 |
| 7 | 7.13 (1H, t, 7.5) | 128.2 | - | 6.93 (1H, t, 8.0) | 128.6 | 6 * |
| 8 | 7.05 (1H, d, 7.5) | 116.0 | 6, 9 | 6.65 (1H, dd, 8.0, 1.0) | 115.6 | 6 |
| 8a | - | 148.6 | 7, 9 *, HO-9 | - | 148.8 | 7 |
| 9 | 5.38 (1H, d, 7.8) | 74.5 | 1, 8, HO-9 | - | 87.4 | 6′, 1, 8 |
| 9a | - | 137.4 | 9 *, HO-9 | - | 137.2 | 1 * |
| MeO-2 | 3.93 (3H, s) | 56.0 | - | 3.77 (3H, s) | 56.0 | - |
| MeO-4 | 4.12 (3H, s) | 61.4 | - | 4.18 (3H, s) | 61.6 | - |
| HO-3 | 7.91 (s) | - | - | 8.11 (s) | - | - |
| HO-5 | 9.44 (s) | - | - | 9.56 (s) | - | - |
| HO-9 | 4.57 (d, 7.8) | - | - | - | - | - |
| 1′ | 6.61 (1H, s) | 105.3 | 10′ | |||
| 2′ | - | 152.9 | 1′ *, MeO-2′ | |||
| 3′ | - | 137.3 | 1′, MeO-3′ | |||
| 4′ | - | 145.3 | - | |||
| 4a′ | - | 114.0 | 1′, 10′ | |||
| 4b′ | - | 120.6 | 6′, 9′ | |||
| 5′ | - | 123.4 | 6′ * | |||
| 6′ | 6.04 (1H, s) | 105.4 | - | |||
| 7′ | - | 146.5 | 6′*, MeO-7′, HO-8′ | |||
| 8′ | - | 143.4 | 6′, 9′, HO-8′ * | |||
| 8a′ | - | 119.2 | 10′, HO-8′ | |||
| 9′ | 3.09 (1H, m), 2.78 (1H, m) | 20.9 | 10′ * | |||
| 10′ | 2.93 (2H, m) | 26.9 | 1′, 9′ * | |||
| 10a′ | - | 128.6 | 1′ *, 9′ | |||
| MeO-2′ | 3.82 (3H, s) | 55.5 | - | |||
| MeO-3′ | 3.37 (3H, s) | 59.6 | - | |||
| MeO-7′ | 3.54 (3H, s) | 55.4 | - | |||
| HO-8′ | - | - | - | 7.61 (s) | - | - |
| Inhibitors | Dose (μM) | Vmax ∆OD/min | Km (mM) | Ki (μM) |
|---|---|---|---|---|
| None | - | 0.12 | 1.55 | |
| 2 | 22 | 0.052 | 1.19 | 20.38 |
| 11 | 0.086 | 1.23 | ||
| Acarbose | 930 | 0.11 | 4.17 | 190.57 |
| 465 | 0.10 | 6.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thant, M.T.; Chatsumpun, N.; Mekboonsonglarp, W.; Sritularak, B.; Likhitwitayawuid, K. New Fluorene Derivatives from Dendrobium gibsonii and Their α-Glucosidase Inhibitory Activity. Molecules 2020, 25, 4931. https://doi.org/10.3390/molecules25214931
Thant MT, Chatsumpun N, Mekboonsonglarp W, Sritularak B, Likhitwitayawuid K. New Fluorene Derivatives from Dendrobium gibsonii and Their α-Glucosidase Inhibitory Activity. Molecules. 2020; 25(21):4931. https://doi.org/10.3390/molecules25214931
Chicago/Turabian StyleThant, May Thazin, Nutputsorn Chatsumpun, Wanwimon Mekboonsonglarp, Boonchoo Sritularak, and Kittisak Likhitwitayawuid. 2020. "New Fluorene Derivatives from Dendrobium gibsonii and Their α-Glucosidase Inhibitory Activity" Molecules 25, no. 21: 4931. https://doi.org/10.3390/molecules25214931