The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries
Abstract
:1. Introduction
2. Circular Economy
3. Popular Technologies for the Re-Use of Food Loss and Waste from the Agri-Food Sector
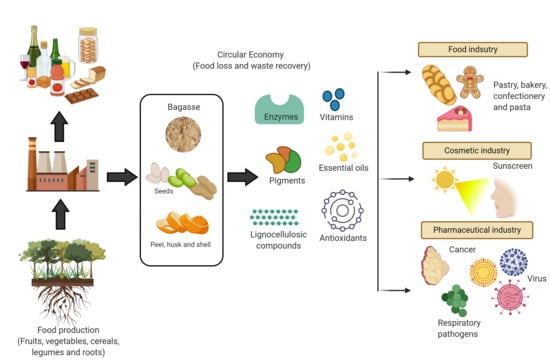
4. Agri-Food Industry
4.1. Fruit and Vegetable Production
4.1.1. Food Loss and Waste (FLW) Products from the Fruit and Vegetable Industrial Processing
4.1.2. Peels
4.1.3. Seeds
4.1.4. Pomace and Core Fruit
4.2. Production of the Root and Tuber Industry
4.2.1. Food Loss and Waste (FLW) Products from the Root and Tuber Industry
4.2.2. Peels
4.2.3. Cassava Bagasse
4.3. Production of the Cereal and Legume Industry
4.3.1. Food Losses and Waste (FLW) Generated from the Cereal and Legume Industry
4.3.2. Husks
4.3.3. Malt Bagasse
4.3.4. Waste Cake
5. Potential of Food Losses and Waste as a Raw Material in the Industry
5.1. Food Industry
5.1.1. Flours
5.1.2. Colorants
5.1.3. Enzymes
5.2. Cosmetic Industry
Antioxidants
5.3. Pharmaceutical Industry
5.3.1. Antibacterial and Anticancer
5.3.2. Antivirals
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability: Samples of the compounds are not available from the authors. |
References
- Tomiyama, J.-M.; Takagi, D.; Kantar, M.B. The effect of acute and chronic food shortage on human population equilibrium in a subsistence setting. Agric. Food Secur. 2020, 9, 1–2. [Google Scholar] [CrossRef]
- World Food Programme Zero Hungry. Available online: https://www.wfp.org/zero-hunger (accessed on 17 November 2020).
- Garza-Reyes, J.A.; Kumar, V.; Batista, L.; Cherrafi, A.; Rocha-Lona, L. From linear to circular manufacturing business models. J. Manuf. Technol. Manag. 2019, 30, 554–560. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, K.R. The Crisis of Consumption of Natural Resources. Int. J. Recent Innov. Acad. Res. 2018, 2, 8–19. [Google Scholar]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Shafiee-Jood, M.; Cai, X. Reducing Food Loss and Waste to Enhance Food Security and Environmental Sustainability. Environ. Sci. Technol. 2016, 50, 8432–8443. [Google Scholar] [CrossRef] [PubMed]
- Van der Werf, P.; Gilliland, J.A. A systematic review of food losses and food waste generation in developed countries. Proc. Inst. Civ. Eng. Waste Resour. Manag. 2017, 170, 66–77. [Google Scholar] [CrossRef]
- Ziolkowska, J.R. Economic and Environmental Costs of Agricultural Food Losses and Waste in the US. Int. J. Food Eng. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Bilali, H.E.; El Bilali, H. Research on food losses and waste in North Africa. N. Afr. J. Food Nutr. Res. 2018, 2, 51–57. [Google Scholar]
- Hoehn, D.; Laso, J.; Cristóbal, J.; Ruiz-Salmón, I.; Butnar, I.; Borrion, A.; Bala, A.; Fullana-i-Palmer, P.; Vázquez-Rowe, I.; Aldaco, R.; et al. Regionalized Strategies for Food Loss and Waste Management in Spain under a Life Cycle Thinking Approach. Foods 2020, 9, 1765. [Google Scholar] [CrossRef]
- Brenes-Peralta, L.; Jiménez-Morales, M.F.; Freire Junior, M.; Belik, W.; Basso, N.; Polenta, G.; Giraldo, C.; Granados, S. Challenges and Initiatives in Reducing Food Losses and Waste: Latin America and the Caribbean; Burleigh Dodds Science Publishing: Cambridge, UK, 2020. [Google Scholar]
- Beretta, C.; Stucki, M.; Hellweg, S. Environmental Impacts and Hotspots of Food Losses: Value Chain Analysis of Swiss Food Consumption. Environ. Sci. Technol. 2017, 51, 11165–11173. [Google Scholar] [CrossRef]
- Porter, S.D.; Reay, D.S.; Higgins, P.; Bomberg, E. A half-century of production-phase greenhouse gas emissions from food loss & waste in the global food supply chain. Sci. Total Environ. 2016, 571, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinach, L.; Parizeau, K.; Fraser, E.D.G. Do food donation tax credits for farmers address food loss/waste and food insecurity? A case study from Ontario. Agric. Human. Values 2020, 37, 383–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards circular economy in the agri-food sector. A systematic literature review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New Light on the Healthy Function of Citrus Fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef]
- Hobbs, J.E. Food supply chains during the COVID-19 pandemic. Can. J. Agric. Econ. Can. D’agroecon. 2020, 68, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Haseeb, M.; Zandi, G.; Hartani, N.H.; Pahi, M.H.; Nadeem., S.U. Environmental Analysis of the Effect of Population Growth Rate on Supply Chain Performance and Economic Growth of Indonesia. Ekoloji Derg. 2019, 28, 417–426. [Google Scholar]
- Maqbool, A.; Khan, S.; Haleem, A.; Khan, M.I. Investigation of Drivers Towards Adoption of Circular Economy: A DEMATEL Approach. In Recent Advances in Mechanical Engineering; Springer: Singapore, 2020; pp. 147–160. [Google Scholar]
- Pagotto, M.; Halog, A. Towards a Circular Economy in Australian Agri-food Industry: An Application of Input-Output Oriented Approaches for Analyzing Resource Efficiency and Competitiveness Potential. J. Ind. Ecol. 2016, 20, 1176–1186. [Google Scholar] [CrossRef]
- Del Borghi, A.; Moreschi, L.; Gallo, M. Circular economy approach to reduce water–energy–food nexus. Curr. Opin. Environ. Sci. Heal. 2020, 13, 23–28. [Google Scholar] [CrossRef]
- Jurgilevich, A.; Birge, T.; Kentala-Lehtonen, J.; Korhonen-Kurki, K.; Pietikäinen, J.; Saikku, L.; Schösler, H. Transition towards Circular Economy in the Food System. Sustainability 2016, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Sharma, Y.K.; Mangla, S.K.; Patil, P.P.; Liu, S. When challenges impede the process: For circular economy-driven sustainability practices in food supply chain. Manag. Decis. 2019, 57, 995–1017. [Google Scholar] [CrossRef]
- Genovese, A.; Acquaye, A.A.; Figueroa, A.; Koh, S.C.L. Sustainable supply chain management and the transition towards a circular economy: Evidence and some applications. Omega 2017, 66, 344–357. [Google Scholar] [CrossRef]
- Lewandowski, M. Designing the Business Models for Circular Economy—Towards the Conceptual Framework. Sustainability 2016, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Cingiz, K.; Wesseler, J. Opportunities and the Policy Challenges to the Circular Agri-Food System. In EU Bioeconomy Economics and Policies; Palgrave Macmillan: Chambridge, UK, 2019; pp. 293–318. [Google Scholar]
- Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M. Mapping of research lines on circular economy practices in agriculture: From waste to energy. Renew. Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- Ingrao, C.; Faccilongo, N.; Di Gioia, L.; Messineo, A. Food waste recovery into energy in a circular economy perspective: A comprehensive review of aspects related to plant operation and environmental assessment. J. Clean. Prod. 2018, 184, 869–892. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The Next Generation of Sustainable Food Packaging to Preserve Our Environment in a Circular Economy Context. Front. Nutr. 2018, 5, 121. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.; Skene, K.; Haynes, K. The Circular Economy: An Interdisciplinary Exploration of the Concept and Application in a Global Context. J. Bus. Ethics 2017, 140, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Macarthur, E. Towards the circular economy. J. Ind. Ecol. 2013, 2, 23–44. [Google Scholar]
- Stahel, W.R. The circular economy. Nature 2016, 7595, 435–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.T.; Buranakarn, V. Emergy indices and ratios for sustainable material cycles and recycle options. Resour. Conserv. Recycl. 2003, 38, 1–22. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Archer, S.A.; Gomes, H.I.; Christgen, B.; Lag-Brotons, A.J.; Purnell, P. Circular economy and the matter of integrated resources. Sci. Total Environ. 2019, 689, 963–969. [Google Scholar] [CrossRef]
- Xue, L.; Liu, G. Introduction to global food losses and food waste. In Saving Food: Production, Supply Chain, Food Waste and Food Consumption; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–31. ISBN 9780128153574. [Google Scholar]
- Fan, Y.; Fang, C. Circular economy development in China-current situation, evaluation and policy implications. Environ. Impact Assess. Rev. 2020, 84, 106441. [Google Scholar] [CrossRef]
- Liu, L.; Liang, Y.; Song, Q.; Li, J. A review of waste prevention through 3R under the concept of circular economy in China. J. Mater. Cycles Waste Manag. 2017, 19, 1314–1323. [Google Scholar] [CrossRef]
- Sze, C.; Lin, K.; Mubofu, E.B.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Salemdeeb, R.; zu Ermgassen, E.K.H.J.; Kim, M.H.; Balmford, A.; Al-Tabbaa, A. Environmental and health impacts of using food waste as animal feed: a comparative analysis of food waste management options. J. Clean. Prod. 2017, 140, 871–880. [Google Scholar] [CrossRef] [Green Version]
- Candida, V.; Fernando, C.; Margarida, G.; Ana Luísa, F. Wastes: Solutions, Treatments and Opportunities III: Selected Papers from e 5th International Conference Wastes 2019, September 4–6, 2019, Lisbon, Portugal; Candida, V., Fernando, C., Margarida, G., Ana Luísa, F., Eds.; CRC Press: Boca Raton, FL, USA, 2019; Volume 3. [Google Scholar]
- Bergesen, H.O.; Parmann, G.; Thommessen, O.B. Convention on the Control of Transboundary Movements of Hazardous Wastes and their Disposal (Basel Convention). Yearb. Int. Coop. Environ. Dev. 2019, 87–89. [Google Scholar] [CrossRef]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; De Vries, H. Critical success and risk factors for circular business models valorising agricultural waste and by-products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Commission, E. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Garske, B.; Heyl, K.; Ekardt, F.; Weber, L.M.; Gradzka, W. Challenges of food waste governance: An assessment of European legislation on food waste and recommendations for improvement by economic instruments. Land 2020, 9, 231. [Google Scholar] [CrossRef]
- Alexa Teigiserova, D.; Hamelin, L.; Thomsen, M.; Hamelin, L. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total Environ. 2019, 706. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.; Bleazard, P.; Okawa, K. Preventing Food Waste: Case Studies of Japan and the United Kingdom. OECD 2015, 76, 50. [Google Scholar]
- Chalak, A.; Abou-Daher, C.; Chaaban, J.; Abiad, M.G. The global economic and regulatory determinants of household food waste generation: A cross-country analysis. Waste Manag. 2016, 48, 418–422. [Google Scholar] [CrossRef]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613–614, 635–643. [Google Scholar] [CrossRef]
- Mena, C.; Adenso-Diaz, B.; Yurt, O. The causes of food waste in the supplier-retailer interface: Evidences from the UK and Spain. Resour. Conserv. Recycl. 2011, 55, 648–658. [Google Scholar] [CrossRef]
- Melikoglu, M.; Sze, C.; Lin, K.; Webb, C. Analysing global food waste problem: pinpointing the facts and estimating the energy content. Cent. Eur. J. Eng. 2013, 3, 157–164. [Google Scholar] [CrossRef]
- Shehzad, A.; Bashir, M.J.K.; Sethupathi, S.; Lim, J.W. An overview of heavily polluted landfill leachate treatment using food waste as an alternative and renewable source of activated carbon. Process Saf. Environ. Prot. 2015, 98, 309–318. [Google Scholar] [CrossRef]
- Ezeah, C.; Byrne, T. A Critical Review of Municipal Solid Waste Legislation and Compliance in Greece-In the Context of the EU Landfill Directive. J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 81–89. [Google Scholar] [CrossRef]
- Angelonidi, E.; Smith, S.R. A comparison of wet and dry anaerobic digestion processes for the treatment of municipal solid waste and food waste. Water Environ. J. 2015, 29, 549–557. [Google Scholar] [CrossRef]
- Duarte, S.; Osuna, S.; Alexandra, J.; Miranda, R.; Pablo, J. Sustainable Use of Organic Solid Waste: A Conceptual Review through the Waste Pickers Organizations. Int. J. Eng. Res. Technol. 2020, 13, 2055–2066. [Google Scholar] [CrossRef]
- Rudnik, E. Compostable Polymer Materials; Newnes: Oxford, UK, 2019. [Google Scholar]
- Pietro, G. Economía Circular e Innovación Tecnológica en Residuos Sólidos: Oportunidades en América Latina; Suárez, J., Ed.; Corporación Andina de Fomento: Buenos Aires, Argentina, 2018. [Google Scholar]
- De Clercq, D.; Wen, Z.; Gottfried, O.; Schmidt, F.; Fei, F. A review of global strategies promoting the conversion of food waste to bioenergy via anaerobic digestion. Renew. Sustain. Energy Rev. 2017, 79, 204–221. [Google Scholar] [CrossRef]
- Ma, H.; Guo, Y.; Qin, Y.; Li, Y.Y. Nutrient recovery technologies integrated with energy recovery by waste biomass anaerobic digestion. Bioresour. Technol. 2018, 269, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies and Industrial Techniques; Academic Press: Cambridge, MA, USA, 2015; ISBN 9780128004197. [Google Scholar]
- Kalogirou, E. Waste-to-Energy Technologies and Global Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tozlu, A.; Özahi, E.; Energy, A.A.-R.U. Waste to energy technologies for municipal solid waste management in Gaziantep. Renew. Sustain. Energy Rev. 2016, 54, 809–815. [Google Scholar] [CrossRef]
- Montejo, C.; Tonini, D.; Márquez, M.d.C.; Fruergaard Astrup, T. Mechanical-biological treatment: Performance and potentials. An LCA of 8 MBT plants including waste characterization. J. Environ. Manag. 2013, 128, 661–673. [Google Scholar] [CrossRef] [Green Version]
- Fei, F.; Wen, Z.; Huang, S.; De Clercq, D. Mechanical biological treatment of municipal solid waste: Energy efficiency, environmental impact and economic feasibility analysis. J. Clean. Prod. 2018, 178, 731–739. [Google Scholar] [CrossRef]
- Ghiffari, R.A. Development of Eucalyptus Oil Agro-industries in Kabupaten Buru. Procedia Soc. Behav. Sci. 2016, 227, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Mullen, A.M.; Álvarez, C.; Pojić, M.; Hadnadev, T.D.; Papageorgiou, M. Classification and target compounds. In Food Waste Recovery: Processing Technologies and Industrial Techniques; Elsevier: Amsterdam, The Netherlands, 2015; pp. 25–57. ISBN 9780128004197. [Google Scholar]
- Paiva, T.; Ribeiro, M.; Coutinho, P. R&D Collaboration, Competitiveness Development, and Open Innovation in R&D. J. Open Innov. Technol. Mark. Complex. 2020, 6, 116. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Lenart, A.; Janowicz, M. Sustainable Development in the Agri-Food Sector in Terms of the Carbon Footprint: A Review. Sustainability 2020, 12, 6463. [Google Scholar] [CrossRef]
- Bachev, H. Risk Management in the Agri-Food Sector. Contemp. Econ. 2013, 7, 45–62. [Google Scholar] [CrossRef] [Green Version]
- United States Departament of Agriculture. World Agricultural Production; United States Departament of Agriculture: Washington, DC, USA, 2020.
- Taylor, D.H.; Soosay, C.; Fearne, A.; Dent, B.; Barber, E. Value chain analysis: an approach to supply chain improvement in agri-food chains. Int. J. Phys. Distrib. Logist. Manag. 2005, 35, 141–162. [Google Scholar] [CrossRef]
- De Moraes, C.C.; de Oliveira Costa, F.H.; Roberta Pereira, C.; da Silva, A.L.; Delai, I. Retail food waste: mapping causes and reduction practices. J. Clean. Prod. 2020, 256, 120124. [Google Scholar] [CrossRef]
- Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300, 122755. [Google Scholar] [CrossRef] [PubMed]
- Al-Rumaihi, A.; McKay, G.; Mackey, H.R.; Al-Ansari, T. Environmental Impact Assessment of Food Waste Management Using Two Composting Techniques. Sustainability 2020, 12, 1595. [Google Scholar] [CrossRef] [Green Version]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 November 2020).
- Pu, M.; Zhong, Y. Rising concerns over agricultural production as COVID-19 spreads: Lessons from China. Glob. Food Sec. 2020, 26, 100409. [Google Scholar] [CrossRef]
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 1–22. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.K.; Kalne, A.A.; Khan, K.A.U. Effects of Processing on Vitamins in Fruits and Vegetables. In Processing of the Fruits and Vegetables: From Farm to Fork; CRC Press: Boca Raton, FL, USA, 2019; p. 324. [Google Scholar]
- Anaya-Esparza, L.M.; Velázquez-Estrada, R.M.; Roig, A.X.; García-Galindo, H.S.; Sayago-Ayerdi, S.G.; Montalvo-González, E. Thermosonication: An alternative processing for fruit and vegetable juices. Trends Food Sci. Technol. 2017, 61, 26–37. [Google Scholar] [CrossRef]
- Peters, K. Technology of Fruits and Vegetable Processing; Scientific e-Resources: 2019; ED-Tech Press: Waltham Abbey, UK, 2019; p. 334. ISBN 1839472626. [Google Scholar]
- Crino, M.; Barakat, T.; Trevena, H.; Neal, B. Systematic Review and Comparison of Classification Frameworks Describing the Degree of Food Processing. Nutr. Food Technol. 2017, 3. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, J.E. Fruit Manufacturing; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Panda, H. Recovery, Fruit Waste Utilization, Waste Utilization of Fruits and Vegetables, Fruit and Vegetable Waste Management, Waste Utilization in Food Industry Method for Quantitative Recovery of…; Asia Pacific Business Press: Delhi, India, 2011. [Google Scholar]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2061–2108. [Google Scholar] [CrossRef]
- Fidelis, M.; de Moura, C.; Kabbas Junior, T.; Pap, N.; Mattila, P.; Mäkinen, S.; Putnik, P.; Bursać Kovačević, D.; Tian, Y.; Yang, B.; et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from By-Products within Circular Economy. Molecules 2019, 24, 3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukruansuwan, V.; Napathorn, S.C. Use of agro-industrial residue from the canned pineapple industry for polyhydroxybutyrate production by Cupriavidus necator strain A-04. Biotechnol. Biofuels 2018, 11, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folinas, D.; Aidonis, D.; Karayannakidis, P. Greening the canned peach production. J. Agric. Inform. 2015, 6, 24–39. [Google Scholar] [CrossRef] [Green Version]
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Raj, D.; Senapati, A. Waste Management in Horticulture Processing Industry. In Commercial Horticulture; Patel, N.L., Chawla, S.L., Ahlawat, T.R., Eds.; New India Publishing Agency: Delhi, India, 2016; pp. 391–401. [Google Scholar]
- Van Dyk, J.S.; Gama, R.; Morrison, D.; Swart, S.; Pletschke, B.I. Food processing waste: Problems, current management and prospects for utilisation of the lignocellulose component through enzyme synergistic degradation. Renew. Sustain. Energy Rev. 2013, 26, 521–531. [Google Scholar] [CrossRef]
- Gülcü, M.; Uslu, N.; Özcan, M.M.; Gökmen, F.; Özcan, M.M.; Banjanin, T.; Gezgin, S.; Dursun, N.; Geçgel, Ü.; Ceylan, D.A.; et al. The investigation of bioactive compounds of wine, grape juice and boiled grape juice wastes. J. Food Process. Preserv. 2019, 43, e13850. [Google Scholar] [CrossRef] [Green Version]
- Ros, M.; Pascual, J.; Ayuso, M.; Morales, A.; Miralles, J. Salidas valorizables de los residuos y subproductos orgánicos de la industria de los transformados de frutas y hortalizas: proyecto Life+ Agrowaste. Agrowaste.Eu 2012, 130, 2–9. [Google Scholar]
- Rico, X.; Gullón, B.; Alonso, J.L.; Yáñez, R. Recovery of high value-added compounds from pineapple, melon, watermelon and pumpkin processing by-products: An overview. Food Res. Int. 2020, 132. [Google Scholar] [CrossRef]
- kumar, V.; Singh, J.; Chandra, S.; Kumar, R.; Singh, K.; Chaudhary, V.; Kumar, P.; Scholar, R.; Professor, A. Post Harvest Technology of Papaya Fruits & its Value Added Products-A Review. Int. J. Pure App. Biosci 2019, 7, 169–181. [Google Scholar] [CrossRef]
- Joglekar, S.N.; Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Process of fruit peel waste biorefinery: a case study of citrus waste biorefinery, its environmental impacts and recommendations. Environ. Sci. Pollut. Res. 2019, 26, 34713–34722. [Google Scholar] [CrossRef] [PubMed]
- Senit, J.J.; Velasco, D.; Gomez Manrique, A.; Sanchez-Barba, M.; Toledo, J.M.; Santos, V.E.; Garcia-Ochoa, F.; Yustos, P.; Ladero, M. Orange peel waste upstream integrated processing to terpenes, phenolics, pectin and monosaccharides: Optimization approaches. Ind. Crop. Prod. 2019, 134, 370–381. [Google Scholar] [CrossRef]
- Dibanda Romelle, F.; Ashwini, R.P.; Manohar, R.S. Chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Salem, R.H. Quality Characteristics of Beef Sausages with Tomato Peel as a Colour and Functional Additive During Frozen Storage. World Appl. Sci. J. 2013, 22, 1085–1093. [Google Scholar] [CrossRef]
- Rincón, A.; Vásquez, A.; Latinoamericanos, F.P.-A.; 2005, U. Chemical composition and bioactive compounds of flour of orange (Citrus sinensis), tangerine (Citrus reticulata) and grapefruit (Citrus paradisi) peels cultivated in. Arch. Latinoam. Nutr. 2005, 55, 305–310. [Google Scholar]
- Silva, I.M.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.; Amboni, R.D. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008. [Google Scholar] [CrossRef]
- Alsayed, H.; Ahmed, A.R.; Al-Sayed, H.M.A. Utilization of watermelon rinds and sharlyn melon peels as a natural source of dietary fiber and antioxidants in cake. J. Food Sci. Technol. 2013, 6, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Raihana, A.R.N.; Marikkar, J.M.N.; Amin, I.; Shuhaimi, M. A Review on Food Values of Selected Tropical Fruits’ Seeds. Int. J. Food Prop. 2015, 18, 2380–2392. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Jorge, N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res. Int. 2014, 66, 493–500. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Jorge, N. Bioactive compounds of oils extracted from fruits seeds obtained from agroindustrial waste. Eur. J. Lipid Sci. Technol. 2017, 119, 1600024. [Google Scholar] [CrossRef]
- Persia, M.E.; Parsons, C.M.; Schang, M.; Azcona, J. Nutritional evaluation of dried tomato seeds. Poult. Sci. 2003, 82, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Studies on the amino acid and fatty acid compositions in the seed and pulpy substance of feral peach (Prunus persica Batsch var. davidiana Max.). J. Life Sci. 2007, 17, 125–131. [Google Scholar] [CrossRef]
- Yu, X.; Van De Voort, F.R.; Li, Z.; Yue, T. Proximate Composition of the Apple Seed and Characterization of Its Oil Proximate Composition of the Apple Seed and Characterization of Its Oil. Int. J. Food Eng. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Felhi, S.; Baccouch, N.; Salah, H.B.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional constituents, phytochemical profiles, in vitro antioxidant and antimicrobial properties, and gas chromatography–mass spectrometry analysis of various solvent extracts from grape seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544. [Google Scholar] [CrossRef]
- Onimawo, I.A.; Oteno, F.; Orokpo, G.; Akubor, P.I. Physicochemical and nutrient evaluation of African bush mango (Irvingia gabonensis) seeds and pulp. Plant Foods Hum. Nutr. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Mallek-Ayadi, S.; Bahloul, N.; Kechaou, N. Chemical composition and bioactive compounds of Cucumis melo L. seeds: Potential source for new trends of plant oils. Process Saf. Environ. Prot. 2018, 113, 68–77. [Google Scholar] [CrossRef]
- Zou, Y.; Ma, K.; Tian, M. Chemical composition and nutritive value of hot pepper seed (Capsicum annuum) grown in Northeast Region of China. Food Sci. Technol. 2015, 35, 659–663. [Google Scholar] [CrossRef] [Green Version]
- El-safy, S.; Salem, R.; Abd El-Ghany, M. Chemical and Nutritional Evaluation of Different Seed Flours as Novel Sources of Protein. World J. Dairy food Sci. 2012, 7, 59–65. [Google Scholar]
- Araújo, A.C.M.A.; Menezes, E.G.T.; Terra, A.W.C.; Dias, B.O.; De Oliveira, É.R.; Queiroz, F. Bioactive compounds and chemical composition of Brazilian Cerrado fruits’ wastes: Pequi almonds, murici, and sweet passionfruit seeds. Food Sci. Technol. 2018, 38, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Kruczek, M.; Drygaś, B.; Habryka, C. Pomace in fruit industry and their contemporary potential application. World Sci. News 2016, 48, 259–265. [Google Scholar]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Castro Sousa, E.; Maria, A.; Uchôa-Thomaz, A.; Osvaldo, J.; Carioca, B.; Maia De Morais, S.; De Lima, A.; Martins, C.G.; Alexandrino, C.D.; Augusto, P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. Camp. 2014, 34, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Grande, L.; Dolino, O.; Montecastro, D.B.; Basilio, A.M.; Ph, A.E. Low-cost Recovery of Bromelain Solids from Industrial Pineapple Peel, Pulp, and Core Wastes Using Ethanolic Cashew Leaf Polyphenol. Philipp. J. Sci. 2020, 149, 581–587. [Google Scholar]
- Ramli, A.N.M.; Aznan, T.N.T.; Illias, R.M. Bromelain: from production to commercialisation. J. Sci. Food Agric. 2017, 97, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.C.; Rescolino, R.; Coelho, D.F.; Zanchetta, B.; Tambourgi, E.B.; Silveira, E. Characterization of Bromelain from Ananas Comosus Agroindustrial Residues Purified by Ethanol Factional Precipitation. Chem. Eng. Trans. 2014, 37, 781–786. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Kumar, A. Properties and Therapeutic Application of Bromelain: A Review. Biotechnol. Res. Int. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.F.; Abu-Ghannam, D.K. Apple pomace as a potential ingredient for the development of new functional foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar]
- Mahmoud, A.E.; Omer, H.A.; Mohammed, A.T.; Ali, M.M. Enhancement of Chemical Composition and Nutritive Value of Some Fruits Pomace by Solid State Fermentation. Egypt. J. Chem 2020, 63, 3713–3720. [Google Scholar] [CrossRef]
- Del Valle, M.; Cámara, M.; Torija, M.E. Chemical characterization of tomato pomace. J. Sci. Food Agric. 2006, 86, 1232–1236. [Google Scholar] [CrossRef]
- Pag An, J.; Ibarz, A. Extraction and rheological properties of pectin from fresh peach pomace. J. Food Eng. 1999, 39, 193–201. [Google Scholar] [CrossRef]
- Elena, M.; Pardo, S.; Ramos Cassellis, E.; Escobedo, R.M.; García, E.J. Chemical Characterisation of the Industrial Residues of the Pineapple (Ananas comosus). J. Agric. Chem. Environ. 2014, 3, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot-A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekara, A.; Josheph Kumar, T. Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. Int. J. Food Sci. 2016, 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Aprianita, A.; Vasiljevic, T.; Bannikova, A.; Kasapis, S. Physicochemical properties of flours and starches derived from traditional Indonesian tubers and roots. J. Food Sci. Technol. 2014, 51, 3669–3679. [Google Scholar] [CrossRef] [Green Version]
- Renub Research. Cassava Starch Market, Consumption & Global Forecast, by Region, Applications and Companies. 2018. Available online: https://www.researchandmarkets.com/research/kk27xb/global_cassava?w=4 (accessed on 13 December 2020).
- Birch, P.R.J.; Bryan, G.; Fenton, B.; Gilroy, E.M.; Hein, I.; Jones, J.T.; Prashar, A.; Taylor, M.A.; Torrance, L.; Toth, I.K. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012, 4, 477–508. [Google Scholar] [CrossRef]
- Ferraro, V.; Piccirillo, C.; Tomlins, K.; Pintado, M.E. Cassava (Manihot esculenta Crantz) and Yam (Dioscorea spp.) Crops and Their Derived Foodstuffs: Safety, Security and Nutritional Value. Crit. Rev. Food Sci. Nutr. 2016, 56, 2714–2727. [Google Scholar] [CrossRef]
- Laycock, B.G.; Halley, P.J. Starch Applications: State of Market and New Trends. In Starch Polymers: From Genetic Engineering to Green Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 381–419. ISBN 9780444537300. [Google Scholar]
- Omohimi, C.; Piccirillo, C.; Ferraro, V.; Roriz, M.C.; Omemu, M.A.; Santos, S.M.D.; Da Ressurreição, S.; Abayomi, L.; Adebowale, A.; Vasconcelos, M.W.; et al. Safety of Yam-Derived (Dioscorea rotundata) Foodstuffs—Chips, Flakes and Flour: Effect of Processing and Post-Processing Conditions. Foods 2019, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Luís, J.; Soto, M.; Venegas González, J.; Bernardino Nicanor, A.; González Cruz, L.; Fernández, J.Y. Chemical Characterization and Nutritional Evaluation of Mountain’s yam (Dioscorea remotiflora Kunth) Tubers. Adv. Biores 2014, 5, 153–160. [Google Scholar] [CrossRef]
- Fakir, M.; Jannat, M.; Mostafa, M.; Seal, H. Starch and flour extraction and nutrient composition of tuber in seven cassava accessions. J. Bangladesh Agric. Univ. 2013, 10, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.K.; Njintang, N.Y.; Singhal, R.S.; Kaushal, P. Tropical Roots and Tubers; Sharma, H.K., Njintang, N.Y., Singhal, R.S., Kaushal, P., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2016; ISBN 9781118992739. [Google Scholar]
- Mohamad Yazid, N.S.; Abdullah, N.; Muhammad, N.; Matias-Peralta, H.M. Application of Starch and Starch-Based Products in Food Industry. J. Sci. Technol. Spec. Issue Appl. Sci. Math. 2018, 10, 144–174. [Google Scholar] [CrossRef] [Green Version]
- IMARC Group. Cassava Starch Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2019–2024. 2019. Available online: https://www.researchandmarkets.com/reports/4752275/cassava-starch-market-global-industry-trends (accessed on 13 December 2020).
- Beltrán-Penagos, M.A.; Sánchez-Camargo, A.D.; Narvaez-Cuenca, C.E. Proximal composition, bioactive compounds and biorefinery approach in potato tubers of Solanum tuberosum Group Phureja: a review. Int. J. Food Sci. Technol. 2020, 55, 2282–2295. [Google Scholar] [CrossRef]
- Liang, S.; McDonald, A.G. Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J. Agric. Food Chem. 2014, 62, 8421–8429. [Google Scholar] [CrossRef] [PubMed]
- Somendrika, M.A.D.; Wickramasinghe, I.; Wansapala, M.A.J.; Peiris, S. Sensory Profile, Nutritional and Shelf-Life Analysis of Cassava Par-Fried Frozen Slices Developed with Raw Cassava Roots. Vidyodaya J. Sci. 2019, 22, 43–52. [Google Scholar]
- Sepelev, I.; Galoburda, R. Industrial potato peel waste application in food production: A review. Investig. para Desarro. Rural 2015, 1, 130–136. [Google Scholar]
- Florencia, V.; López, O.V.; García, M.A. Exploitation of by-products from cassava and ahipa starch extraction as filler of thermoplastic corn starch. Compos. Part B Eng. 2020, 182, 107653. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Kleekayai, T.; Suntornsuk, W. Production and characterization of chitosan obtained from Rhizopus oryzae grown on potato chip processing waste. World J. Microbiol. Biotechnol. 2011, 27, 1145–1154. [Google Scholar] [CrossRef]
- Abedini, A.; Amiri, H.; Karimi, K. Efficient biobutanol production from potato peel wastes by separate and simultaneous inhibitors removal and pretreatment. Renew. Energy 2020, 160, 269–277. [Google Scholar] [CrossRef]
- Albis, A.; Martínez, J.; Severiche, M.; Garcia, J. Remoción de plomo de soluciones acuosas usando cáscara de yuca modificada con ácido cítrico. Av. Investig. Ing. 2016, 13. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.H.M.M.; Akter, M. Euphorbiaceae (Spurge) Family of Rajshahi, Bangladesh. Res. Plant Sci. 2013, 1, 74–80. [Google Scholar] [CrossRef]
- Faezah, O.N.; Aishah, H.; Kalsom, Y. Umi Comparative evaluation of organic and inorganic fertilizers on total phenolic, total flavonoid, antioxidant activity and cyanogenic glycosides in cassava (Manihot esculenta). Afr. J. Biotechnol. 2016, 12, 2414–2421. [Google Scholar]
- Chaves-López, C.; Serio, A.; Grande-Tovar, C.D.; Cuervo-Mulet, R.; Delgado-Ospina, J.; Paparella, A. Traditional Fermented Foods and Beverages from a Microbiological and Nutritional Perspective: The Colombian Heritage. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- Daud, Z.; Sari, A.; Kassim, M.; Aripin, A.M.; Awang, H.; Zainuri, M.; Hatta, M. Chemical Composition and Morphological of Cocoa Pod Husks and Cassava Peels for Pulp and Paper Production. Aust. J. Basic Appl. Sci. 2013, 7, 406–411. [Google Scholar]
- Akinyele, B.J.; Akinyosoye, F.A. Effect of Volvariella volvacea cultivaton on the chemical composition of agrowastes. Afr. J. Biotechnol. 2005, 4, 979–983. [Google Scholar] [CrossRef]
- Aruna, T.E.; Aworh, O.C.; Raji, A.O.; Olagunju, A.I. Protein enrichment of yam peels by fermentation with Saccharomyces cerevisiae (BY4743). Ann. Agric. Sci. 2017, 62, 33–37. [Google Scholar] [CrossRef]
- Arapoglou, D.; Varzakas, T.; Vlyssides, A.; Israilides, C. Ethanol production from potato peel waste (PPW). Waste Manag. 2010. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, G.; Zhang, L.; Sun, Z. Preparation of high performance H2S removal biochar by direct fluidized bed carbonization using potato peel waste. Process Saf. Environ. Prot. 2017, 107, 281–288. [Google Scholar] [CrossRef]
- de Fatima Araújo, L.; Felix, R.A.A.R.; Aguiar, E.M.; Coelho, R.R.P. Evaluation of the Potentiality of Maniocresidues (Manihot esculenta Crantz) in animal feeding. Int. J. Adv. Eng. Res. Sci. 2020, 7, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Travalini, A.P.; Lamsal, B.; Magalhães, W.L.E.; Demiate, I.M. Cassava starch films reinforced with lignocellulose nanofibers from cassava bagasse. Int. J. Biol. Macromol. 2019, 139, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Wongskeo, P.; Rangsunvigit, P.; Chavadej, S. Production of Glucose from the Hydrolysis of Cassava Residue using Bacteria Isolates from Thai Higher Termites. World Acad. Sci. Eng. Technol. 2012, 6, 275–278. [Google Scholar]
- Koehler, P.; Wieser, H. Chemistry of cereal grains. In Handbook on Sourdough Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 11–45. ISBN 9781461454250. [Google Scholar]
- Margier, M.; Georgé, S.; Hafnaoui, N.; Remond, D.; Nowicki, M.; Du Chaffaut, L.; Amiot, M.-J.; Reboul, E. Nutritional Composition and Bioactive Content of Legumes: Characterization of Pulses Frequently Consumed in France and Effect of the Cooking Method. Nutrients 2018, 10, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, T.G.; Nunes, M.A.; Bessada, S.M.F.; Costa, H.S.; Oliveira, M.B.P.P. Biologically active and health promoting food components of nuts, oilseeds, fruits, vegetables, cereals, and legumes. In Chemical Analysis of Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 609–656. [Google Scholar]
- Campos-Vega, R.; Nieto-Figueroa, K.H.; Oomah, B.D. Cocoa (Theobroma cacao L.) pod husk: Renewable source of bioactive compounds. Trends Food Sci. Technol. 2018, 81, 172–184. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pegels, N.; González, I.; García, T.; Martín, R. Authenticity testing of wheat, barley, rye and oats in food and feed market samples by real-time PCR assays. LWT Food Sci. Technol. 2015, 60, 867–875. [Google Scholar] [CrossRef]
- OCDE/FAO Cereales. Perspectivas Agricolas 2017–2026; OECD Publishing: Paris, Franch, 2017. [Google Scholar]
- Galati, A.; Oguntoyinbo, F.A.; Moschetti, G.; Crescimanno, M.; Settanni, L. The Cereal Market and the Role of Fermentation in Cereal-Based Food Production in Africa. Food Rev. Int. 2014, 30, 317–337. [Google Scholar] [CrossRef]
- Sudheesh, S.; Verma, P.; Forster, J.; Cogan, N.; Kaur, S. Generation and Characterisation of a Reference Transcriptome for Lentil (Lens culinaris Medik.). Int. J. Mol. Sci. 2016, 17, 1887. [Google Scholar] [CrossRef]
- Singh, J.; Kanaujia, R.; Kumar, J.; Singh, F.; Ak, S.; Singh, N.P. Genetic Variability for Antioxidant Activity and Total Phenolic Content in Four Major Pulse crops. Tech. Nutr. Food Sci. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Śmiglak-Krajewska, M.; Wojciechowska-Solis, J.; Viti, D. Consumers’ Purchasing Intentions on the Legume Market as Evidence of Sustainable Behaviour. Agriculture 2020, 10, 424. [Google Scholar] [CrossRef]
- Duodu, K.G. Effects of processing on antioxidant phenolics of cereal and legume grains. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; American Chemical Society: Washington, DC, USA, 2011; Volume 1089, pp. 31–54. [Google Scholar]
- Oghbaei, M.; Prakash, J. Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food Agric. 2016, 2, 1136015. [Google Scholar] [CrossRef] [Green Version]
- Akanbi, T.O.; Dare, K.O.; Aryee, A.N.A. High-Value Products from Cereal, Nuts, Fruits, and Vegetables Wastes. In Byproducts from Agriculture and Fisheries; Wiley: Hoboken, NJ, USA, 2019; pp. 347–368. [Google Scholar]
- Branca, C.; Di Blasi, C.; Galgano, A. Pyrolytic conversion of wastes from cereal, protein and oil-protein crops. J. Anal. Appl. Pyrolysis 2017, 127, 426–435. [Google Scholar] [CrossRef]
- Arendt, E.; Zannini, E. Cereal Grains for the Food and Beverage Industries; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Owens, G. Cereals Processing Technology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Franzen, M.; Borgerhoff Mulder, M. Ecological, economic and social perspectives on cocoa production worldwide. Biodivers. Conserv. 2007, 16, 3835–3849. [Google Scholar] [CrossRef]
- Afoakwa, E. Cocoa Production and Processing Technology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, X.; Lv, G.; Li, X.; Chi, Y.; Yan, J.; Liu, X.; Buekens, A. TG-pyrolysis and FTIR analysis of chocolate and biomass waste. J. Therm. Anal. Calorim. 2014, 117, 343–353. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. State-of-the-Art Chocolate Manufacture: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1313–1344. [Google Scholar] [CrossRef] [Green Version]
- Langridge, P. Economic and Academic Importance of Barley. In The Barley Genome; Springer: Chambridge, UK, 2018; pp. 1–10. [Google Scholar]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide Research Trends on Wheat and Barley: A Bibliometric Comparative Analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Rodman, A.D.; Gerogiorgis, D.I. Multi-objective process optimisation of beer fermentation via dynamic simulation. Food Bioprod. Process. 2016, 100, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Loomis, G.; Dari, B.; Rogers, C.W.; Sihi, D. Evaluation of residue management practices on barley residue decomposition. PLoS ONE 2020, 15, e0232896. [Google Scholar] [CrossRef]
- Martínez, M.L.; Eliche, D.; Cruz, N.; Corpas, F.A. Utilization of bagasse from the beer industry in clay brick production for building. Materconstrucc. Revistas. Csic. 2012, 62, 465–2746. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, M.; Fernández-Gil, M.; Churruca, I.; Miranda, J.; Lasa, A.; Navarro, V.; Simón, E. Evolution of Gluten Content in Cereal-Based Gluten-Free Products: An Overview from 1998 to 2016. Nutrients 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H. Current status of food waste generation and management in China. Bioresour. Technol. 2019, 273, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Satusap, P.; Chavasit, V.; Kriengsinyos, W.; Judprasong, K. Development of cereal and legume based food products for the elderly. Springerplus 2014, 3, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awarikabey, E.; Amponsah, I.K.; Woode, M.Y. The value of the cocoa bean shell (hull) and the effect of various processing methods on the phyto-constituents and antioxidant activity of the nib and shell. J. Nat. Prod. Plant Resour. 2014, 4, 58–64. [Google Scholar]
- Ramachandran, S.; Singh, K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications-A review. Bioresour. Technol. 2007, 98. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.; Tari, C. Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Ind. Crop. Prod. 2014, 54, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Rosentrater, K.A. A review of corn masa processing residues: Generation, properties, and potential utilization. Waste Manag. 2006, 26, 284–292. [Google Scholar] [CrossRef]
- Alexandri, M.; López-Gómez, J.P.; Olszewska-Widdrat, A.; Venus, J. Valorising agro-industrial wastes within the circular bioeconomy concept: The case of defatted rice bran with emphasis on bioconversion strategies. Fermentation 2020, 6, 42. [Google Scholar] [CrossRef]
- Tassoni, A.; Tedeschi, T.; Zurlini, C.; Cigognini, I.M.; Petrusan, J.-I.; Rodríguez, Ó.; Neri, S.; Celli, A.; Sisti, L.; Cinelli, P.; et al. State-of-the-Art Production Chains for Peas, Beans and Chickpeas—Valorization of Agro-Industrial Residues and Applications of Derived Extracts. Molecules 2020, 25, 1383. [Google Scholar] [CrossRef] [Green Version]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of Cocoa Husk Feeding on the Composition of Swine 2 Intestinal Microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef]
- Mendes, C.A.; Adnet, F.A.; Leite, M.C.; Furtado, C.G.; Sousa, A.M. Chemical, physical, mechanical, thermal and morphological characteriz.ation of corn husk residue. Cellul. Chem. Technol. 2015, 49, 727–735. [Google Scholar]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Aruwayo, A. Chemical composition of some selected non-conventional feed resources katsina. In Proceedings of the Asian-Nias Joint Annual Meeting, Abuja, Nigeria, 1–4 November 2020. [Google Scholar]
- Hansen, M.J.; Chwalibog, A.; Tauson, A.-H. Influence of different fibre sources in diets for growing pigs on chemical composition of faeces and slurry and ammonia emission from slurry. Anim. Feed Sci. Technol. 2007, 134, 326–336. [Google Scholar] [CrossRef]
- Barana, D.; Salanti, A.; Orlandi, M.; Danish Ali, S.; Ali, D.S.; Zoia, L. Biorefinery process for the simultaneous recovery of lignin, hemicelluloses, cellulose nanocrystals and silica from rice husk and Arundo donax. Ind. Crop. Prod. 2016. [Google Scholar] [CrossRef]
- Oomah, B.D.; Corbé, A.; Balasubramanian, P. Antioxidant and Anti-inflammatory Activities of Bean (Phaseolus vulgaris L.) Hulls. J. Agric. Food Chem 2010, 58, 8225–8230. [Google Scholar] [CrossRef]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources-A positive approach for valorization of fruit and vegetable wastes. Sustainablity 2020, 12, 5401. [Google Scholar] [CrossRef]
- Schmitz, E.; Nordberg Karlsson, E.; Adlercreutz, P. Warming weather changes the chemical composition of oat hulls. Plant Biol. 2020, 22, 1086–1091. [Google Scholar] [CrossRef]
- Mello, L.R.P.F.; Mali, S. Use of malt bagasse to produce biodegradable baked foams made from cassava starch. Ind. Crop. Prod. 2014, 55, 187–193. [Google Scholar] [CrossRef]
- dos Santos Mathias, T.R.; de Mello, P.P.; Servulo1, E.F.C. Solid wastes in brewing process: A review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef]
- Woyengo, T.A.; Patterson, R.; Levesque, C.L. Nutritive value of extruded or multi-enzyme supplemented cold-pressed soybean cake for pigs. J. Anim. Sci. 2016, 94, 5230–5238. [Google Scholar] [CrossRef]
- Tyapkova, O.; Osen, R.; Wagenstaller, M.; Baier, B.; Specht, F.; Zacherl, C. Replacing fishmeal with oilseed cakes in fish feed—A study on the influence of processing parameters on the extrusion behavior and quality properties of the feed pellets. J. Food Eng. 2016, 191, 28–36. [Google Scholar] [CrossRef]
- Ancuța, P.; Sonia, A. Oil Press-Cakes and Meals Valorization through Circular Economy Approaches: A Review. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Otles, S.; Despoudi, S.; Bucatariu, C.; Kartal, C. Food waste management, valorization, and sustainability in the food industry. In Food Waste Recovery: Processing Technologies and Industrial Techniques; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 3–23. ISBN 9780128004197. [Google Scholar]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kibler, K.M.; Reinhart, D.; Hawkins, C.; Motlagh, A.M.; Wright, J. Food waste and the food-energy-water nexus: A review of food waste management alternatives. Waste Manag. 2018, 74, 52–62. [Google Scholar] [CrossRef]
- Strazza, C.; Magrassi, F.; Gallo, M.; Del Borghi, A. Life Cycle Assessment from food to food: A case study of circular economy from cruise ships to aquaculture. Sustain. Prod. Consum. 2015, 2, 40–51. [Google Scholar] [CrossRef]
- Zeković, I.; Lenhardt, L.; Dramićanin, T.; Dramićanin, M.D. Classification of Intact Cereal Flours by Front-Face Synchronous Fluorescence Spectroscopy. Food Anal. Methods 2012, 5, 1205–1213. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Ribotta, P.D.; León, A.E.; Pérez, G.T. Incorporation of several additives into gluten free breads: Effect on dough properties and bread quality. J. Food Eng. 2012, 111, 590–597. [Google Scholar] [CrossRef]
- CARLSON, B.L.; KNORR, D.; Watkins, T.R. Influence of Tomato Seed Addition on the Quality of Wheat Flour Breads. J. Food Sci. 1981, 46, 1029–1031. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. Potential of grape byproducts as functional ingredients in baked goods and pasta. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2473–2505. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Brennan, C.S.; Jaganmohan, R.; Surabi, A.; Alagusundaram, K. Utilisation of pigeon pea (Cajanus cajan L) byproducts in biscuit manufacture. LWT Food Sci. Technol. 2011, 44, 1533–1537. [Google Scholar] [CrossRef]
- Sainz, R.L.; Szezecinski, A.C.S.F.; Fontana, M.; Bosenbecker, V.K.; Ferri, V.C.; do Nascimento, C.O. Uso de harina de baya de uva en la producción de cookies. BIO Web Conf. 2019, 12, 04003. [Google Scholar] [CrossRef]
- Pitre, A.M.; Andrade, A.; García, L.; Londoño, P. Desarrollo de una galleta a partir del orujo de uva variedad criolla negra. Anales 2011, 11, 191–205. [Google Scholar]
- Koca, I.; Tekguler, B.; Yilmaz, V.A.; Hasbay, I.; Koca, A.F. The use of grape, pomegranate and rosehip seed flours in Turkish noodle (erişte) production. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef] [Green Version]
- Masli, M.D.P.; Gu, B.J.; Rasco, B.A.; Ganjyal, G.M. Fiber-Rich Food Processing Byproducts Enhance the Expansion of Cornstarch Extrudates. J. Food Sci. 2018, 83, 2500–2510. [Google Scholar] [CrossRef]
- Ying, D.Y.; Hlaing, M.M.; Lerisson, J.; Pitts, K.; Cheng, L.; Sanguansri, L.; Augustin, M.A. Physical properties and FTIR analysis of rice-oat flour and maize-oat flour based extruded food products containing olive pomace. Food Res. Int. 2017, 100, 665–673. [Google Scholar] [CrossRef]
- Lohani, U.C.; Muthukumarappan, K. Effect of Extrusion Processing Parameters on Antioxidant, Textural and Functional Properties of Hydrodynamic Cavitated Corn Flour, Sorghum Flour and Apple Pomace-Based Extrudates. J. Food Process Eng. 2017, 40. [Google Scholar] [CrossRef]
- Aparecida Damasceno, K.; Alvarenga Gonçalves, C.A.; Dos Santos Pereira, G.; Lacerda Costa, L.; Bastianello Campagnol, P.C.; Leal De Almeida, P.; Arantes-Pereira, L. Development of Cereal Bars Containing Pineapple Peel Flour (Ananas comosus L. Merril). J. Food Qual. 2016, 39, 417–424. [Google Scholar] [CrossRef]
- Rosales Soto, M.U.; Brown, K.; Ross, C.F. Antioxidant activity and consumer acceptance of grape seed flour-containing food products. Int. J. Food Sci. Technol. 2012, 47, 592–602. [Google Scholar] [CrossRef]
- Wani, A.A.; Sogi, D.S.; Singh, P.; Khatkar, B.S. Influence of watermelon seed protein concentrates on dough handling, textural and sensory properties of cookies. J. Food Sci. Technol. 2015, 52, 2139–2147. [Google Scholar] [CrossRef] [Green Version]
- MarketsandMarkets. Natural Food Colors & Flavors Market by Color Type (Caramel, Carotenoids, Anthocyanins, Curcumin, Annatto, and Copper Chlorophyllin), Flavor Type (Natural Extracts, Aroma Chemicals, & Essential Oils), Application & Region-Global Forecast to 2025. 2020. Available online: https://www.marketsandmarkets.com/Market-Reports/natural-colors-flavors-market-676.html (accessed on 13 December 2020).
- Mordor Intelligence. Global Food Colorants Market, Growth, Trends, Forecast. 2020. Available online: https://www.mordorintelligence.com/industry-reports/food-colorants-market (accessed on 13 December 2020).
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Rymbai, H.; Sharma, R.R.; Srivastav, M. Biocolorants and Its Implications in Health and Food Industry—A Review. J. Pharmacol. Res. 2011, 3, 2228–2244. [Google Scholar]
- Koubaa, M.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Koubaa, W.; Boussetta, N.; Vorobiev, E. Recovery of colorants from red prickly pear peels and pulps enhanced by pulsed electric field and ultrasound. Innov. Food Sci. Emerg. Technol. 2016, 37, 336–344. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Shi, X. A fuzzy model for aggregative food safety risk assessment in food supply chains. Prod. Plan. Control 2012, 23, 377–395. [Google Scholar] [CrossRef]
- Backes, E.; Pereira, C.; Barros, L.; Prieto, M.A.; Genena, A.K.; Barreiro, M.F.; Ferreira, I.C.F.R. Recovery of bioactive anthocyanin pigments from Ficus carica L. peel by heat, microwave, and ultrasound based extraction techniques. Food Res. Int. 2018, 113, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Parra-Campos, A.; Ordóñez-Santos, L.E. Natural pigment extraction optimization from coffee exocarp and its use as a natural dye in French meringue. Food Chem. 2019, 285, 59–66. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT Food Sci. Technol. 2017, 84, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Mourtzinos, I.; Prodromidis, P.; Grigorakis, S.; Makris, D.P.; Biliaderis, C.G.; Moschakis, T. Natural food colorants derived from onion wastes: Application in a yoghurt product. Electrophoresis 2018, 39, 1975–1983. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Szabo, K.; Teleky, B.-E.; Mureșan, V.; Rusu, A.-V.; Socol, C.-T.; Vodnar, D.-C. Poly(vinyl alcohol)-Based Biofilms Plasticized with Polyols and Colored with Pigments Extracted from Tomato By-Products. Polymers 2020, 12, 532. [Google Scholar] [CrossRef] [Green Version]
- Rizk, E.M.; Bedier, S.H.; Elgendy, M.A. Utilization of carotenoid pigments extracted from tomato peel as natural antioxidants and colorants in sunflower oil and spaghetti. Egypt. J. Agric. Res. 2014, 92, 309–321. [Google Scholar]
- Noronha Matos, K.A.; Praia Lima, D.; Pereira Barbosa, A.P.; Zerlotti Mercadante, A.; Campos Chisté, R. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- Kantifedaki, A.; Kachrimanidou, V.; Mallouchos, A.; Papanikolaou, S.; Koutinas, A.A. Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 2018, 185, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Hackett, M.M.; Lee, J.H.; Francis, D.; Schwartz, S.J. Thermal stability and isomerization of lycopene in tomato oleoresins from different varieties. J. Food Sci. 2004, 69, 536–541. [Google Scholar] [CrossRef]
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Charactrization of carotenoids (lyco-red) extracted from tomato peels and its uses as natural colorants and antioxidants of ice cream. Ann. Agric. Sci. 2014, 59, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yang, X.; Yang, S.; Zhu, M.; Structural, X.W.-C.U. Technology prospecting on enzymes: application, marketing and engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209017. [Google Scholar] [CrossRef] [Green Version]
- MarketsandMarkets. Industrial Enzymes Market Growth & Trends: Size and Share. 2020. Available online: https://www.researchandmarkets.com/reports/4520168/industrial-enzymes-market-growth-trends-and (accessed on 13 December 2020).
- Mordor Intelligence. Food Enzymes Market—Growth, Trends and Forecasts (2020—2025). 2020. Available online: https://www.mordorintelligence.com/industry-reports/global-food-enzymes-market-industry (accessed on 13 December 2020).
- Zaidi, K.; Ali, A.; Ali, S.; International, I.N.-B.U. Microbial tyrosinases: promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. Biochem. Res. Int. 2014, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- De Lencastre Novaes, L.C.; de Carvalho Santos Ebinuma, V.; Mazzola, P.G.; Júnior, A.P. Polymer-based alternative method to extract bromelain from pineapple peel waste. Biotechnol. Appl. Biochem. 2013, 60, 527–535. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Vicente, F.A.; Lario, L.D.; Pessoa, A.; Ventura, S.P.M. Recovery of bromelain from pineapple stem residues using aqueous micellar two-phase systems with ionic liquids as co-surfactants. Process Biochem. 2016, 51, 528–534. [Google Scholar] [CrossRef]
- Guo, J.; Miao, Z.; Wan, J.; Guo, X. Pineapple peel bromelain extraction using gemini surfactant-based reverse micelle—Role of spacer of gemini surfactant. Sep. Purif. Technol. 2018, 190, 156–164. [Google Scholar] [CrossRef]
- Wan, J.; Guo, J.; Miao, Z.; Guo, X. Reverse micellar extraction of bromelain from pineapple peel—Effect of surfactant structure. Food Chem. 2016, 197, 450–456. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Valetti, N.W.; Pastrana-Castro, L.M.; Teixeira, J.A.; Picó, G.A.; Pintado, M.M. Optimization of bromelain isolation from pineapple byproducts by polysaccharide complex formation. Food Hydrocoll. 2019, 87, 792–804. [Google Scholar] [CrossRef] [Green Version]
- Okino-Delgado, C.H.; Pereira, M.S.; da Silva, J.V.I.; Kharfan, D.; do Prado, D.Z.; Fleuri, L.F. Lipases obtained from orange wastes: Commercialization potential and biochemical properties of different varieties and fractions. Biotechnol. Prog. 2019, 35, e2734. [Google Scholar] [CrossRef] [Green Version]
- Silveira, E.A.; Tardioli, P.W.; Farinas, C.S. Valorization of Palm Oil Industrial Waste as Feedstock for Lipase Production. Appl. Biochem. Biotechnol. 2016, 179, 558–571. [Google Scholar] [CrossRef]
- Moftah, O.A.S.; Grbavčić, S.; Žuža, M.; Luković, N.; Bezbradica, D.; Knežević-Jugović, Z. Adding value to the oil cake as a waste from oil processing industry: Production of lipase and protease by Candida utilis in solid state fermentation. Appl. Biochem. Biotechnol. 2012, 166, 348–364. [Google Scholar] [CrossRef]
- Pereira, A.D.S.; Fontes-Sant’Ana, G.C.; Amaral, P.F. Mango agro-industrial wastes for lipase production from Yarrowia lipolytica and the potential of the fermented solid as a biocatalyst. Food Bioprod. Process. 2019, 115, 68–77. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Kholany, E.A.; Abo-Mosalum, E.M.R. Production of α-amylase by Aspergillus niger isolated from mango kernel. Middle East J. Appl. Sci. 2019, 9, 134–141. [Google Scholar]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Effect of grape seed extract on skin fibroblasts exposed to UVA light and its photostability in sunscreen formulation. J. Cosmet. Dermatol. 2020. [Google Scholar] [CrossRef]
- Garcia, R. Comercialization and productive internacionalization in the cosmetic industry: competitive challenges for brazilian firms. Production 2005, 15, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Matwiejczuk, N.; Galicka, A.; Brzóska, M.M. Review of the safety of application of cosmetic products containing parabens. J. Appl. Toxicol. 2020, 40, 176–210. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Khan, S.T. Potential industrial use of compounds from by-products of fruits and vegetables. In Health and Safety Aspects of Food Processing Technologies; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 273–307. ISBN 9783030249038. [Google Scholar]
- Herranz-López, M.; Barrajón-Catalán, E. Antioxidants and Skin Protection. Antioxidants 2020, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Ferreira, M.; Oliveira, A.S.; Magalhães, C.; Sousa, M.E.; Pinto, M.; Sousa Lobo, J.M.; Almeida, I.F. Evolution of the use of antioxidants in anti-ageing cosmetics. Int. J. Cosmet. Sci. 2019, 41, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Gabros, S.; Nessel, T.A.; Zito, P.M. Sunscreens And Photoprotection; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Moo-Huchin, V.M.; Moo-Huchin, M.I.; Estrada-León, R.J.; Cuevas-Glory, L.; Estrada-Mota, I.A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lourith, N.; Kanlayavattanakul, M.; Chingunpitak, J. Development of sunscreen products containing passion fruit seed extract. Braz. J. Pharm. Sci. 2017, 53, 16116. [Google Scholar] [CrossRef] [Green Version]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from Agroindustrial by-Products as Natural Ingredients for Cosmetics: Chemical Structure and In Vitro Sunscreen and Cytotoxic Activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef] [Green Version]
- Marto, J.; Gouveia, L.F.; Chiari, B.G.; Paiva, A.; Isaac, V.; Pinto, P.; Simões, P.; Almeida, A.J.; Ribeiro, H.M. The green generation of sunscreens: Using coffee industrial sub-products. Ind. Crop. Prod. 2016, 80, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Milani, L.P.G.; Garcia, N.O.S.; Morais, M.C.; Dias, A.L.S.; Oliveira, N.L.; Conceição, E.C. Extract from byproduct Psidium guajava standardized in ellagic acid: additivation of the in vitro photoprotective efficacy of a cosmetic formulation. Rev. Bras. Farmacogn. 2018, 28, 692–696. [Google Scholar] [CrossRef]
- Yarovaya, L.; Khunkitti, W. Anti-inflammatory activity of grape seed extract as a natural sun protection enhancer for broad-spectrum sunscreen. In Proceedings of the Cosmetic & Beauty International Conference 2019 Sustainable Cosmetic & Beauty Innovations; Mae Fa Luang University, Chiang Rai, Thailand, 7–9 October 2019. [Google Scholar]
- Mota, M.D.; da Boa Morte, A.N.; Silva, L.C.R.C.; Chinalia, F.A. Sunscreen protection factor enhancement through supplementation with Rambutan (Nephelium lappaceum L) ethanolic extract. J. Photochem. Photobiol. B Biol. 2020, 205, 111837. [Google Scholar] [CrossRef]
- De Lima Yamaguchi, K.K.; Dos, L.; Santarém, S.; Lamarão, C.V.; Lima, E.S.; Florêncio Da Veiga-Junior, V. Avaliação in vitro da Atividade Fotoprotetora de Resíduos de Frutas Amazônicas. Sci. Amaz. 2016, 5, 109–116. [Google Scholar]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Medicin. Chem. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- MarketsandMarkets. Healthcare BPO Market-Global Forecasts to 2022|by Outsourcing Approaches, Pharmaceutical, Provider & Models|MarketsandMarkets. 2017. Available online: https://www.marketsandmarkets.com/Market-Reports/healthcare-outsourcing-bpo-market-472.html (accessed on 13 December 2020).
- IQVIA. The Global Use of Medicine in 2019 and Outlook to 2023. 2019. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023 (accessed on 13 December 2020).
- Aminov, R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharmacol. 2017, 133, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Kardan Yamchi, J.; Haeili, M.; Gizaw Feyisa, S.; Kazemian, H.; Hashemi Shahraki, A.; Zahednamazi, F.; Imani Fooladi, A.A.; Feizabadi, M.M. Evaluation of efflux pump gene expression among drug susceptible and drug resistant strains of Mycobacterium tuberculosis from Iran. Infect. Genet. Evol. 2015, 36, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of evidence available on hesperidin-rich products as potential tools against COVID-19 and hydrodynamic cavitation-based extraction as a method of increasing their production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Passamonti, S.; Tramer, F.; Ziberna, L.; Boligon, A.A.; Athayde, M.L. Phenolic composition of orange peels and modulation of redox status and matrix metalloproteinase activities in primary (Caco-2) and metastatic (LoVo and LoVo/ADR) colon cancer cells. Eur. Food Res. Technol. 2016, 242, 1949–1959. [Google Scholar] [CrossRef]
- Churata-Oroya, D.E.; Ramos-Perfecto, D.; Moromi-Nakata, H.; Martínez-Cadillo, E.; Castro-Luna, A.; Garcia, R. Antifungal Eff ect of Citrus paradisi “grapefruit” on strains of Candida albicans isolated from patients with denture stomatitis. Rev Estomatol Hered. 2016, 26, 78–84. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- De la Rosa, J.P.; Ruiz-Palomino, P.; Arriola-Guevara, E.; García-Fajardo, J.; Sandoval, G.; Guatemala-Morales, G. A Green Process for the Extraction and Purification of Hesperidin from Mexican Lime Peel (Citrus aurantifolia Swingle) that is Extendible to the Citrus Genus. Processes 2018, 6, 266. [Google Scholar] [CrossRef] [Green Version]
- Hilali, S.; Fabiano-Tixier, A.-S.; Ruiz, K.; Hejjaj, A.; Ait Nouh, F.; Idlimam, A.; Bily, A.; Mandi, L.; Chemat, F. Green Extraction of Essential Oils, Polyphenols, and Pectins from Orange Peel Employing Solar Energy: Toward a Zero-Waste Biorefinery. ACS Sustain. Chem. Eng. 2019, 7, 11815–11822. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; Beatriz, L.; Nascimento, S.; et al. Real-Scale Integral Valorization of Waste Orange Peel via Hydrodynamic Cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef] [Green Version]
- Albanese, L.; Meneguzzo, F. Hydrodynamic cavitation technologies: A pathway to more sustainable, healthier beverages, and food supply chains. In Processing and Sustainability of Beverages; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Extraction of Natural Antioxidants from the Thelephora ganbajun Mushroom by an Ultrasound-Assisted Extraction Technique and Evaluation of Antiproliferative Activity of the Extract against Human Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Lingampeta, P. Solid wastes of fruit peels as source of low cost broad spectrum natural antimicrobial compounds- Furanone, Furfural and Benezenetriol. Int. J. Res. Eng. Technol. 2014, 3, 2321–7308. [Google Scholar]
- Santos, T.R.J.; de Aquino Santana, L.C.L. Antimicrobial potential of exotic fruits residues. S. Afr. J. Bot. 2019, 124, 338–344. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Passamonti, S.; Tramer, F.; Ziberna, L.; Boligon, A.A.; Athayde, M.L. Inhibition of metalloproteinase and proteasome activities in colon cancer cells by citrus peel extracts. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Meiyanto, E.; Hermawan, A. Anindyajati Natural products for cancer-targeted therapy: Citrus flavonoids as potent chemopreventive agents. Asian Pac. J. Cancer Prev. 2012, 13, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of Extraction, Purification and Therapeutic Applications. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.S.H.; Mohammed, A.S.; Abdullah, R.; Mirghani, M.E.S.; Al-Qubaisi, M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [Green Version]
- Gawlik-Dziki, U.; Jezyna, M.; Świeca, M.; Dziki, D.; Baraniak, B.; Czyz, J. Effect of bioaccessibility of phenolic compounds on in vitro anticancer activity of broccoli sprouts. Food Res. Int. 2012, 49, 469–476. [Google Scholar] [CrossRef]
- De Sales, N.F.F.; Da Costa, L.S.; Carneiro, T.I.A.; Minuzzo, D.A.; Oliveira, F.L.; Cabral, L.M.C.; Torres, A.G.; El-Bacha, T. Anthocyanin-rich Grape Pomace Extract (Vitis vinifera L.) from wine industry affects mitochondrial bioenergetics and glucose metabolism in human hepatocarcinoma HepG2 cells. Molecules 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Srisukh, V.; Tribuddharat, C.; Nukoolkarn, V.; Bunyapraphatsara, N.; Chokephaibulkit, K.; Phoomniyom, S.; Chuanphung, S.; Srifuengfung, S. Antibacterial activity of essential oils from citrus hystrix (makrut lime) against respiratory tract pathogens. Sci. Asia 2012, 38, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Francki, R.; Fauquet, C.; Knudson, D.; Brown, F. Classification and Nomenclature of Viruses: Fifth Report of the International Committee on Taxonomy of Viruses. Virology Division of the International Union; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Muhire, B.; Martin, D.P.; Brown, J.K.; Navas-Castillo, J.; Moriones, E.; Murilo Zerbini, F.; Rivera-Bustamante, R.; Malathi, V.G.; Briddon, R.W.; Varsani, A.; et al. A genome-wide pairwise-identity-based proposal for the classification of viruses in the genus Mastrevirus (family Geminiviridae). Arch. Virol. 2013, 158, 1411–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupovic, M. Recombination between RNA viruses and plasmids might have played a central role in the origin and evolution of small DNA viruses. BioEssays 2012, 34, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, M.D.; Fradet-Turcotte, A. Virus DNA Replication and the Host DNA Damage Response. Annu. Rev. Virol. 2018, 5, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibacterial, antiviral, and antifungal properties of wines and winery byproducts in relation to their flavonoid content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Kuo, C.T. Hesperidin, nobiletin, and tangeretin are collectively responsible for the anti-neuroinflammatory capacity of tangerine peel (Citri reticulatae pericarpium). Food Chem. Toxicol. 2014, 71, 176–182. [Google Scholar] [CrossRef]
- Tang, K.; He, S.; Zhang, X.; Guo, J.; Chen, Q.; Yan, F.; Banadyga, L.; Zhu, W.; Qiu, X.; Guo, Y. Tangeretin, an extract from Citrus peels, blocks cellular entry of arenaviruses that cause viral hemorrhagic fever. Antivir. Res. 2018, 160, 87–93. [Google Scholar] [CrossRef]
- Xu, J.J.; Liu, Z.; Tang, W.; Wang, G.C.; Chung, H.Y.; Liu, Q.Y.; Zhuang, L.; Li, M.M.; Li, Y.L. Tangeretin from Citrus reticulate Inhibits Respiratory Syncytial Virus Replication and Associated Inflammation in Vivo. J. Agric. Food Chem. 2015, 63, 9520–9527. [Google Scholar] [CrossRef]
- Shie, P.-H.; Huang, R.-L.; Lay, H.-L. Pharmaceutica Analytica Acta an open access journal The Flavonoids in Citrus madurensis Lour and their Anti-Hepatitis B Virus Activity. Pharm Anal Acta 2013, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Hu, J.; Ren, F.; Xu, H.; Tan, M.; Wang, Q.; Ren, J. Nobiletin, a novel inhibitor, inhibits HBsAg production and hepatitis B virus replication. Biochem. Biophys. Res. Commun. 2020, 523, 802–808. [Google Scholar] [CrossRef]
- Lin, S.; Chen, M.; Li, S.; Lin, C.; Wang, T. Antiviral activity of nobiletin against chikungunya virus in vitro. Antivir. Ther. 2017, 22, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, A.; Pant, A.B. Efforts at COVID-19 Vaccine Development: Challenges and Successes. Vaccines 2020, 8, 739. [Google Scholar] [CrossRef] [PubMed]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 13 December 2020).
- Gadhvi, K.; Vyas, S.; Karetha, K. Peelings of citrus fruits as a precious resource of phytochemical and vital bioactive medicines during Covid: 19 periods. Int. J. Bot. Stud. 2020, 5, 342–344. [Google Scholar]
- Haggag, Y.A.; El-Ashmawy, N.E.; Okasha, K.M. Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection? Med. Hypotheses 2020, 144. [Google Scholar] [CrossRef]








| Technology | Countries | Advantage | Disadvantage | Product | References |
|---|---|---|---|---|---|
| Anaerobic digestion | United States, Germany, Switzerland, Italy, Spain, and Norway | An utterly harmless end product rich in nutrients is obtained and can be used in agriculture. Solid matter is reduced in the digestion. The gas generated in the stabilization of solids can be used as an energy source. As the tanks are closed, there are no terrible odors outside the premises. During the stabilization process, pathogens and individual parasitic organisms are eliminated | Requires high amounts of water. It is a slow process that requires more time. Requires more energy in processes | Biogas and liquid effluent used as compost | [60,63,64,65] |
| Composting and vermicomposting | Germany, Switzerland, Italy, Spain, Norway, South Korea, Japan, and several Latin American countries | Reduces the use of inorganic fertilizers. Saves irrigation water due to the water holding capacity of the compost. Provides the necessary nutrients for the development of plants naturally | In the more advanced industrial processes, the use of energy can be considerable, presenting high costs | Fertilizer and compost for crops | [60,63,65] |
| WTE-Waste to Energy | Germany, Switzerland, Italy, Spain, Norway, South Korea, and Japan | Avoid methane emissions caused by landfills. They offset greenhouse gas emissions caused by the production of electricity from fossil fuels. Recover/recycle valuable resources | It is an expensive process. It pollutes the environment due to incinerators that produce smoke during the combustion process | Energy | [60,66,67] |
| MBT- Mechanical Biological Treatment | Germany, Spain, Switzerland, Korea, and Japan | Confined material is inert. Conservation of resources and reduction of emissions harmful to the environment | Potential for odor problems. A variety of occupational health and safety problems | A stabilized organic fraction, recovered combustible solid products, materials ferrous/non-ferrous and biogas | [60,68,69] |
| Feed production | Germany, France, Italy, Korea, and Japan | Reduce pollution and environmental impact that these products generate when they are thrown away as waste. Possibility of reducing production costs in specific diets. Provide the animal with a rich source of protein and energy | Seasonality and variability of production There must be a previous study for the compatibility between the animal and the feed. There must be a regulation for detailed control of food, traceability, and food safety | Animal feeding | [60,63,65] |
| Industrial Product | Process | By-Product | Fraction of Raw Material in Fresh Weight (%) | References |
|---|---|---|---|---|
| Canned pineapple | Peeling | Peel | 44 | [93] |
| Core remove | Core | 15 | ||
| Canned peaches | Peeling | Peel | 8.6 | [94] |
| Cutting | Seed | 11.4 | ||
| Dried apple | Core remove | Core | 2–4 | [92,95] |
| Tomato paste | Peeling | Peel | 3 | [95,96] |
| Pulping | Seed | 7 | ||
| Straining | Pomace | 10–20 | ||
| Apple juice | Peeling | Peel | 5 | [95,96] |
| Pulping | Seed | 2–4 | ||
| Straining | Pomace | 20–30 | ||
| Pineapple juice | Core remove | Core | 11 | [95] |
| Peeling | Peel | 40 | ||
| Straining | Pomace | 8 | ||
| Mango juice | Peeling | Peel | 12–15 | [96] |
| Pulping | Seed | 15–20 | ||
| Straining | Pomace | 5–10 | ||
| Peach juice | Pulping | Seed | 4–11 | [96] |
| Grapefruit juice | Peeling | Peel | 30 | [96,97] |
| Pulping | Seed | 20 | ||
| Grape juice | Straining | Pomace | 13 | [92,98] |
| Pulping | Seed | 5 | ||
| Orange juice | peeling | peel | 30–40 | [96,99] |
| Pulping | Seed | 2 | ||
| Straining | Pomace | 28 | ||
| Carrot juice | Straining | Pomace | 30–40 | [95] |
| Passion fruit juice | Peeling | Peel | 40 | [96] |
| Pulping | Seed | 10 | ||
| Pineapple jams | Peeling | Peel | 44 | [93,100] |
| Core remove | Core | 11 | ||
| Pepper jams | Core remove | Seed | 50–60 | [99] |
| Frozen mango | Peeling | Peel | 12–15 | [88,96] |
| Pulping | Seed | 13.5 | ||
| Cutting | Discarded pulp | 5–10 | ||
| Frozen melon | Peeling | Peel | 8 | [100] |
| Cutting | Seed | 5 |
| Peels | Protein Crude (g/100 g) | Fiber Crude (g/100 g) | Ash (g/100 g) | Lipid (g/100 g) | Carbohydrate (g/100 g) | Reference |
|---|---|---|---|---|---|---|
| Pineapple (f.w.) | 5.11 | 14.80 | 4.39 | 5.31 | 55.52 | [104] |
| Tomato (d.w.) | 11.17 | 76.13 | 3.54 | 4.48 | - | [105] |
| Apple (f. w.) | 2.80 | 13.95 | 1.39 | 9.96 | 59.96 | [104] |
| Mango (f.w.) | 5.00 | 15.43 | 3.24 | 4.72 | 63.80 | [104] |
| Grapefruit (d.w.) | 4.22 | 1.61 | 2.99 | 2.01 | 46.44 | [106] |
| Orange (f.w.) | 9.73 | 14.19 | 5.17 | 8.70 | 53.27 | [104] |
| Passion fruit (d.w.) | 4.05 | 19.20 | 7.52 | 0.10 | 21.28 | [107] |
| Melon (f.w.) | 9.07 | 29.59 | 11.09 | 1.58 | 48.67 | [108] |
| Seeds | Protein Crude (g/100 g) | Fiber (g/100 g) | Ash (g/100 g) | Lipid (g/100 g) | Reference |
|---|---|---|---|---|---|
| Tomato (d.w.) | 24.5 | 33.9 | 3.0 | 20.0 | [112] |
| Peach (d.w.) | 27.85 | 60.92 | 3.41 | 1.21 | [113] |
| Apple (d.w.) | 34.00 | 24.00 | 4.1 | 27.7 | [114] |
| Grape (f.w.) | 6.93 | 38.60 | 4.50 | 2.87 | [115] |
| Mango (f.w.) | 7.70 | 10.23 | 2.26 | 65.46 | [116] |
| Melon (d.w.) | 27.41 | 25.32 | 4.83 | 30.65 | [117] |
| Pepper (d.w.) | 21.29 | 38.76 | 4.94 | 23.65 | [118] |
| Orange (d.w.) | 3.06 | 5.50 | 2.50 | 54.20 | [119] |
| Passion fruit (f. w) | 15.00 | 41.33 | 1.40 | 29.60 | [120] |
| Fruit | Protein (g/100 g) | Fiber (g/100 g) | Ash (g/100 g) | Lipid (g/100 g) | Reference |
|---|---|---|---|---|---|
| Grape pomace (d.w.) | 8.49 | 46.17 | 4.65 | 8.16 | [123] |
| Apple pomace (f.w.) | 3.30 | 42.10 | 1.10 | 2.30 | [128] |
| Mango pomace (d.w.) | 10.59 | 6.14 | 2.76 | 1.74 | [129] |
| Tomato pomace (d.w.) | 19.27 | 59.03 | 3.92 | 5.85 | [130] |
| Peach pomace (d.w.) | 7.50 | 54.20 | 3.00 | 3.00 | [131] |
| Pineapple core (f.w.) | 0.85 | 47.60 | 1.30 | 3.17 | [132] |
| Carrot pomace (d.w.) | 20.90 | 55.80 | 5.50 | 1.30 | [133] |
| Industrial Product | Process | By-Product | Fraction of Raw Material in Fresh Weight (%) | References |
|---|---|---|---|---|
| Frozen potato | Peeling | peel | 6–10 | [146,147] |
| Frozen yucca | Peeling | Peel | 3–5 | [148,149] |
| Cassava starch | Peeling | Peel | 3–5 | [150] |
| Sifted | Bagasse | 10 | ||
| Potato starch | Peeling | Peel | 10–15 | [97] |
| Yam starch | Peeling | Peel | 4–5 | [97] |
| Cassava flour | Peeling | Peel | 3–5 | [151] |
| Sifted | Bagasse | 6 | ||
| Potato chips and snacks | Peeling | Peel | 15–20 | [152,153] |
| Cassava chips and snacks | Peeling | Peel | 7–10 | [65] |
| Peels | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | Protein (%) | Lipid (%) | References |
|---|---|---|---|---|---|---|---|
| Cassava (f.w.) | 37.90 | 23.90 | 7.50 | 4.50 | 4.73 | 0.19 | [158,159] |
| Yam (f.w.) | - | - | - | 4.45 | 6.60 | 1.12 | [160] |
| Potato (f.w.) | 15.00 | 39.00 | 10.00 | 6.34 | 8.00 | 2.60 | [161,162] |
| Bagasse | Cellulose (g/100 g) | Hemicellulose (g/100 g) | Lignin (g/100 g) | Ash (g/100 g) | Protein (g/100 g) | Lipid (g/100 g) | Reference |
|---|---|---|---|---|---|---|---|
| Cassava | 21.47 | 12.97 | 21.86 | 12.60 | 13.31 | 1.18 | [165] |
| Industrial Products | Process | By-Product | Raw Material Fraction (%) | Reference |
|---|---|---|---|---|
| Chocolate (f.w.) | Deshelling | Shell | 10–12 | [196] |
| Soy oil (d.w.) | Deshelling | Shell | 8 | [197] |
| Extraction | Cake | 60 | ||
| Beer (d.w.) | Filtering | Bagasse | 20–25 | [192] |
| Wheat flour (f.w.) | Deshelling | Shell | 15–20 | [198] |
| Corn flour (d.w.) | Deshelling | Shell | 6 | [199] |
| Rice flour (f.w.) | Deshelling | Shell | 28 | [200] |
| Canned beans (f.w.) | Deshelling | Shell | 6–8 | [201] |
| Canned peas (f.w.) | Deshelling | Shell | 10 | [201] |
| Husk | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | Protein (%) | Lipid (%) | References |
|---|---|---|---|---|---|---|---|
| Cocoa (d.w.) | 35.40 | 37.00 | 14.70 | 12.30 | 16.70 | 36.9 | [158,202] |
| Corn (d.w.) | 31.00 | 34.00 | 14.30 | 6.8 | - | - | [203] |
| Wheat (d.w.) | 36.00 | 18.00 | 16.00 | - | 6.00 | 5.00 | [204] |
| Soybean (d.w.) | 5.10 | 19.14 | 2.10 | 13.85 | 23.80 | 47.00 | [205,206] |
| Rice (d.w.) | 40.00 | 21.00 | 22.00 | 16.00 | - | - | [207] |
| Beans d.w.) | 50.25 | 1.23 | - | 3.51 | - | - | [208] |
| Pea (d.w.) | 17.00 | 33.00 | 2.50 | - | 14.30 | - | [209] |
| Oat (d.w.) | 17.20 | 33.10 | 25.40 | 6.00 | 1.70 | 0.50 | [210] |
| Bagasse | Fiber (%) | Carbohydrate (%) | Protein (%) | Lipid (%) | Ash (%) | Reference |
|---|---|---|---|---|---|---|
| Malt (dry weight) | 63.84 | 73.84 | 23.60 | 4.44 | 2.78 | [211] |
| Cake | Protein (%) | Fiber (%) | Ash (%) | Calcium (%) | Phosphorus (%) | Reference |
|---|---|---|---|---|---|---|
| Soybean (dry weight) | 47.50 | 5.10 | 6.40 | 0.13 | 0.64 | [197] |
| By-Product | Application | Added Amount (%) | Effect of By-Product Flours on Functional Foods | Reference |
|---|---|---|---|---|
| Seed, pomace, and grape peel | Baked goods and pasta | 2, 4, and 6 (seeds); 3, 6, and 9 (pomace); 2, 5, and 10 (pomace), respectively | Increase of functional ingredients in the bakery, pastry, and pasta industries without damaging the quality of the products | [225] |
| Pigeon pea cotyledon | Biscuit | 95, 90, and 85 of wheat flour; 5, 10, and 15 pigeon pea flour | The protein content of the cookies enriched with pigeon pea cotyledon 1.4 times and a significant increase in fiber content | [226] |
| Grape peel, seeds, and remains of the pulp | Biscuit | 30 and 40 replacement with wheat flour | The flour presented physicochemical characteristics within the nutritional standards, with an acceptance of 92.6% of the consumers evaluated | [227] |
| Grape pomace | Biscuit | 10, 20, 30, 40, and 50 replacement of grape pomace flour with wheat flour | The protein, fiber, and ash content increased considerably in the product, with consumer acceptability of 100% | [228] |
| Pomegranate, grape, and rosehip seeds | Turkish noodles | 10, 20, and 30 inclusion of grape pomegranate and rosehip, respectively | The addition of 10% of grape, pomegranate, and rosehip seeds increased the antioxidant activity of the product between 5.7 and 8.4 times | [229] |
| Apple pomace and sugarcane bagasse | Corn extrudates (high fiber croquettes) | 15–30 cornmeal substitution | The inclusion of apple pomace and sugarcane bagasse showed the potential to produce extrudates with significant expansion, with relatively lower energy inputs and high fiber content | [230] |
| Olive pomace | Oat and rice extrudates | 5 and 10 substitutions of rice flour and oats | Favorable impact on the physical characteristics of the extruded product for the base of rice flour and oats. High content of fiber, protein, and polyphenols through the incorporation of olive pomace | [231] |
| Apple pomace | Sorghum and corn extrudates | 10, 20, and 30 substitutions with sorghum and cornflour | Extruded products with better phenolic content, antioxidant activity, textural, and functional properties with greater inclusion of apple pomace were obtained | [232] |
| Pineapple peels | Cereal bars | 3, 6, and 9 inclusion of pineapple peel flour | Adding up to 6% pineapple peel flour to cereal bars did not alter sensory acceptance and increased fiber content | [233] |
| Grape seeds | Cereals, pancakes, and noodles | Substitution of 25 and 30 of grape seed flour in pancakes, 20 in noodles, and 5 in cereal bars | The cereal bars, pancakes, and noodles had a high acceptance by the consumer | [234] |
| Watermelon seeds | Biscuit | Substitution with 5–10 of watermelon seed flour in Biscuits | Watermelon in concentrations of 5–7.5% in cookies improves quality and increases the protein content | [235] |
| Pigment | By-Product | Extraction Method | Production Scale | Applications in Food Products | Reference |
|---|---|---|---|---|---|
| Cyanidin | Ficus carica L. peel | Heat, microwave, and ultrasound | Laboratory scale | Additive and natural coloring in pastry and confectionery | [242] |
| Cyanidin 3-glucoside | Coffee exocarp | Solvent Extraction (SC) and Solid Solvent (SSR) | Laboratory scale | Natural coloring applied to French meringue | [243] |
| Cyanidin 3-glucoside | The by-product of the mulberry industry | By-product mixture with water 1: 3 (w/v) stirred for eight hours in the dark, filtered through a sieve, and evaporated to 1/3 of the initial volume | Laboratory scale | Additive and natural coloring | [244] |
| Quercetin, Cyanidin 3-O- glucoside | Solid waste and onion leaves | Extraction by ecological solvents, such as water and glycerol. 2-Hydroxypropyl-β-cyclodextrin was also used as a co-solvent to increase the extraction yield. | Pilot-scale | Natural coloring applied to yogurts, providing antioxidant properties | [245] |
| β-carotene | Tomato peel and seed | Ultrasound extraction and application in biofilms | Laboratory scale | Edible biofilm coloring | [246] |
| Bethany and iso-betanin | Peel and waste pulp of red prickly pear | Pulsed electric field and ultrasound | Pilot-scale | Natural dyes. Protection against low-density lipoprotein (LDL) oxidative modifications | [240] |
| Lycopene, phytoene, phytofluene, and β-carotene | Tomato peel | Extraction using organic solvents (ethanol and acetone) | Laboratory scale | Natural colors in sunflower oil and spaghetti | [247] |
| β-carotene, γ-carotene, and δ-carotene | Astrocaryum vulgare and Bactris gasipaes peel | Extraction using organic solvents (ethanol and acetone) | Laboratory scale | Natural coloring, in addition to being a source of vitamin A | [248] |
| Lutein and zeaxanthin | Orange peels | Use of the fungi Monascus purpureus and Penicillium purpurogenum by fermentation in solid-state (SSF) and semi-solid (ST) and aqueous solutions of ethanol 70% v/v and isopropanol 95% v/v | Laboratory scale | Natural coloring in pastry | [249] |
| Lycopene, phytoene, phytofluene, β-carotene, cis-lycopene, and lutein | Tomato peel | Dried at 40 °C, ground and passed through 0.15 mm, using ethanol as solvent and acetone/hexane (50:50 v/v). The method described by Hackett et al. [250] | Laboratory scale | Natural coloring and antioxidant in ice cream | [251] |
| Enzyme | By-Product | Method | Result | References |
|---|---|---|---|---|
| Bromelain | Pineapple peel | An aqueous biphasic system consisting of polyethylene glycol (PEG) and polyacrylic acid | Bromelain extraction achieved a yield of 335.27% with a purification factor of 25.78 in PEG | [256] |
| Pineapple stems | Aqueous two-phase micellar systems with ionic liquids as co-surfactants | A high bromelain recovery (90%) was found for the low micelle phase for all systems | [258] | |
| Pineapple peel | Gemini surfactant-based reverse micelle | Activity recovery, purification fold, and protein extraction efficiency were 160 to 163%, 2.7 to 3.3, and 59%, respectively | [259,260] | |
| Pineapple stems and skins | Precipitation with carrageenan using a factorial experimental design | High recovery yield 80–90% of active bromelain for both (stems and peels), making it possible to obtain approximately 0.3 g of bromelain from 100 g of pineapple by-products | [261] | |
| Peel, pulp residues, and pineapple core | Extraction with ethanolic polyphenol from cashew leaves | Solids were obtained from pineapple waste with the following protease activities: 5343 CDU/g (core), 5147 CDU/g (shell), and 5732 CDU/g (pulp) | [124] | |
| Lipases | Bagasse and shell of Pear var. Hamlin, Valencia, and Natal | Emulsified olive oil and on p-nitrophenyl substrates | The bagasse and peel lipases from different orange varieties were obtained at an optimum pH between 6.0–8.0 and an optimum range temperature between 30 to 60 °C | [262] |
| Industrial waste from palm oil | Use of selected strain of Aspergillus niger grown in solid-state (SSF) and submerged fermentation (SMF) | Lipase activity levels of up to 15.41 UI/mL were reached in the palm oil extract by SSF | [263] | |
| Olive oil cake | Use of Candida utilis in solid-state fermentation | An alkaline substrate treatment appeared to be effective, leading to 39% increases in lipase production | [264] | |
| Mango seeds and peel | Use of Yarrowia lipolytica in submerged fermentation | The highest lipase activity observed was 3500 UI/L of the solid residue | [265] | |
| Amylases | Mango seeds | Use of Aspergillus niger isolated | The maximum α-amylase activity was obtained | [266] |
| By-Product | Study | Result | Reference |
|---|---|---|---|
| Passion fruit seed | Formulation of sunscreen as foundation and correction makeup | The 3% passion fruit polar extract correctors had a sun protection factor (SPF) of 18.09 ± 1.48 and 18.60 ± 1.21. Did not cause skin irritation when evaluated in human volunteers | [275] |
| Grapeseed | Evaluation of the protective effects of grape seed on fibroblasts irradiated with UV light | Grape seed increased cell viability and effectively protected fibroblasts from UV damage, also improving UV filter absorbency and overall formulation efficacy | [267] |
| Oat shell and walnut | Determination of functional properties such as antioxidant power and UV absorption capacity of lignans extracted from by-products | The extracted lignans showed adequate protection against UV radiation, a property of great interest to block the entire ultraviolet spectrum | [276] |
| Industrial coffee waste | Biological effects of the by-product in the development of a new generation of solar filters | The emulsion containing 35% (w/w) of the coffee fraction showed improvements in water performance with a broad spectrum of sun protection (SPF) compared to an emulsion containing 35% (w/w) of green coffee that improved the SPF in physical sunscreens | [277] |
| Waste from the guava industry | Formulation of a cosmetic product as a sunscreen | The polar extract showed synergy with the UV chemical filter (Ethylhexyl methoxycinnamate), enhancing the sun protection factor by 17.99% | [278] |
| Grape seeds | By-product effect and its UV and visible light protection capacity | The by-product showed the ability to absorb a wide range of the solar spectrum, including ultraviolet and blue light. At a concentration of 100 µg/mL, it significantly inhibited nitrous oxide production in RAW 264.7 cells (commonly used model of mouse macrophages for the study of cellular responses) | [279] |
| Rambutan skin | Develop a sunscreen based on rambutan skin polar extracts | The by-product can synergistically increase the sun protection factor (SPF) values of synthetic organic sunscreens and lower costs in a sunscreen formulation | [280] |
| Shells and seeds Amazonian fruits | Determine the protection capacity of polar seed extracts against UV light | The polar extracts showed high capacity, especially Caryocar villosum, Garcinia madruno, and Bertholletia excelsa with UV absorption peaks, and piquiá, bacurizinho, and açaí seeds (Euterpe oleracea and E. precatoria) | [281] |
| By-Product | Study | Result | Reference |
|---|---|---|---|
| Orange peel (Citrus sinensis) | Phenolic extracts of the orange peel against oxidative stress in primary human colon tumor (Caco-2) and metastatic cell lines (LoVo and LoVo/ADR) | Orange peels showed antioxidant properties by inhibiting the activities of total matrix metalloproteinases (MMP), preventing the progression of cancer and the tumor cell metastasizing | [289] |
| Orange peel (Citrus sinensis) | Matrix metalloproteinase (MMP) and proteasome activities in cells by peel extracts for colon cancer | The extracts inhibited proteasome (responsible for the degradation of cell regulatory proteins, found in excess in tumor cells) activity in extract-treated cells, inhibiting the progression of cancer | [299] |
| Peel of Citrus aurantifolia and Citrus reticulata | Citrus peel like a natural model for cancer prevention and treatment | Hesperidin and naringenin increased the cytotoxicity of doxorubicin in cancer cells (MCF-7 and HeLa). Hesperidin can lower the concentration of liver and serum lipids and reduce osteoporosis | [300] |
| Pineapple peel (Ananas comosus) | Determination of extraction methods, purification, and therapeutic applications of bromelain | Medicinal applications: inhibition of platelet aggregation, antithrombotic, anti-inflammatory, modulation of cytokines, and immunity | [301] |
| Mango seed (Mangifera indica) | Cytotoxic effect of the by-product extract on MDA-MB-231 and MCF-7 cancer cells | The extract induced cytotoxicity in MDA-MB-231 and MCF-7 cells with IC50 values of 30 and 15 μg/mL, respectively | [302] |
| Broccoli residues (Brassica oleracea var. Italica) | Inhibitory effect of broccoli sprout extracts on prostate cancer cell phones with low (AT-2) and high (MAT-LyLu) metastatic potential | Efficiency in the inhibition of cell proliferation, motility, and cell coupling was obtained using the extracts | [303] |
| Grape pomace (Vitis vinifera) | By-product effects on metabolism and redox status in HepG2 human hepatocarcinoma cells | Long-term metabolic effects generated cytotoxicity, and cells died from necrosis, and it was not toxic to non-cancerous human fibroblasts | [304] |
| Grapefruit peel (Citrus paradisi) | Grapefruit peel essential oil as an active component against strains of Candida albicans isolated from patients with sub-protein stomatitis | Citrus paradisi essential oil at 25% and 12.5% presented a limited antifungal activity, and that presents inhibition halos of greater diameter | [290] |
| Peel and leaves of kafir lime (Citrus hystrix) | Inhibitory effect of essential oils of by-products against respiratory pathogens (Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and Acinetobacter baumannii) and evaluation of their active components | Twenty-five components identified in the extract, limonene as the main compound with yields of 91.5 and 88.6% of antibacterial activity | [305] |
| By-Product | Compounds | Virus | Result | Reference |
|---|---|---|---|---|
| Citrus reticulate peel | Tangeretin | Human respiratory syncytial virus (RSV) | Inhibition of RSV replication and decreased inflammation in lungs | [313] |
| Peels of Citrus madurensis Lour | Hesperidin, diosmin, neohesperidin, nobiletin, tangeretin | Hepatitis B virus | Significantly inhibited the surface antigen of the hepatitis B virus | [314] |
| Citrus fruit peel | Nobiletin | Hepatitis B virus | Inhibited surface antigen and hepatitis B virus | [315] |
| Citrus reticulate peel | Nobiletin | Chikungunya virus | Reduced the load of the virus and exerts a positive effect on the immune system | [316] |
| By-Product | Study | Result | Reference |
|---|---|---|---|
| Peels of lime (C. Aurantifolia), grapefruit (C. maxima), lemon (C. jambhiri), lime (C. limetta), grapefruit (C. medica L.), mandarin (C. reticulata), and mandarin (C. reticulate white) | Identification of the added value of the by-product through different extraction methods and its potential utility against COVID-19 | The amount of iron and phenols was higher in grapefruit and grapefruit peel compared to other species, obtaining bioactive compounds and medicinal phytochemicals such as flavonoids for the treatment of COVID-19 | [322] |
| Assorted citrus peels | Review of varieties of citrus by-products as a source of hesperidin, an active compound against COVID-19 and its extraction process | Hesperidin has a strong binding affinity for all significant viruses such as SARS-CoV and their mutations with the potential to prevent it from spreading to cells; likewise, hesperetin (hesperidin aglycone) and naringin stop the pro-inflammatory reaction of the immune system | [288] |
| Orange peel (Citrus × sinensis) | Study of hesperidin as a source to counteract the SARS-CoV-2 virus from citrus fruits | Hesperidin showed a good safety profile, with a median lethal dose (LD50) of 4837.5 mg/kg. A dose administration of 500 mg/kg of flavanone did not induce any abnormality in body weight, clinical signs, symptoms, and blood parameters | [18] |
| Assorted citrus peels | The feasibility of hesperidin for the treatment of COVID-19 | Hesperidin targets the root of the infection by binding the protein spike | [323] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. https://doi.org/10.3390/molecules26020515
Osorio LLDR, Flórez-López E, Grande-Tovar CD. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules. 2021; 26(2):515. https://doi.org/10.3390/molecules26020515
Chicago/Turabian StyleOsorio, Lady Laura Del Rio, Edwin Flórez-López, and Carlos David Grande-Tovar. 2021. "The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries" Molecules 26, no. 2: 515. https://doi.org/10.3390/molecules26020515