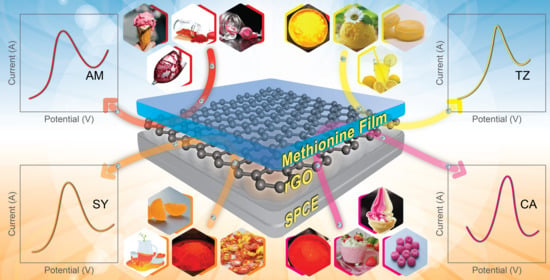
Disposable Electrochemical Sensor for Food Colorants Detection by Reduced Graphene Oxide and Methionine Film Modified Screen Printed Carbon Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology of Modified Electrode
2.2. Electrochemical Characterization of Electrode Modification
2.3. Electrochemical Behavior of Amaranth, Tartrazine, Sunset Yellow and Carminic Acid on Modified Electrode
2.4. Effect of pH of Electrolyte
2.5. Effect of Scan Rate
2.6. Effect of Accumulation Time
2.7. Determination of Linear Range and Detection Limit
2.8. Interference Test
2.9. Real Sample Analysis
2.10. Reproducibility Test
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Synthesis of rGO
3.3. Preparation of rGO-Methionine Modified SPCE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pogacean, F.; Rosu, M.C.; Coros, M.; Magerusan, L.; Moldovan, M.; Sarosi, C.; Porav, A.-S.; Stefan-van Staden, R.-I.; Pruneanu, S. Graphene/TiO2-Ag Based Composites Used as Sensitive Electrode Materials for Amaranth Electrochemical Detection and Degradation. J. Electrochem. Soc. 2018, 165, B3054–B3059. [Google Scholar] [CrossRef]
- Stevens, L.J.; Kuczek, T.; Burgess, J.R.; Stochelski, M.A.; Arnold, L.E.; Galland, L. Mechanisms of behavioral, atopic, and other reactions to artificial food colors in children. Nutr. Rev. 2013, 71, 268–281. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authorit. Refined exposure assessment for amaranth (E 123). EFSA J. 2013, 11, 3442. [Google Scholar]
- EFSA ANS Panel (Panel on Food Additives and Nutrient Sources Added to Food). Scientific opinion on the reconsideration of the temporary ADI and refined exposure assessment for Sunset Yellow FCF (E 110). EFSA J. 2014, 12, 3765. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the re-evaluation Tartrazine (E 102). EFSA J. 2009, 7, 1331. [Google Scholar] [CrossRef]
- Lipskikh, O.I.; Korotkova, E.I.; Khristunova, Y.P.; Barek, J.; Kratochvil, B. Sensors for voltammetric determination of food azo dyes—A critical review. Electrochim. Acta 2018, 260, 974–985. [Google Scholar] [CrossRef]
- Borges, M.E.; Tejera, R.L.; Díaz, L.; Esparza, P.; Ibáñez, E. Natural dyes extraction from cochineal (Dactylopius coccus). New extraction methods. Food Chem. 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food. Scientific Opinion on the re-evaluation of cochineal, carminic acid, carmines (E 120) as a food additive. EFSA J. 2015, 13, 4288. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Gras, C. 18—The “Carmine Problem” and Potential Alternatives11Both Authors Contributed Equally to this Chapter, in Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 385–428. [Google Scholar]
- Hashem, E.; Saleh, M.S.; Al-Salahi, N.O.; Youssef, A.K. Advanced Spectrophotometric Analysis of Sunset Yellow Dye E110 in Commercial Food Samples. Food Anal. Methods 2017, 10, 865–875. [Google Scholar] [CrossRef]
- Heidarizadi, E.; Tabaraki, R. Simultaneous spectrophotometric determination of synthetic dyes in food samples after cloud point extraction using multiple response optimizations. Talanta 2016, 148, 237–246. [Google Scholar] [CrossRef]
- Rovina, K.; Siddiquee, S.; Shaarani, S.M. Extraction, Analytical and Advanced Methods for Detection of Allura Red AC (E129) in Food and Beverages Products. Front. Microbiol. 2016, 7, 798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.-J.; Liu, C.-T.; Li, W.; Tang, A.-N. Dispersive solid-phase microextraction and capillary electrophoresis separation of food colorants in beverages using diamino moiety functionalized silica nanoparticles as both extractant and pseudostationary phase. Talanta 2015, 132, 366–372. [Google Scholar] [CrossRef]
- Ashwin Karthick, N.; Thangappan, R.; Arivanandhan, M.; Gnanamani, A.; Jayavel, R. A Facile Synthesis of Ferrocene Functionalized Graphene Oxide Nanocomposite for Electrochemical Sensing of Lead. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1021–1028. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Lin, Y.; Liu, M.; Fang, G.; Wang, S. Coral-like Au1Pt3 alloy nanoparticles with multiple surface defects modified by poly(L-methionine) membrane for the selective detection of dopamine in biological samples. J. Alloy. Compd. 2020, 815, 152643. [Google Scholar] [CrossRef]
- Thomas, D.; Rasheed, Z.; Jagan, J.S.; Kumar, K.G. Study of kinetic parameters and development of a voltammetric sensor for the determination of butylated hydroxyanisole (BHA) in oil samples. J. Food Sci. Technol. 2015, 52, 6719–6726. [Google Scholar] [CrossRef] [Green Version]
- Ojani, R.; Alinezhad, A.; Abedi, Z. A highly sensitive electrochemical sensor for simultaneous detection of uric acid, xanthine and hypoxanthine based on poly(l-methionine) modified glassy carbon electrode. Sens. Actuators B Chem. 2013, 188, 621–630. [Google Scholar] [CrossRef]
- Wang, S.-F.; Du, D.; Zou, Q.-C. Electrochemical behavior of epinephrine at l-cysteine self-assembled monolayers modified gold electrode. Talanta 2002, 57, 687–692. [Google Scholar] [CrossRef]
- He, J.; Zhongrong, S.O.N.G.; Zhang, S.; Lin, W.A.N.G.; Zhang, Y.; Ri, Q.I.U. Methionine—Au Nanoparticle Modified Glassy Carbon Electrode: A Novel Platform for Electrochemical Detection of Hydroquinone. Medziagotyra 2014, 20, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, Z.; Zhang, Y.; Zheng, Z.; Wang, C.; Du, Y.; Ye, W. Simultaneous electrochemical determination of uric acid, xanthine and hypoxanthine based on poly(l-arginine)/graphene composite film modified electrode. Talanta 2012, 93, 320–325. [Google Scholar] [CrossRef]
- Hu, Q.Q.; Gao, H.; Wang, Y.M.; Ma, W.; Sun, D.M. Simultaneous Determination of Carmine and Amaranth Based on a Poly(L-Arginine)–Graphene Modified Electrode. J. Anal. Chem. 2018, 73, 817–823. [Google Scholar] [CrossRef]
- Manjunatha, J.G. A novel voltammetric method for the enhanced detection of the food additive tartrazine using an electrochemical sensor. Heliyon 2018, 4, e00986. [Google Scholar] [CrossRef] [Green Version]
- Chao, M.; Ma, X. Convenient Electrochemical Determination of Sunset Yellow and Tartrazine in Food Samples Using a Poly(L-Phenylalanine)-Modified Glassy Carbon Electrode. Food Anal. Methods 2015, 8, 130–138. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Liu, G.; Sun, D. Determination of sunset yellow and tartrazine using silver and poly (L-cysteine) composite film modified glassy carbon electrode. Indian J. Chem. Sect. A 2016, 55, 298–303. [Google Scholar]
- Zhang, K.; Luo, P.; Wu, J.; Wang, W.; Ye, B. Highly sensitive determination of Sunset Yellow in drink using a poly (L-cysteine) modified glassy carbon electrode. Anal. Methods 2013, 5, 5044. [Google Scholar] [CrossRef]
- Gördük, Ö.; Şahin, Y. Poly(L-Cysteine) Modified Pencil Graphite Electrode for Determination of Sunset Yellow in Food and Beverage Samples by Differential Pulse Voltammetry. Int. J. Electrochem. Sci. 2018, 13, 159–174. [Google Scholar]
- Gao, Y.; Wang, L.; Zhang, Y.; Zou, L.; Li, G.; Ye, B. Electrochemical behavior of amaranth and its sensitive determination based on Pd-doped polyelectrolyte functionalized graphene modified electrode. Talanta 2017, 168, 146–151. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, H.; Zhao, L.; Qu, L.; Yu, L. Electrochemical Sensor Based on Poly(Sodium 4-Styrenesulfonate) Functionalized Graphene and Co3O4 Nanoparticle Clusters for Detection of Amaranth in Soft drinks. Food Anal. Methods 2017, 10, 3149–3157. [Google Scholar] [CrossRef]
- Wang, M.; Cui, M.; Zhao, M.; Cao, H. Sensitive determination of Amaranth in foods using graphene nanomeshes. J. Electroanal. Chem. 2018, 809, 117–124. [Google Scholar] [CrossRef]
- Arvand, M.; Gaskarmahalleh, A.A.; Hemmati, S. Enhanced-Oxidation and Highly Sensitive Detection of Tartrazine in Foodstuffs via New Platform Based on Poly(5-Sulfosalicylic Acid)/Cu(OH)2 Nanoparticles. Food Anal. Methods 2017, 10, 2241–2251. [Google Scholar] [CrossRef]
- Nuñez-Dallos, N.; Macías, M.A.; García-Beltrán, O.; Calderón, J.A.; Nagles, E.; Hurtado, J. Voltammetric determination of amaranth and tartrazine with a new double-stranded copper (I) helicate-single-walled carbon nanotube modified screen printed electrode. J. Electroanal. Chem. 2018, 822, 95–104. [Google Scholar] [CrossRef]
- Arvand, M.; Erfanifar, Z.; Ardaki, M.S. A New Core@Shell Sili00CA-Coated Magnetic Molecular Imprinted Nanoparticles for Selective Detection of Sunset Yellow in Food Samples. Food Anal. Methods 2017, 10, 2593–2606. [Google Scholar] [CrossRef]
- Chebotarev, A.; Koicheva, A.; Bevziuk, K.; Pliuta, K.; Snigur, D. Simultaneous determination of Sunset Yellow and Tartrazine in soft drinks on carbon-paste electrode modified by silica impregnated with cetylpyridinium chloride. J. Food Meas. Charact. 2019, 13, 1964–1972. [Google Scholar] [CrossRef]
- Arslan, E.; Çakır, S. Electrochemical fabrication of polyproline modified graphite electrode decorated with Pd–Au bimetallic nanoparticles: Application for determination of carminic acid. J. Electroanal. Chem. 2016, 760, 32–41. [Google Scholar] [CrossRef]
- Wang, M.; Sun, Y.; Yang, X.; Zhao, J. Sensitive determination of Amaranth in drinks by highly dispersed CNT in graphene oxide “water” with the aid of small amounts of ionic liquid. Food Chem. 2015, 179, 318–324. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the re-evaluation of Amaranth (E 123) as a food additive. EFSA J. 2010, 8, 1649. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Scientific Opinion on the re-evaluation of Sunset Yellow FCF (E 110) as a food additive. EFSA J. 2009, 7, 1330. [Google Scholar] [CrossRef]
- Sheikhshoaie, M.; Karimi-Maleh, H.; Sheikhshoaie, I.; Ranjbar, M. Voltammetric amplified sensor employing RuO2 nano-road and room temperature ionic liquid for amaranth analysis in food samples. J. Mol. Liq. 2017, 229, 489–494. [Google Scholar] [CrossRef]
- Bijad, M.; Karimi-Maleh, H.; Farsi, M.; Shahidi, S.A. Simultaneous Determination of Amaranth and Nitrite in Foodstuffs via Electrochemical Sensor Based on Carbon Paste Electrode Modified with CuO/SWCNTs and Room Temperature Ionic Liquid. Food Anal. Methods 2017, 10, 3773–3780. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, M.; Hu, W. N-methyl-2-pyrrolidone-exfoliated graphene nanosheets as sensitive determination platform for amaranth at the nanomolar level. Ionics 2017, 23, 241–246. [Google Scholar] [CrossRef]
- Mazlan, S.Z.; Lee, Y.H.; Hanifah, S.A. A New Laccase Based Biosensor for Tartrazine. Sensors 2017, 17, 2859. [Google Scholar] [CrossRef] [Green Version]
- Karimi, M.A.; Aghaei, V.H.; Nezhadali, A.; Ajami, N. Graphitic Carbon Nitride as a New Sensitive Material for Electrochemical Determination of Trace Amounts of Tartrazine in Food Samples. Food Anal. Methods 2018, 11, 2907–2915. [Google Scholar] [CrossRef]
- Sakthivel, M.; Sivakumar, M.; Chen, S.M.; Pandi, K. Electrochemical synthesis of poly(3,4-ethylenedioxythiophene) on terbium hexacyanoferrate for sensitive determination of tartrazine. Sens. Actuators B Chem. 2018, 256, 195–203. [Google Scholar] [CrossRef]
- Ding, Z.; Deng, P.; Wu, Y.; Tian, Y.; Li, G.; Liu, J.; He, Q. A Novel Modified Electrode for Detection of the Food Colorant Sunset Yellow Based on Nanohybrid of MnO2 Nanorods-Decorated Electrochemically Reduced Graphene Oxide. Molecules 2019, 24, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.P.V.; Rajkumar, C.; Chen, S.M.; Thirumalraj, B.; Lin, K.C. Activated porous carbon supported rhenium composites as electrode materials for electrocatalytic and supercapacitor applications. Electrochim. Acta 2018, 271, 433–447. [Google Scholar]
- Peña-Gonzalez, A. Detection of Sunset Yellow by Adsorption Voltammetry at Glassy Carbon Electrode Modified with Chitosan. Int. J. Electrochem. Sci. 2018, 13, 5005–5015. [Google Scholar] [CrossRef]
- Shetti, N.; Nayak, D.; Malode, S. Electrochemical behavior of azo food dye at nanoclay modified carbon electrode-a nanomolar determination. Vacuum 2018, 155, 524–530. [Google Scholar] [CrossRef]
- Rozi, N.; Ahmad, A.; Yook Heng, L.; Shyuan, L.K.; Hanifah, S.A. Electrochemical Sunset Yellow Biosensor Based on Photocured Polyacrylamide Membrane for Food Dye Monitoring. Sensors 2018, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, B.; Zhang, K.; Bin, D.; Shiraishi, Y.; Yang, P.; Du, Y. Highly sensitive electrochemical determination of Sunset Yellow based on the ultrafine Au-Pd and reduced graphene oxide nanocomposites. J. Colloid Interface Sci. 2016, 481, 229–235. [Google Scholar] [CrossRef]
- Yilmaz, U.T.; Ergun, F.; Yilmaz, H. Determination of the food dye carmine in milk and candy products by differential pulse polarography. J. Food Drug Anal. 2014, 22, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Pakapongpan, S.; Tuantranont, A.; Poo-arporn, R.P. Magnetic Nanoparticle-Reduced Graphene Oxide Nanocomposite as a Novel Bioelectrode for Mediatorless-Membraneless Glucose Enzymatic Biofuel Cells. Sci. Rep. 2017, 7, 12882. [Google Scholar] [CrossRef] [Green Version]
- Poo-arporn, Y.; Pakapongpan, S.; Chanlek, N.; Poo-arporn, R.P. The development of disposable electrochemical sensor based on Fe3O4-doped reduced graphene oxide modified magnetic screen-printed electrode for ractopamine determination in pork sample. Sens. Actuators B Chem. 2019, 284, 164–171. [Google Scholar] [CrossRef]









| Dye | Electrode | Dye Concentration (µM) | Oxidative Current (µA) |
|---|---|---|---|
| Amaranth | Bare SPCE | 0.09 ± 0.01 | |
| rGO/SPCE | 10 | 0.94 ± 0.08 | |
| rGO-methionine/SPCE | 1.05 ± 0.12 | ||
| Tartrazine | Bare SPCE | 0.41 ± 0.01 | |
| rGO/SPCE | 50 | 1.45 ± 0.20 | |
| rGO-methionine/SPCE | 3.28 ± 0.51 | ||
| Sunset yellow | Bare SPCE | 0.45 ± 0.04 | |
| rGO/SPCE | 50 | 1.52 ± 0.22 | |
| rGO-methionine/SPCE | 1.77 ± 0.37 | ||
| Carminic acid | Bare SPCE | 0.35 ± 0.01 | |
| rGO/SPCE | 50 | 1.49 ± 0.23 | |
| rGO-methionine/SPCE | 1.76 ± 0.33 |
| Dyes | Modification | Method | Electrode | Linear Range (µM) | LOD (nM) | Ref. |
|---|---|---|---|---|---|---|
| AM | PSS-GR-Pd/GCE | DPV | GCE | 0.1–9 | 7 | [27] |
| CPE/RuO2/NR/DPIBr | SWV | CPE | 0.008–550 | 3 | [38] | |
| PLA-ERGO/GCE | DPV | GCE | 0.75–75 | 250 | [21] | |
| 1-M-3-BIBR/CuO/SWCNTs/CPE | SWV | CPE | 0.004–750 | 1 | [39] | |
| GS/GCE | DPV | GCE | 2500–125,000 | 0.75 | [40] | |
| Au/GTA | LSV | AuE | 0.3–100 | 100 | [1] | |
| rGO-methionine/SPCE (This work) | DPV | SPCE | 1–10 10–100 | 57 | - | |
| TZ | Microspheres-laccase/AuNPs/SPE | DPV | SPCE | 0.2–14 | 40 | [41] |
| PGMCPE | CV | CPE | 1–27 35–87 | 283 | [22] | |
| g-C3N4/Graphite electrode | DPV | Graphite | 0.1–10 | 210 | [42] | |
| PEDOT@TbHCF/GCE | DPV | GCE | 0.1–206 | 32 | [43] | |
| H-SWCNT/SPCE | SWAdV | SPCE | 1–8.5 | 60 | [31] | |
| rGO-methionine/SPCE (This work) | DPV | SPCE | 1–10 10–85 | 41 | - | |
| SY | MnO2 NRs-ERGO/GCE | LSV | GCE | 0.01–2 2–10 10–100 | 2 | [44] |
| Re@CDACs/SPCE | Amperometry | SPCE | 0.05–390 | 16 | [45] | |
| CS/GCE | SWAdV | GCE | 0.25–3.25 | 98 | [46] | |
| NC-CPE | SWV | CPE | 0.001–0.1 | 0.2 | [47] | |
| Poly(AAm-co-EMA)/Lac/GCE | DPV | GCE | 0.08–10 | 20 | [48] | |
| AuNPs/PANI-co-PoAN-co-PoT/GO/AuE | SWV | AuE | 5–500 | 14.2 | [49] | |
| rGO-methionine/SPCE (This work) | DPV | SPCE | 1–10 10–50 | 48 | - | |
| CA | Unmodified | DPP | DME | 1–90 | 160 | [50] |
| Pd-AuNPs/Poly(Pr)/GE | SWV | Graphite | 0.01–1 | 5.9 | [34] | |
| rGO-methionine/SPCE (This work) | DPV | SPCE | 1–20 20–60 | 36 | - |
| Substance | Variance | |||
|---|---|---|---|---|
| Amaranth | Tartrazine | Sunset Yellow | Carminic Acid | |
| Without substance | 100.00 | 100.00 | 100.00 | 100.00 |
| Glucose 1000x | 104.73 | 96.97 | 99.73 | 98.30 |
| Sucrose 1000x | 102.74 | 95.77 | 98.20 | 99.94 |
| NaCl 100x | 97.16 | 97.25 | 100.05 | 96.77 |
| KCl 100x | 99.14 | 95.82 | 99.62 | 96.48 |
| Glycine 10x | 95.30 | 102.53 | 99.93 | 97.43 |
| Ascorbic acid 10x | 98.48 | 101.49 | 101.51 | 95.95 |
| Citric acid 10x | 95.80 | 102.65 | 98.08 | 104.08 |
| Substance | Variance | |||
|---|---|---|---|---|
| Amaranth | Tartrazine | Sunset Yellow | Carminic Acid | |
| Without substance | 100.00 | 100.00 | 100.00 | 100.00 |
| Glucose 1000x | 104.01 | 97.84 | 103.33 | 97.24 |
| Sucrose 1000x | 99.38 | 100.95 | 103.37 | 96.61 |
| NaCl 100x | 101.41 | 103.76 | 96.94 | 100.61 |
| KCl 100x | 104.21 | 99.62 | 99.33 | 102.94 |
| Glycine 10x | 99.57 | 98.84 | 98.94 | 103.60 |
| Ascorbic acid 10x | 98.36 | 97.24 | 98.98 | 104.08 |
| Citric acid 10x | 95.36 | 104.11 | 97.42 | 102.54 |
| Sample | Added (µM) | Expected (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|---|
| Sprite | 0.00 | Not found | ||
| 1.50 | 1.50 | 1.49 (1.41) | 99.45 (94.02) | |
| 2.00 | 2.00 | 2.01 (1.90) | 100.44 (95.01) | |
| 2.50 | 2.50 | 2.51 (2.41) | 100.36 (96.30) | |
| SPY classic red | 0.00 | 0.52 | ||
| 1.50 | 2.02 | 1.95 | 96.44 | |
| 2.00 | 2.52 | 2.45 | 97.29 | |
| 2.50 | 3.02 | 3.10 | 102.87 |
| Sample | Added (µM) | Expected (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|---|
| Sprite | 0.00 | Not found | ||
| 1.50 | 1.50 | 1.55 (1.48) | 103.43 (98.34) | |
| 2.00 | 2.00 | 2.01 (2.05) | 100.48 (102.36) | |
| 2.50 | 2.50 | 2.49 (2.40) | 99.53 (96.12) | |
| 100 Plus | 0.00 | 0.98 | ||
| 3.00 | 3.98 | 4.15 | 104.21 | |
| 4.00 | 4.98 | 4.91 | 98.46 | |
| 5.00 | 5.98 | 6.27 | 104.85 | |
| Sponsor lemon lime | 0.00 | 1.82 | ||
| 4.00 | 5.82 | 5.58 | 95.90 | |
| 5.00 | 6.82 | 6.42 | 94.14 | |
| 6.00 | 7.82 | 7.89 | 100.82 |
| Scheme | Added (µM) | Expected (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|---|
| Sprite | 0.00 | Not found | ||
| 1.50 | 1.50 | 1.29 (1.57) | 85.98 (104.76) | |
| 2.00 | 2.00 | 1.92 (1.99) | 96.12 (99.70) | |
| 2.50 | 2.50 | 2.40 (2.48) | 95.99 (99.17) | |
| Mirinda (orange) 4 fold dilution | 0.00 | 1.10 | ||
| 3.00 | 4.10 | 4.24 | 103.38 | |
| 4.00 | 5.10 | 5.18 | 101.50 | |
| 5.00 | 6.10 | 6.20 | 101.63 | |
| Fanta (orange) | 0.00 | 2.71 | ||
| 3.00 | 5.71 | 5.71 | 100.01 | |
| 5.00 | 7.71 | 7.26 | 94.06 | |
| 7.00 | 9.71 | 8.88 | 91.44 |
| Sample | Added (µM) | Expected (µM) | Found (µM) | Recovery (%) |
|---|---|---|---|---|
| Sprite | 0.00 | 0.00 | ||
| 5.00 | 5.00 | 5.48 (4.87) | 109.63 (97.40) | |
| 10.00 | 10.00 | 11.13 (9.72) | 111.29 (97.24) | |
| 15.00 | 15.00 | 15.58 (14.28) | 103.87 (95.23) | |
| Betagan strawberry flavour | 0.00 | 0.46 | ||
| 3.00 | 3.46 | 3.14 | 90.75 | |
| 4.00 | 4.46 | 4.14 | 92.95 | |
| 5.00 | 5.46 | 4.85 | 88.93 | |
| Ice-cream Nestle Eskimo (2 fold dilution) | 0.00 | 0.95 | ||
| 3.00 | 3.95 | 3.31 | 83.83 | |
| 4.00 | 4.95 | 4.36 | 88.12 | |
| 5.00 | 5.95 | 4.86 | 81.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkapinyo, C.; Subannajui, K.; Poo-arporn, Y.; Poo-arporn, R.P. Disposable Electrochemical Sensor for Food Colorants Detection by Reduced Graphene Oxide and Methionine Film Modified Screen Printed Carbon Electrode. Molecules 2021, 26, 2312. https://doi.org/10.3390/molecules26082312
Akkapinyo C, Subannajui K, Poo-arporn Y, Poo-arporn RP. Disposable Electrochemical Sensor for Food Colorants Detection by Reduced Graphene Oxide and Methionine Film Modified Screen Printed Carbon Electrode. Molecules. 2021; 26(8):2312. https://doi.org/10.3390/molecules26082312
Chicago/Turabian StyleAkkapinyo, Chutimon, Kittitat Subannajui, Yingyot Poo-arporn, and Rungtiva P. Poo-arporn. 2021. "Disposable Electrochemical Sensor for Food Colorants Detection by Reduced Graphene Oxide and Methionine Film Modified Screen Printed Carbon Electrode" Molecules 26, no. 8: 2312. https://doi.org/10.3390/molecules26082312