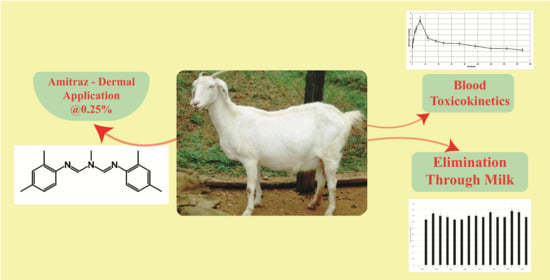
Disposition Kinetics of Amitraz in Lactating Does
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Corta, E.; Bakkali, A.; Berrueta, L.A.; Gallo, B.; Vicente, F. Kinetics and mechanism of amitraz hydrolysis in aqueous media by HPLC and GC-MS. Talanta 1999, 48, 189–199. [Google Scholar] [CrossRef]
- Ravindran, R.; Subramanian, H. Ivermectin, amitraz, diflubenzuron and karanji oil in the treatment of notoedric mange in rabbits. Ind. Vet. J. 2000, 77, 986–987. [Google Scholar]
- Ghubash, R. Parasitic Miticidal Therapy. Clin. Tech. Small Anim. Pract. 2006, 21, 135–144. [Google Scholar] [CrossRef]
- Ravindran, R.; Jyothimol, G.; Amithamol, K.K.; Sunil, A.R.; Chandrasekhar, L.; Lenka, D.R.; Amritha, A.; Sreelekha, K.; Sathish, N.; Udayan, D.; et al. In vitro efficacy of amitraz, coumaphos, deltamethrin and lindane against engorged female Rhipicephalus (Boophilus) annulatus and Haemaphysalis bispinosa ticks. Exp. Appl. Acarol. 2018, 75, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Sreelekha, K.; Chandrasekhar, L.; Kartha, H.S.; Ravindran, R.; Juliet, S.; Ajithkumar, K.G.; Nair, S.N.; Ghosh, S. Ultrastructural analysis of oocytes of Rhipicephalus (Boophilus) annulatus during postengorgement period as a tool to evaluate the cytotoxic effects of amitraz and deltamethrin on the germinative cells. Vet. Parasitol. 2017, 247, 113–120. [Google Scholar] [CrossRef]
- Kanapadinchareveetil, S.; Chandrasekhar, L.; Pious, A.; Kartha, H.S.; Ravindran, R.; Juliet, S.; Nair, S.N.; Ajithkumar, K.G.; Ghosh, S. Molecular, histological and ultrastructural characterization of cytotoxic effects of amitraz on the ovaries of engorged females of Rhipicephalus (Boophilus) annulatus. Exp. Parasitol. 2019, 204, 107732. [Google Scholar] [CrossRef]
- Bonsall, J.L.; Turnbull, G.J. Extrapolation from safety data to management of poisoning with reference to amitraz (a formamidine pesticide) and xylene. Hum. Toxicol. 1983, 2, 587–592. [Google Scholar] [CrossRef]
- Cullen, L.K.; Reynoldson, J.A. Central and peripheral α-adrenoceptor actions of amitraz in the dog. J. Vet. Pharmacol. Ther. 1990, 13, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Amitraz. In Veterinary Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 599–603. [Google Scholar] [CrossRef]
- Del Pino, J.; Moyano-Cires, P.V.; Anadon, M.J.; Díaz, M.J.; Lobo, M.; Capo, M.A.; Frejo, M.T. Molecular mechanisms of amitraz mammalian toxicity: A comprehensive review of existing data. Chem. Res. Toxicol. 2015, 28, 1073–1094. [Google Scholar] [CrossRef]
- Kumar, P.; Kher, S.K.; Dwivedi, S. An analytical study of livestock in Jammu and Kashmir. Int. J. Plant Anim. Environ. Sci. 2012, 2, 169–177. [Google Scholar]
- Perry, B.D.; Randolph, T.F.; Mcdermott, J.J.; Sones, K.R.; Thornton, P.K. Investing in Animal Health Research to Alleviate Poverty; International Livestock Research Institute: Nairobi, Kenya, 2002; p. 148. [Google Scholar]
- Minjauw, B.; McLeod, A. Tick-Borne Diseases and Poverty: The Impact of Ticks and Tick-Borne Diseases on the Livelihoods of Small-Scale and Marginal Livestock Owners in India and Eastern and Southern Africa Research Report, DFID Animal Health Programme; Centre for Tropical Veterinary Medicine, University of Edinburgh: Edinburgh, UK, 2003. [Google Scholar]
- Kumar, A.; Rawat, B.S.; Saxena, A.K.; Agarwal, G.P. Prevalence of ectoparasites on goats in Dehradun (India). Appl. Parasitol. 1994, 35, 227–236. [Google Scholar]
- Soundararajan, C.; Latha, B.R.; Pandian, S.S. Prevalence of tick infestation in goats under different system of management. Int. J. Agric. Sci. Vet. Med. 2014, 2, 2. [Google Scholar]
- Larzen, J.J. Amitraz. INCHEM (World Health Organization) International Programme on Chemical Safety (IPCS). Available online: http://www.inchem.org/documents/jmpr/jmpmono/v098pr02.htm (accessed on 3 July 2021).
- Dhooria, S.; Agarwal, R. Amitraz, an under recognized poison: A systematic review. Indian J. Med. Res. 2016, 144, 348–358. [Google Scholar] [CrossRef] [Green Version]
- Hugnet, C.; Buronfusse, F.; Pineau, X.; Cadore, J.L.; Lorgue, G.; Berney, P.J. Toxicity and kinetics of amitraz in dogs. Am. J. Vet. Res. 1996, 57, 1506–1510. [Google Scholar]
- Pass, M.A.; Mogg, T.D. Pharmacokinetics and metabolism of amitraz in ponies and sheep. J. Vet. Pharmacol. Ther. 1995, 18, 210–215. [Google Scholar] [CrossRef]
- Hu, S.X.; Benner, C.P.; White, J.A.; Martin, R.A.; Feenstra, K.L. Pharmacokinetics and brain distribution of amitraz and its metabolites in rats. Environ. Toxicol. Pharmacol. 2019, 65, 40–45. [Google Scholar] [CrossRef]
- Yilmaz, H.L.; Yildizdas, D.R. Amitraz poisoning, an emerging problem: Epidemiology, clinical features, management, and preventive strategies. Arch. Dis. Child. 2003, 88, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Jorens, P.G.; Zandijk, E.; Belmans, L.; Schepens, P.J.; Bossaert, L.L. An unusual poisoning with the unusual pesticide amitraz. Hum. Exp. Toxicol. 1997, 16, 600–601. [Google Scholar] [CrossRef] [Green Version]
- Ulukaya, S.; Demirag, K.; Moral, A.R. Acute amitraz intoxication in human. Intensive Care Med. 2001, 27, 930–933. [Google Scholar] [CrossRef]
- Proudfoot, A.T. Poisoning with amitraz. Toxicol. Rev. 2003, 22, 71–74. [Google Scholar] [CrossRef]
- Cullen, L.K.; Reynoldson, J.A. Cardiovascular and respiratory effects of the acaricide amitraz. J. Vet. Pharmacol. Ther. 1987, 10, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Hsu, W.H. Influence of the formamidine pesticide amitraz and its metabolites on porcine myometrial contractility involvement of alpha (2)-adrenoceptors and Ca2+ channels. Toxicol. Appl. Pharmacol. 1994, 128, 45–49. [Google Scholar] [CrossRef]
- Leung, V.K.; Chan, T.Y.; Yeung, V.T. Amitraz poisoning in humans. J. Toxicol. Clin. Toxicol. 1999, 37, 513–514. [Google Scholar] [CrossRef] [PubMed]
- DeLay, R.L.; Lacoste, E.; Mezzasalma, T.; Blond-Riou, F. Pharmacokinetics of metaflumizone and amitraz in the plasma and hair of dogs following topical application. Vet. Parasitol. 2007, 150, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Riviere, J.E.; Brooks, J.D.; Collard, W.T.; Deng, J.; de Rose, G.; Mahabir, S.P.; Marchiondo, A.A. Prediction of formulation effects on dermal absorption of topically applied ectoparasiticides dosed in-vitro on canine and porcine skin using a mixture-adjusted quantitative structure permeability relationship. J. Vet. Pharmacol. Ther. 2014, 37, 435–444. [Google Scholar] [CrossRef]
- McDougall, K.W.; Heath, A.B.; Black, R.R. Residues of amitraz in the tissues, milk and butter of cattle dipped in Taktic®. Aust. J. Exp. Agric. 1979, 19, 663–665. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR—Hydrophobic, Electronic, and Steric Constants; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Chen, X.; Li, H.; Tian, L.; Li, Q.; Luo, J.; Zhang, Y. Analysis of the physicochemical properties of acaricides based on Lipinski’s Rule of Five. J. Comput. Biol. 2020, 27, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- George, J.E.; Davey, R.B.; Ahrens, E.H.; Pound, J.M.; Drummond, R.O. Efficacy of amitraz (Taktic 12.5% EC) as a dip for the control of Boophilus microplus (Canestrini) (Acari: Ixodidae) on cattle. Prev. Vet. Med. 1998, 37, 55–67. [Google Scholar] [CrossRef]
- Rugg, D.; Hair, J.A.; Everett, R.E.; Cunningham, J.R.; Carter, L. Confirmation of the efficacy of a novel formulation of metaflumizone plus amitraz for the treatment and control of fleas and ticks on dogs. Vet. Parasitol. 2007, 150, 209–218. [Google Scholar] [CrossRef]
- Bouhsira, E.; Yoon, S.S.; Roques, M.; Manavella, C.; Vermot, S.; Cramer, L.G.; Ollagnier, C.; Franc, M. Efficacy of fipronil, amitraz and (S)-methoprene combination spot-on for dogs against adult dog fleas (Ctenocephalides canis, Curtis, 1826). Vet. Parasitol. 2011, 179, 351–353. [Google Scholar] [CrossRef]
- Mandal, T.K.; Chakraborty, A.K.; Bhattacharya, A.; Ghosh, R.K.; Majumder, S. The disposition kinetics and residues of fenvalerate in tissues following single dermal application to Black Bengal goats. Vet. Res. Commun. 1996, 20, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Roudaut, B.; Garnier, M. Sulphonamide residues in eggs following drug administration via the drinking water. Food Addit. Contam. 2002, 19, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Gibbaldi, M.; Perrier, D. Pharmacokinetics, 2nd ed.; Informa Health Care; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S.P.K. Solver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1994. [Google Scholar]




| Parameters | Units | Mean ± SE |
|---|---|---|
| λz | 1/h | 0.006 ± 0.004 |
| t1/2 λz | h | 111 ± 31 |
| Tmax | h | 12 ± 5 |
| Cmax | μg/mL | 8 ± 3 |
| Clast/Cmax | 0.26 ± 0.05 | |
| AUC0–t | μg/mL·h | 539 ± 211 |
| AUC0–∞ | μg/mL·h | 874 ± 254 |
| AUC0–t/0–∞ | 0.62 ± 0.34 | |
| AUMC0–inf | μg/mL·h2 | 146,808 ± 24,363 |
| MRT0–inf | h | 168 ± 39 |
| Vdss | mL/gm | 92 ± 36 |
| Cl | mL/kg/h | 0.57 ± 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanjundappa, S.; Nair, S.N.; Udayan, D.; Kanapadinchareveetil, S.; Jacob, M.; Ravindran, R.; Juliet, S. Disposition Kinetics of Amitraz in Lactating Does. Molecules 2021, 26, 4769. https://doi.org/10.3390/molecules26164769
Nanjundappa S, Nair SN, Udayan D, Kanapadinchareveetil S, Jacob M, Ravindran R, Juliet S. Disposition Kinetics of Amitraz in Lactating Does. Molecules. 2021; 26(16):4769. https://doi.org/10.3390/molecules26164769
Chicago/Turabian StyleNanjundappa, Sathish, Suresh Narayanan Nair, Darsana Udayan, Sreelekha Kanapadinchareveetil, Mathew Jacob, Reghu Ravindran, and Sanis Juliet. 2021. "Disposition Kinetics of Amitraz in Lactating Does" Molecules 26, no. 16: 4769. https://doi.org/10.3390/molecules26164769