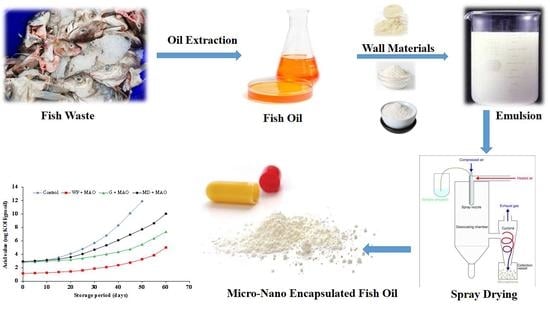
The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physiochemical Characteristics of Extracted Fish Oils
2.2. Fatty Acid Composition (%) of Extracted Oils
2.3. Physical Properties of Encapsulated Fish Oils
2.3.1. Moisture Content of Encapsulated Fish Oils
2.3.2. Encapsulation Efficiency of Encapsulated Fish Oil Powders
2.3.3. The Flowability of Powders
2.4. Oxidative Stability of Encapsulated Fish Oils
2.4.1. Peroxide Values (PV) of Encapsulated Fish Oils during Storage at 4 °C for 2 Months
2.4.2. TBA Value of Encapsulated Fish Oils during Storage at 4 °C in the Refrigerator
2.4.3. Acid Value (AV) of Encapsulated Fish Oils during Storage at 4 ± 0.25 °C
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Extraction of the Oil from Fish Wastes
3.2.2. Physicochemical Analysis of Extracted Oils
Physical Analysis of Extracted Oils
Color Analysis of Extracted Oils
Chemical Analysis of Extracted Oils
- (a)
- Identification and determination of fatty acids in oils by gas chromatography (GC-MS).
- (b)
- Peroxide values and acid values were estimated as described by A.O.C.S. [48].
- (c)
- Thiobarbituric acid (TBA) value.
Encapsulation of Extracted Fish Oils
- (a)
- Preparation of emulsions
- (b)
- Spray-drying
Moisture Content and Encapsulation Efficiency of Encapsulated Oils
- (a)
- Moisture content
- (b)
- Encapsulation efficiency (EE)
Determination of Flow Properties of Encapsulated Oils
- (a)
- Bulk Density (ρB)
- (b)
- (c)
- Powder flowability:
Determination of Oxidative Stability of Encapsulated Fish Oils
- (a)
- Thiobarbituric acid (TBA)
- (b)
- Acid value (AV)
3.2.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghaly, A.E.; Dave, D.; Brooks, M.S.; Budge, S.M. Production of biodiesel by enzymatic trans-esterification: A critical review. Am. J. Biochem. Biotechnol. 2010, 6, 54–76. [Google Scholar] [CrossRef]
- Renuka, V.; Anandan, R.; Suseela, M.; Ravishankar, C.N.; Sivaraman, G.K. Fatty acid profile of yellow fin tuna eye (Thunnus albacares) and oil sardine muscle (Sardinella longiceps). Fish. Technol. 2016, 53, 151–154. [Google Scholar]
- Arab-Tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of omega-3 long-chain polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Janarthanan, G.; Chouksey, M.K.; Venkateshwarlu, G. Effect of fish oil encapsulates incorporation on the physico-chemical and sensory properties of cookies. J. Food Sci. Technol. 2015, 53, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Encina, C.; Vergara, C.; Márquez-Ruiz, G.; Holgado, F.; Robert, P.; Giménez, B. Influence of solvent and lecithin in microencapsulation of fish oil by spray-drying. RSC Adv. 2018, 8, 4172–4181. [Google Scholar] [CrossRef] [Green Version]
- Harris, W.S. Fish oil supplementation: Evidence for health benefits. Clevel. Clin. J. Med. 2004, 71, 208–210. [Google Scholar] [CrossRef]
- Cho, Y.H.; Shim, H.K.; Park, J. Encapsulation of Fish Oil by an Enzymatic Gelation Process Using Transglutaminase Cross-linked Proteins. J. Food Sci. 2003, 68, 2717–2723. [Google Scholar] [CrossRef]
- Yu, F.; Li, Z.; Zhang, T.; Wei, Y.; Xue, Y.; Xue, C. Influence of encapsulation techniques on the structure, physical properties, and thermal stability of fish oil microcapsules by spray drying. J. Food Process. Eng. 2017, 40, e12576. [Google Scholar] [CrossRef]
- Badr, A.N.; El-Said, M.; Elmessery, T.; Abdel-Razek, A.G. Non-traditional Oils Encapsulation as Novel Food Additive Enhanced Yogurt Safety Against Aflatoxins. Pak. J. Biol. Sci. 2019, 22, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, A.N.; Ali, H.S.; Abdel-Razek, A.G.; Shehata, M.G.; Albaridi, N.A. Bioactive Components of Pomegranate Oil and Their Influence on Mycotoxin Secretion. Toxins 2020, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Bao, S.-S.; Hu, X.-C.; Zhang, K.; Xu, X.-K.; Zhang, H.-M.; Huang, H. Characterization of spray-dried microalgal oil encapsulated in cross-linked sodium caseinate matrix induced by microbial trans-glutaminase. J. Food Sci. 2011, 76, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Augustin, M.A.; Sanguansri, L.; Cheng, L.J. Oxidative stability of microencapsulated fish oil powders stabilized by blends of chitosan, modified starch, and glucose. J. Agric. Food Chem. 2010, 58, 4487–4493. [Google Scholar] [CrossRef]
- Kolanowski, W.; Laufenberg, G.; Kunz, B. Fish oil stabilization by microencapsulation with modified cellulose. Int. J. Food Sci. Nutr. 2004, 55, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Ben Rebah, F.; Gargouri, Y.; Miled, N. Lipid composition and antioxidant activity of liver oils from ray species living in Tunisian coasts. Arab. J. Chem. 2018, 11, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Aryee, A.N.; van de Voort, F.R.; Simpson, B.K. FTIR determination of free fatty acids in fish oils intended for biodiesel production. Process. Biochem. 2009, 44, 401–405. [Google Scholar] [CrossRef]
- Boran, G.; Karaçam, H.; Boran, M. Changes in the quality of fish oils due to storage temperature and time. Food Chem. 2006, 98, 693–698. [Google Scholar] [CrossRef]
- Smichi, N.; Kharrat, N.; Achouri, N.; Gargouri, Y.; Miled, N.; Fendri, A. Physicochemical Characterization and Nutritional Quality of Fish By-Products: In vitro Oils Digestibility and Synthesis of Flavor Esters. J. Food Process. Technol. 2016, 7, 602–611. [Google Scholar] [CrossRef]
- Okada, T.; Morrissey, M.T. Recovery and Characterization of Sardine Oil Extracted by pH Adjustment. J. Agric. Food Chem. 2007, 55, 1808–1813. [Google Scholar] [CrossRef]
- Yi, J.; Andersen, M.L.; Skibsted, L.H. Interactions between tocopherols, tocotrienols and carotenoids during autoxidation of mixed palm olein and fish oil. Food Chem. 2011, 127, 1792–1797. [Google Scholar] [CrossRef]
- Négre-Sadargues, G.; Castillo, R.; Segonzac, M. Carotenoid pigments and trophic behavior of deep-sea shrimps from a hydrothermal area of the mid-Atlantic ridge. Comp. Biochem. Physiol. A Mol. Integr. Physiol 2000, 127, 293–300. [Google Scholar] [CrossRef]
- Srigley, C.T.; Rader, J.I. Content and Composition of Fatty Acids in Marine Oil Omega-3 Supplements. J. Agric. Food Chem. 2014, 62, 7268–7278. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Ghorbani, M.; Jafari, S.M.; Mahoonak, A.S. Encapsulation of EPA and DHA concentrate from Kilka fish oil by milk proteins and evaluation of its oxidative stability. J. Food Sci. Technol. 2018, 56, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, M.I.; Sushchik, N.N.; Gubanenko, G.A.; Demirchieva, S.M.; Kalachova, G.S. Effect of way of cooking on content of essential polyunsaturated fatty acids in muscle tissue of humpback salmon (Oncorhynchus gorbuscha). Food Chem. 2006, 96, 446–451. [Google Scholar] [CrossRef]
- Benguendouz, A.; Bouderoua, K.; Bouterfa, A.; Belabes, M.; Bekada, A.; Sioriki, E.; Zabetakis, I. Fatty acid profile and assessment of heavy metals content of Sardina pilchardus captured in the Algerian coast. Iran. J. Fish. Sci. 2017, 16, 1021–1029. [Google Scholar] [CrossRef]
- Rahnan, S.A.; Huah, T.S.; Nassan, O.; Daud, N.M. Fatty acid composition of some Malaysian fresh water fish. Food Chem. 1995, 54, 45–49. [Google Scholar] [CrossRef]
- Gámez-Meza, N.; Higuera-Ciapara, I.; de la Barca, A.M.C.; Vázquez-Moreno, L.; Noriega-Rodríguez, J.; Angulo-Guerrero, O. Seasonal variation in the fatty acid composition and quality of sardine oil from sardinops sagax caeruleus of the gulf of California. Lipids 1999, 34, 639–642. [Google Scholar] [CrossRef]
- Wu, T.H.; Bechtel, P.J. Salmon by-product storage and oil extraction. Food Chem. 2008, 111, 868–871. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schwarz, K. Chemical stabilization of oils rich in long-chain polyunsaturated fatty acids during homogenization, microencapsulation and storage. Food Chem. 2009, 113, 1106–1112. [Google Scholar] [CrossRef]
- Moreno, P.J.G.; Pelayo, A.; Yu, S.; Busolo, M.; Chronakis, I.S.; Jacobsen, C. Physicochemical characterization and oxidative stability of fish oil loaded electro sprayed capsules: Combined use of whey protein and carbohydrates as wall materials. J. Food Eng. 2018, 231, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Rahmani-Manglano, N.E.; González-Sánchez, I.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Jacobsen, C.; Guadix, E.M. Development of Fish Oil-Loaded Microcapsules Containing Whey Protein Hydrolysate as Film-Forming Material for Fortification of Low-Fat Mayonnaise. Foods 2020, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.A.; McNamee, B.F.; O’Riordan, D.; O’Sullivan, M. Emulsification and Microencapsulation Properties Sodium Caseinate/Carbohydrate Blends. Int. Dairy J. 2001, 11, 137–144. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Kothari, D.C.; Venkateshwarlu, G. Oxidative Stability of Microencapsulated Fish Oil during Refrigerated Storage. J. Food Process. Preserv. 2015, 39, 12433. [Google Scholar] [CrossRef]
- Binsi, P.K.; Nayak, N.; Sarkar, P.C.; Jeyakumari, A.; Muhamed, A.P.; Ninan, G.; Ravishankar, C.N. Structural and oxidative stabilization of spray dried fish oil microencapsulates with gum Arabic and sage polyphenols: Characterization and release kinetics. Food Chem. 2017, 219, 158–168. [Google Scholar] [CrossRef]
- Kim, E.H.-J.; Chen, X.D.; Pearce, D. Effect of Surface Composition on the Flow Ability of Industrial Spray-Dried Dairy Powders. Colloid Sur. B Bioint. 2005, 46, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.J.; Ahrné, L. Food powder handling and processing: Industry problems, knowledge barriers and research opportunities. Chem. Eng. Process. Process. Intensif. 2005, 44, 209–214. [Google Scholar] [CrossRef]
- Turchiuli, C.; Munguia, M.J.; Sanchez, M.H.; Cortés-Ferré, H.E.; Dumoulin, E. Use of different supports for oil encapsulation in powder by spray drying. Powder Technol. 2014, 255, 103–108. [Google Scholar] [CrossRef]
- Anwar, S.H.; Kunz, B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. J. Food Eng. 2011, 105, 367–378. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Madadlou, A.; Rafiee, S. Fish oil microencapsulation as influenced by spray dryer operational variables. Int. J. Food Sci. Technol. 2013, 48, 1707–1713. [Google Scholar] [CrossRef]
- Drusch, S.; Serfert, Y.; Scampicchio, M.; Schmidt-Hansberg, B.; Schwarz, K. Impact of Physicochemical Characteristics on the Oxidative Stability of Fish Oil Microencapsulated by Spray-Drying. J. Agric. Food Chem. 2007, 55, 11044–11051. [Google Scholar] [CrossRef] [PubMed]
- Klinkesorn, U.; Sophanodora, P.; Chinachoti, P.; McClements, D.J.; Decker, E.A. Stability of Spray-Dried Tuna Oil Emulsions Encapsulated with Two-Layered Interfacial Membranes. J. Agric. Food Chem. 2005, 53, 8365–8371. [Google Scholar] [CrossRef] [PubMed]
- Sprague, M.; Cooper, S.; Tocher, D.R.; Betancor, M. Encapsulated Fish Oil Products Available in the UK Meet Regulatory Guidelines with Respect to EPA and DHA Contents and Oxidative Status. Eur. J. Lipid. Sci. Technol. 2018, 120, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pravinkumar, M.; Eugien, L.X.; Viswanathan, C.; Raffi, S.M. Extraction of fish body oil from Sardinella longiceps by employing direct steaming method and its quantitative and qualitative assessment. J. Coastal Life Med. 2015, 3, 962–966. [Google Scholar] [CrossRef]
- AOAC International. Association of Official Analytical Chemists, 18th ed.; North Frederick Avenue: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Fasina, O.O.; Hallman, H.; Craig-Schmidt, M.; Clements, C. Predicting temperature-dependence viscosity of vegetable oils from fatty acid composition. J. Am. Oil Chem. Soc. 2006, 83, 899–903. [Google Scholar] [CrossRef]
- Anandan, R.; Chatterjee, N.S.; Sivakumar, R.; Mathew, S.; Asha, K.K.; Ganesan, B. Dietary chitosan supplementation ameliorates isoproterenol-induced aberrations in membrane-bound ATP and mineral status of rat myocardium. Biol. Trace Element Res. 2015, 167, 103–109. [Google Scholar] [CrossRef]
- American Oil Chemists’ Society. American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society, Method Cd 8-53; American Oil Chemists’ Society: Urbana, IL, USA, 2009. [Google Scholar]
- Wan, Y.; Bankston, J.D.; Bechtel, P.J.; Sathivel, S. Microencapsulation of Menhaden Fish Oil Containing Soluble Rice Bran Fiber Using Spray Drying Technology. J. Food Sci. 2011, 76, E348–E356. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; Bhandari, B.; He, Y. Nano-particle encapsulation of fish oil by spray drying. Food Res. Int. 2008, 41, 172–183. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Bae, E.K.; Lee, S.J. Microencapsulation of avocado oil by spray drying using whey protein and maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef]
- Chinta, D.D.; Graves, R.A.; Pamujula, S.; Praetorius, N.; Bostanian, L.A.; Mandal, T.K. Spray-Dried Chitosan as a Direct Compression Tableting Excipient. Drug Dev. Ind. Pharm. 2008, 35, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhong, Q. Determination of thiobarbituric acid reactive substances in microencapsulated products. Food Chem. 2010, 123, 794–799. [Google Scholar] [CrossRef]










| Parameter | Mackerel Oil | Sardine Oil | Sand Smelt Oil |
|---|---|---|---|
| Crude lipids | 17.11 ± 0.10 a | 12.70 ± 0.28 b | 6.44 ± 0.14 c |
| Specific gravity (SG) | 0.912 ± 0.002 b | 0.947 ± 0.022 a | 0.928 ± 0.003 ab |
| Refractive Index (RI) | 1.479 ± 0.0021 a | 1.480 ± 0.01 a | 1.470 ± 0.001 a |
| Flow time (sec.) | 5.70 ± 0.01 b | 6.79 ± 0.01 a | 6.15 ± 0.06 ab |
| Total carotenoids (µg/g) | 128.34 ± 3.78 a | 98.12 ± 1.56 c | 105.26 ± 2.18 b |
| Acid value (AV) | 2.80 ± 0.07 a | 2.97 ± 0.05 a | 2.78 ± 0.01 a |
| Peroxid value (PV) | 2.67 ± 0.03 a | 2.67 ± 0.11 a | 2.28 ± 0.04 b |
| Iodine value (IV) | 182.89 ± 3.01 b | 194.33 ± 0.58 a | 109.67 ± 1.53 c |
| Compounds | Mackerel Oil | Sardine Oil | Sand Smelt Oil |
|---|---|---|---|
| Capric acid 10:0 | 0.02 | Nd | 0.025 |
| Myristic acid 14:0 | 13.18 | Nd | 8.9 |
| Palmitic acid 16:0 | Nd | Nd | Nd |
| Palmitoleic acid 16:1 | 14.12 | Nd | 11.9 |
| Stearic 18:0 | 7.04 | 7.16 | 11.82 |
| Oleic 18:1 | 11.65 | Nd | 30.59 |
| Linoleic 18:2 | 3.71 | 5.69 | 21.69 |
| Linolenic 18:3 | 1.29 | 1.68 | 1.19 |
| Gondoic acid 20:1 | 3.36 | 4.04 | 5.3 |
| Arachidonic 20:4 | 3.97 | 4.55 | 1.09 |
| Eicosapentaenoic acid (EPA) 20:5 | 18.63 | 23.27 | 1.7 |
| Docosahexaenoic acid (DHA) 22:6 | 15.49 | 16.27 | 5.4 |
| Total saturated fatty acids (SFA) | 20.24 | 7.16 | 20.80 |
| Total unsaturated (UnSFA) | 72.22 | 55.5 | 78.86 |
| Total monounsaturated fatty acids (MUFA) | 29.13 | 4.04 | 47.79 |
| Total polyunsaturated fatty acids (PUFA) | 43.09 | 51.46 | 31.07 |
| SFA/UnSFA | 0.29 | 0.12 | 0.26 |
| Sample | Moisture % | Encapsulation Efficiency % | Flowability |
|---|---|---|---|
| WP + SSO | 2.04 ± 0.09 c | 64.71 ± 0.26 c | Fair |
| WP + MAO | 1.93 ± 0.02 c | 71.16 ± 0.16 a | Fair |
| WP + SRO | 2.03 ± 0.07 c | 68.61 ± 0.24 b | Fair |
| AG + SSO | 3.45 ± 0.02 a | 47.06 ± 0.16 g | Passable |
| AG + MAO | 3.21 ± 0.08 a | 60.63 ± 0.33 d | Passable |
| AG + SRO | 3.51 ± 0.01 a | 56.98 ± 0.11 e | Passable |
| MD + SSO | 2.88 ± 0.02 b | 45.55 ± 0.10 h | Poor |
| MD + MAO | 2.49 ± 0.01 b | 57.25 ± 0.16 e | Poor |
| MD + SRO | 2.83 ± 0.05 b | 50.57 ± 0.51 f | Poor |
| Sample | Bulk Density (pB) | Tapped Density (pT) | Carr’s Index | Hausner Ratio | Flowability |
|---|---|---|---|---|---|
| WP + SSO | 0.26 ± 0.05 ab | 0.32 ± 0.02 ab | 17.51 ± 1.18 ef | 1.21 ± 0.01 ef | Fair |
| WP + MAO | 0.22 ± 0.02 e | 0.27 ± 0.03 c | 16.24 ± 0.74 f | 1.19 ± 0.01 f | Fair |
| WP + SRO | 0.28 ± 0.05 a | 0.34 ± 0.01 ab | 18.64 ± 1.23 e | 1.23 ± 0.02 e | Fair |
| AG + SSO | 0.27 ± 0.05 ab | 0.35 ± 0.01 a | 23.08 ± 0.39 cd | 1.29 ± 0.06 c | Passable |
| AG + MAO | 0.26 ± 0.04 ab | 0.34 ± 0.02 ab | 21.77 ± 0.95 d | 1.27 ± 0.03 d | Passable |
| AG+ SRO | 0.26 ± 0.01 abc | 0.34 ± 0.02 ab | 23.89 ± 0.64 c | 1.32 ± 0.01 c | Passable |
| MD + SSO | 0.23 ± 0.15 de | 0.31 ± 0.02 b | 27.66 ± 0.43 b | 1.38 ± 0.07 b | Poor |
| MD + MAO | 0.24 ± 0.01 cde | 0.33 ± 0.01 ab | 26.51 ± 0.73 b | 1.36 ± 0.01 b | Poor |
| MD + SRO | 0.25 ± 0.02 bcd | 0.35 ± 0.02 a | 30.19 ± 0.42 a | 1.43 ± 0.01 a | Poor |
| Flowability | Carr’s Index | Hausner Ratio |
|---|---|---|
| Excellent | <10 | 1.00–1.11 |
| Good | 11–15 | 1.12–1.18 |
| Fair | 16–20 | 1.19–1.25 |
| Passable | 21–25 | 1.26–1.34 |
| Poor | 26–31 | 1.35–1.45 |
| Very poor | 32–37 | 1.46–1.59 |
| Awful | >38 | >1.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selim, K.A.; Alharthi, S.S.; Abu El-Hassan, A.M.; Elneairy, N.A.; Rabee, L.A.; Abdel-Razek, A.G. The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils. Molecules 2021, 26, 6109. https://doi.org/10.3390/molecules26206109
Selim KA, Alharthi SS, Abu El-Hassan AM, Elneairy NA, Rabee LA, Abdel-Razek AG. The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils. Molecules. 2021; 26(20):6109. https://doi.org/10.3390/molecules26206109
Chicago/Turabian StyleSelim, Khaled A., Salman S. Alharthi, Abdelmonam M. Abu El-Hassan, Nady A. Elneairy, Laila A. Rabee, and Adel G. Abdel-Razek. 2021. "The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils" Molecules 26, no. 20: 6109. https://doi.org/10.3390/molecules26206109