Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
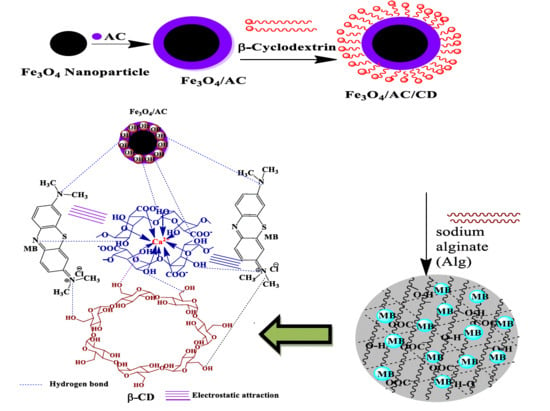
2.2. Preparation of Fe3O4-AC Nanocomposites
2.3. Preparation of β-CD Coated Fe3O4-AC Nanocomposite
2.4. Preparation of Fe3O4/AC/CD/Alg Polymer Composite Gel Beads
2.5. Analytical Procedures
2.6. Adsorption Experiments
2.7. Desorption and Reusability
3. Results and Discussion
3.1. Characterization of the Nanocomposites
3.1.1. Transmission Electron Microscopy (TEM)
3.1.2. Scanning Electron Microscopy with Energy Dispersive X-ray Spectrometry (SEM-EDX)
3.1.3. X-ray Diffraction (XRD)
3.1.4. Fourier Transform Infrared (FTIR)
3.1.5. Vibrating Sample Magnetometry (VSM)
3.1.6. Textural Analysis
3.2. Effect of pH on Methylene Blue Adsorption
3.3. Effect of Adsorbent Amount
3.4. Effect of Initial Dye Concentration
3.5. Effect of Contact Time on MB Adsorption
3.6. Effect of Temperature
3.7. Adsorption Isotherms
3.8. Adsorption Kinetics
3.9. Thermodynamic Studies
3.10. Desorption and Reusability Study of Fe3O4/AC/CD/Alg Polymer Beads
3.11. Comparison with other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mittal, A.; Kurup, L.; Mittal, J. Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazard. Mater. 2007, 146, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taziwa, R.; Meyer, E.L.; Sideras-Haddad, E.; Erasmus, R.M.; Manikandan, E.; Mwakikunga, B.W. Effect of carbon modification on the electrical, structural, and optical properties of electrodes and their performance in labscale dye-sensitized solar cells. Int. J. Photoenergy 2012, 2012, 904323. [Google Scholar] [CrossRef]
- Doble, M.; Kumar, A. Biotreatment of Industrial Effluents; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Turabik, M. Adsorption of basic dyes from single and binary component systems onto bentonite: Simultaneous analysis of Basic Red 46 and Basic Yellow 28 by first order derivatives spectrophotometric analysis method. J. Hazard. Mater. 2008, 158, 52–64. [Google Scholar] [CrossRef] [PubMed]
- El-Sewify, I.M.; Shenashen, M.A.; Shahat, A.; Selim, M.M.; Khalil, M.M.H.; El-Safty, S.A. Sensitive and selective fluorometric determination and monitoring of Zn2+ions using supermicroporous Zr-MOFs chemosensors. Microchem. J. 2018, 139, 24–33. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Review on dye removal from its aqueous solution in to alternative cost effective and non-conventional adsorbents. J. Chem. Process Eng. 2014, 1, 1–7. [Google Scholar]
- Chen, D.; Chen, J.; Luan, X.; Ji, H.; Xia, Z. Characterization of anion–cationic surfactants modified montmorillonite and its application for the removal of methyl orange. Chem. Eng. J. 2011, 171, 1150–1158. [Google Scholar] [CrossRef]
- Dahlan, N.A.; Ng, S.L.; Pushpamalar, J. Adsorption of methylene blue onto powdered activated carbon immobilized in a carboxymethyl sagopulp hydrogel. J. Appl. Polym. Sci. 2017, 134, 44271. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Agarwal, S.; Sadegh, H.; Monajjemi, M.; Hamdy, A.S.; Ali, G.A.M.; Memar, A.O.H.; Shahryari-ghoshekandi, R.; Tyagi, I.; Gupta, V.K. Efficient removal of toxic bromothymol blue and methylene blue from wastewater by polyvinylalcohol. J. Mol. Liq. 2016, 218, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Mahdavinia, G.R.; Massoudi, A.; Baghban, A.; Shokri, E. Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels. J. Environ. Chem. Eng. 2014, 2, 1578–1587. [Google Scholar] [CrossRef]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Balarak, D.; Jaafari, J.; Hassani, G.; Mahdavi, Y.; Tyagi, I.; Agarwal, S.; Gupta, V.K. The use of low-cost adsorbent (Canolaresidues) for the adsorption of methylene blue from aqueous solution: Isotherm, kinetic and thermodynamic studies. Colloid Interface Sci. Commun. 2015, 7, 16–19. [Google Scholar] [CrossRef]
- Coruh, S.; Geyikci, F.; Ergun, O.N. Adsorption of basic dye from wastewater using raw and activated red mud. Environ. Technol. 2011, 32, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- El-Mekkawi, D.M.; Ibrahim, F.A.; Selim, M.M. Removal of methylene blue from water using zeolites prepared from Egyptian kaolins collected from different sources. J. Environ. Chem. Eng. 2016, 4, 1417–1422. [Google Scholar] [CrossRef]
- Gomaa, H.; El-Safty, S.A.; Shenashen, M.A.; Abdelmottaleb, M.; Cheira, M.F. Three-dimensional, Vertical Platelets of ZnO Carriers for Selective Extraction of Cobalt Ions from Waste Printed Circuit Boards. ACS Sustain. Chem. Eng. 2018, 6, 13813–13825. [Google Scholar] [CrossRef]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Monolithic Scaffolds for Highly Selective Ion Sensing/Removal of Co(II),Cu(II),and Cd(II)Ions in Water. Analyst 2014, 139, 6393–6405. [Google Scholar] [CrossRef]
- Han, Z.; Jin, J.; Wang, Y.; Zhang, Z.; Gu, J.; Ou, M.; Xu, X. Encapsulating TiO2 into Polyvinyl Alcohol Coated Polyacrylonitrile Composite Beads for the Effective Removal of Methylene Blue. J. Braz. Chem. Soc. 2019, 30, 211–223. [Google Scholar] [CrossRef]
- Balkız, G.; Pingo, E.; Kahya, N.; Kaygusuzet, H.; Erim, F.B. Graphene Oxide/Alginate Quasi-Cryogels for Removal of Methylene Blue. Water Air Soil Pollut. 2018, 229, 131–139. [Google Scholar] [CrossRef]
- Raghunath, S.; Anand, K.; Gengan, R.M.; Nayunigari, M.K.; Maity, A. Sorption isotherms, kinetic and optimization process of amino acid proline based polymer nanocomposite for the removal of selected textiles dyes from industrial wastewater. J. Photochem. Photobiol. B 2016, 165, 189–201. [Google Scholar] [CrossRef]
- Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Hu, G.; Jamal, L.; Jahangir Alam, S.M. Preparation and Characterization of Magadiite–Magnetite Nanocomposite with Its Sorption Performance Analyses on Removal of Methylene Blue from Aqueous Solutions. Polymers 2019, 11, 607. [Google Scholar] [CrossRef] [Green Version]
- Nair, V.; Panigrahy, A.; Vinu, R. Development of Novel Chitosan-Lignin Composites for Adsorption of Dyes and Metal Ions from Wastewater. Chem. Eng. J. 2014, 254, 491–502. [Google Scholar] [CrossRef]
- Manikandan, G.; Senthil, K.P.; Saravanan, A. Modelling and analysis on the removal of methylene blue dye from aqueous solution using physically/chemically modified Ceibapentandra seeds. J. Ind. Eng. Chem. 2018, 62, 446–461. [Google Scholar]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Park, G.D.; Spector, R.; Goldberg, M.J.; Johnson, G.F. Expanded role of charcoal therapy in the poisoned and over dosed patient. Arch. Intern. Med. 1986, 146, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zuo, F.; Zheng, Z.; Ding, X.; Peng, Y. A Novel Approach to Molecular Recognition Surface of Magnetic Nanoparticles Based on Host–Guest Effect. Nanoscale Res. Lett. 2009, 4, 738–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crini, G. Kinetic and equilibrium studies on removal of cationic dyes from aqueous solutions by adsorption onto a cyclodextrin polymer. Dyes Pigm. 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Crini, G. Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Jiang, X.; Yu, J.; Chen, X. Adsorption and removal of malachite green from aqueous solution using magnetic beta-cyclodextrin-graphene oxide nanocomposites as adsorbents. Colloids Surf. A Phys. Eng. Asp. 2015, 466, 166–173. [Google Scholar] [CrossRef]
- Fan, L.; Li, M.; Lv, Z.; Sun, M.; Luo, C.; Lu, F.; Qiu, H. Fabrication of magnetic chitosan nanoparticles grafted with β-cyclodextrin as effective adsorbents toward hydroquinol. Colloids Surf. B Biointerfaces 2012, 95, 42–49. [Google Scholar] [CrossRef]
- Tanasa, E.; Zaharia, C.; Radu, I.-C.; Surdu, V.-A.; Vasile, B.S.; Damian, C.-M.; Andronescu, E. Novel Nanocomposites Based on Functionalized Magnetic Nanoparticles and Polyacrylamide: Preparation and Complex Characterization. Nanomaterials 2019, 9, 1384. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhao, X.; Li, Y.; Zhao, C.; Du, Q.; Sun, J.; Wang, Y.; Peng, X.; Xia, Y.; Wang, Z.; et al. Adsorption of ciprofloxacin onto biocomposite fibres of graphine oxide/calcium alginate. Chem. Eng. J. 2013, 230, 389–395. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Removal of heavy metals from their aqueous solutions through had sorption onto natural polymers. Carbohydr. Polym. 2011, 84, 454–458. [Google Scholar] [CrossRef]
- Rocher, V.; Bee, A.; Siaugue, J.-M.; Cabuil, V. Dye removal from aqueous solution by magnetic alginate beads crosslinked with epichlorohydrin. J. Hazard. Mater. 2010, 178, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Yong, L.; Sheng, H.; Jie, M. Adsorptive removal of ciprofloxacin by sodium alginate/graphene oxide composite beads from aqueous solution. J. Colloid Interface Sci. 2016, 484, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Badruddoza, A.Z.M.; Shawon, Z.B.Z.; Daniel, T.W.J.; Hidajat, K.; Uddin, M.S. Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohydr. Polym. 2013, 91, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Janaki, V.; Vijayaraghavan, K.; Oh, B.T.; Lee, K.J.; Muthuchelian, K.; Ramasamy, A.K.; Kannan, S.K. Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent. Carbohydr. Polym. 2012, 90, 1437–1444. [Google Scholar] [CrossRef]
- Swaminathan, S.; Muthumanickkam, A.; Imayathamizhan, N.M. An effective removal of methylene blue dye using polyacrylonitrile yarn waste/graphene oxide nanofibrous composite. Int. J. Environ. Sci. Technol. 2015, 12, 3499–3508. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Song, X.; Sun, Y. Attapulgite Nanofiber-Cellulose Nanocomposite with Core-Shell Structure for Dye Adsorption. Int. J. Polym. Sci. 2016, 2016, 2081734. [Google Scholar] [CrossRef] [Green Version]
- Parlayici, S. Alginate-coated perlite beads for the efficient removal of methylene blue, malachite green, and methyl violet from aqueous solutions: Kinetic, thermodynamic, and equilibrium studies. J. Anal. Sci. Technol. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Bahrami, H. Preparation, characterization and dye adsorption properties of biocompatible composite (alginate/titania nanoparticle). Desalination 2011, 275, 93–101. [Google Scholar] [CrossRef]
- Asadi, S.; Eris, S.; Azizian, S. Alginate-Based Hydrogel Beads as a Biocompatible and Efficient Adsorbent for Dye Removal from Aqueous Solutions. ACS Omega 2018, 3, 15140–15148. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, A.; Navgire, M.; Sarma, K.C.; Gogoi, P. Synthesis and characterization of β-cyclodextrin coated Fe3O4/carbon nanocomposite for adsorption of tea catechin from aqueous solutions, Indian. J. Chem. Technol. 2017, 24, 498–507. [Google Scholar]
- Asthana, A.; Verma, R.; Singh, A.K.; Susan, M.A.B.H. Glycine functionalized magnetic nanoparticle entrapped calcium alginate beads: A promising adsorbent for removal of Cu(II)ions. J. Environ. Chem. Eng. 2016, 4, 1985–1995. [Google Scholar] [CrossRef]
- Marto, A.; Halim, A.A.; Awang, H.; Latiff, A.A.A.; Kadir, A.A.; Abubakar, M.; Daud, Z. Batch Study on COD and Ammonia Nitrogen Removal Using Granular Activated Carbon and Cockle Shells. Int. J. Eng. 2017, 30, 937–944. [Google Scholar]
- Sun, L.; Fugetsu, B. Graphene oxide captured for green use: Influence on the structures of calcium alginate and macroporous alginic beads and their application to aqueous removal of acridine orange. Chem. Eng. J. 2014, 240, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Cullity, B.D. Elements of X-ray Diffraction, 2nd ed.; Addison-Wesley: Boston, MA, USA, 1978. [Google Scholar]
- Wu, Y.; Qi, H.; Shi, C.; Ma, R.; Liu, S.; Huang, Z. Preparation and adsorption behaviors of sodium alginate-based adsorbent-immobilized β-cyclodextrin and graphene oxide. RSC Adv. 2017, 7, 31549–31557. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.J.; Guo, S.J.; Zhai, Y.M.; Dong, S.J.; Wang, E.K. Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: Synthesis and host-guest inclusion for enhanced electrochemical performance. ACS Nano 2010, 4, 4001–4010. [Google Scholar] [CrossRef]
- Algothmi, W.M.; Bandaru, N.M.; Yu, Y.; Shapter, J.G.; Ellis, A.V. Alginate-graphene oxide hybrid gel beads: An efficient copper adsorbent material. J. Colloid Interface Sci. 2013, 397, 32–38. [Google Scholar] [CrossRef]
- Jeyagowri, B.; Yamuna, R.T. Biosorption of methylene blue from aqueous solutions by modified mesoporous Simaroubaglauca seed shell powder. Glob. Nest J. 2015, 17, 701–715. [Google Scholar]
- Hassan, H.S.; Elkady, M.F.; Shazly, A.H.E.; Bamufleh, H.S. Formulation of Synthesized Zinc Oxide Nano powder into Hybrid Beads for Dye Separation. J. Nanomater. 2014, 2014, 967492. [Google Scholar]
- Gusmao, K.A.G.; Gurgel, L.V.A.; Melo, T.M.S.; Gil, L.F. Adsorption studies of methylene blue and gentian violet on sugarcane bagasse modified with EDTA dianhydride (EDTAD) in aqueous solutions: Kinetic and equilibrium aspects. J. Environ. Manag. 2013, 118, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamuna, R.T.; Namasivayam, C. Color removal from aqueous solution by biogas residual slurry. Toxicol. Environ. Chem. 1993, 38, 131–143. [Google Scholar] [CrossRef]
- Inbraj, B.S.; Sulochana, N. Basic dye adsorption on a low cost carbonaceous sorbent-kinetic and equilibrium studies. Indian J. Technol. 2002, 9, 201–208. [Google Scholar]
- Srivastava, V.; Sillanpää, M. Synthesis of malachite@clay nanocomposite for rapid scavenging of cationic and anionic dyes from synthetic wastewater. J. Environ. Sci. 2017, 51, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The Constitution and fundamental properties of solids and liquids part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Uberdie adsorption in losungen. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Nemr, A.E. Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: Kinetic and isotherm studies. J. Hazard. Mater. 2009, 161, 132–141. [Google Scholar] [CrossRef]
- Gupta, S.; Babu, B.V. Removal of toxic metal Cr(VI) from aqueous solutions using saw dust as adsorbent: Equilibrium, kinetics and regeneration studies. Chem. Eng. J. 2009, 150, 352–365. [Google Scholar] [CrossRef]
- Kumar, K.Y.; Muralidhara, H.B.; Nayaka, Y.A.; Balasubramanyam, J.; Hanu-manthappa, H. Low-cost synthesis of metal oxide nanoparticles and their application in adsorption of commercial dye and heavy metal ion in aqueous solution. Powder Technol. 2013, 246, 125–136. [Google Scholar] [CrossRef]
- Aygun, A.; Yenisoy-Karakas, S.; Duman, I. Production of granular activated carbon from fruit stones and nut shells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Tseng, R.L.; Tseng, S.K.; Wu, F.C. Preparation of high surface area carbons from Corncob with KOH etching plus CO2 gasification for the adsorption of dyes and phenols from water. Colloids Surf. A 2006, 279, 69–78. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L. High adsorption capacity NaOH-activated carbon for dye removal from aqueous solution. J. Hazard. Mater. 2008, 152, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Salisua, A.; Sanagi, M.M.; Naim, A.A.; Karim, K.J. Removal of methylene blue dye from aqueous solution using alginate grafted polyacrylonitrile beads. Pharma Chem. 2015, 7, 237–242. [Google Scholar]
- Nasuha, N.; Hameed, B.H. Adsorption of methylene blue from aqueous solution onto NaOH-modified rejected tea. Chem. Eng. J. 2011, 166, 783–786. [Google Scholar] [CrossRef]












| Substance | Most Intense Peak (2θ, degree) | Most Intense Peak (θ, degree) | hkl | FWHM * of Most Intense Peak (β, Radians) | Size of the Particles (D, nm) |
|---|---|---|---|---|---|
| Fe3O4/AC | 35.71 | 17.86 | 311 | 0.79 | 11.04 |
| Fe3O4/AC/CD | 30.41 | 15.20 | 220 | 3.11 | 2.71 |
| Fe3O4/AC/CD/Alg | 35.72 | 17.86 | 311 | 1.55 | 5.63 |
| BET Analysis of the Fe3O4/AC/CD/AlgNanocomposite | |
|---|---|
| Specific surface area | 8 m2 g−1 |
| Total pore volume | 0.02 cm3 g−1 |
| Mean pore diameter | 1.47 nm |
| Adsorbent | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | R2 | RL | KF | n | R2 | ||
| Fe3O4/AC/CD/Alg polymer gel beads | 2.304 | 2.079 | 0.992 | 0.086 | 12.941 | 2.232 | 0.971 |
| Dry polymer beads | 0.595 | 10.638 | 0.976 | 0.336 | 17.458 | 1.597 | 0.963 |
| Adsorbent | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g/mg min−1) | R2 | |||
| Fe3O4/AC/CD/Alg polymer gel beads | 0.0483 | 99.083 | 0.980 | 1.243 | 0.168 | 0.987 |
| Dry polymer beads | 0.071 | 200.44 | 0.876 | 0.072 | 1.215 | 0.880 |
| Adsorbent | ΔH (KJ/mol) | ΔS (J/Kmol) | ΔG (KJ/mol) | ||
|---|---|---|---|---|---|
| 308 K | 318 K | 328 K | |||
| Fe3O4/AC/CD/Alg polymer gel beads | 15.77 | 56.80 | −4.90 | −5.591 | −7.962 |
| Dry polymer beads | 0.024 | 92.867 | −3.894 | −5.062 | −5.767 |
| Adsorbents | Adsorption Capacity (mg/g) | References |
|---|---|---|
| Walnut shell-activated carbon | 3.53 | [62] |
| Almond shell-activated carbon | 1.33 | [62] |
| Corncob derived activated carbon | 0.84 | [63] |
| Fir wood derived activated carbon | 1.21 | [64] |
| Alginate grafted polyacrylonitrile beads | 3.51 | [65] |
| NaOH-modified rejected tea | 2.44 | [66] |
| Fe3O4/AC/CD/Alg polymer beads | 2.079 | This study |
| Dry polymer beads | 10.638 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Carabineiro, S.A.C.; Susan, M.A.B.H. Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite. Nanomaterials 2020, 10, 170. https://doi.org/10.3390/nano10010170
Yadav S, Asthana A, Chakraborty R, Jain B, Singh AK, Carabineiro SAC, Susan MABH. Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite. Nanomaterials. 2020; 10(1):170. https://doi.org/10.3390/nano10010170
Chicago/Turabian StyleYadav, Sushma, Anupama Asthana, Rupa Chakraborty, Bhawana Jain, Ajaya Kumar Singh, Sónia A. C. Carabineiro, and Md. Abu Bin Hasan Susan. 2020. "Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite" Nanomaterials 10, no. 1: 170. https://doi.org/10.3390/nano10010170