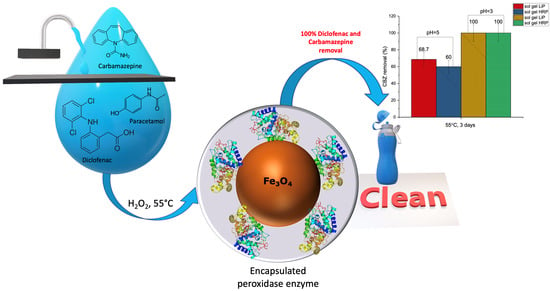
Removal of Diclofenac, Paracetamol, and Carbamazepine from Model Aqueous Solutions by Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Magnetite Synthesis
2.2. Immobilization of Enzymes and Their Encapsulation into Silica Matrix
2.3. ABTS Oxidation Test and Enzyme Activity
2.4. Drug Solutions
2.5. Drugs Decomposition by Native Enzymes
2.6. Drugs Degradation by Non-Encapsulated Enzymes
2.7. Drug Decomposition by Hybrid Composites
2.8. Enzyme Leaching from Sol–Gel Encapsulated Composites
2.9. NMR Experiments
2.10. UV–Vis Spectroscopy
2.11. SEM
2.12. FTIR
2.13. AFM
2.14. Transmission Electron Microscopy
2.15. TGA
2.16. Differential Scanning Calorimetry (DSC)
2.17. Statistical Analysis
3. Results and Discussion
3.1. Transmission Electron Microscopy
3.2. Atomic Force Microscopy
3.3. Scanning Electron Microscopy
3.4. Thermogravimetric Analysis
3.5. FTIR
3.6. Activity of the Composites
3.7. Enzyme Leaching from Sol–Gel Encapsulated Composites
3.8. Activity in Drug Decomposition and NMR Study of Decomposition Products
3.8.1. Drug Degradation by Non-Encapsulated Enzymes
3.8.2. Degradation of Diclofenac by Non-Immobilized Enzymes
3.8.3. Degradation of Diclofenac by Sol–Gel Encapsulated Enzymes at pH 5
3.8.4. Degradation of Diclofenac by Sol–Gel Encapsulated Enzymes at pH 3
3.8.5. Degradation of Paracetamol by Non-Immobilized Enzymes
3.8.6. Degradation of Paracetamol by Sol–Gel Encapsulated Enzymes at pH 5
3.8.7. Degradation of Paracetamol by Sol–Gel Encapsulated Enzymes at pH 3
3.8.8. Degradation of Carbamazepine by Non-Immobilized Enzymes
3.8.9. Degradation of Carbamazepine by Sol–Gel Encapsulated Enzymes at pH 5
3.8.10. Degradation of Carbamazepine by Sol–Gel Encapsulated Enzymes at pH 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Ritter, L.; Solomon, K.R.; Forget, J.; Stemeroff, M.; O’Leary, C. “Persistent organic pollutants” (PDF). United Nations Environment Programme. Archived from the original (PDF) on 2007-09-26. Retrieved 2007-09-16.
- Ebele, A.J.; Abdallah, M.A.-E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Tixier, C.; Singer, H.P.; Oellers, S.; Müller, S.R. Occurrence and Fate of Carbamazepine, Clofibric Acid, Diclofenac, Ibuprofen, Ketoprofen, and Naproxen in Surface Waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef]
- Buser, H.R.; Poiger, T.; Muller, M.D. Occurrence and fate of the pharmaceutical drug diclofenac in surface waters: Rapid photodegradation in a lake. Environ. Sci. Technol. 1998, 32, 3449–3456. [Google Scholar] [CrossRef]
- Aldekoa, J.; Medici, C.; Osorio, V.; Perez, S.; Marce, R.; Barcelo, D.; Frances, F. Modelling the emerging pollutant diclofenac with the GREAT-ER model: Application to the Llobregat River Basin. J. Hazard. Mater. 2013, 263 Pt 1, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Läkemedelsverket. Miljöindikatorer inom Ramen för Nationella Läkemedelsstrategin (NLS); Swedish Medical Products Agency: Uppsala, Sweden, 2015. [Google Scholar]
- Hallgren, P.; Wallberg, P. Background report on pharmaceutical concentrations and effects in the Baltic Sea. In Policy Area Hazards of the EU Strategy for the Baltic Sea Region; Swedish Environmental Protection Agency: Stockholm, Sweden, 2015. [Google Scholar]
- Tröger, R.; Klöckner, P.; Ahrens, L.; Wiberg, K. Micropollutants in drinking water from source to tap-Method development and application of a multiresidue screening method. Sci. Total Environ. 2018, 627, 1404–1432. [Google Scholar] [CrossRef] [PubMed]
- Dalahmeh, S.; Ahrens, L.; Gros, M.; Wiberg, K.; Pell, M. Potential of biochar filters for onsite sewage treatment: Adsorption and biological degradation of pharmaceuticals in laboratory filters with active, inactive and no biofilm. Sci. Total Environ. 2018, 612, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, F. Application of Ozone in Wastewater Treatment: Oxidation of Pharmaceuticals and Filamentous Bulking Sludge; Department of Chemical Engineering, Lund University: Lund, Sweden, 2017. [Google Scholar]
- Available online: http://www.water.se/kommuner (accessed on 5 February 2020).
- Canonica, S.; Meunier, L.; von Gunten, U. Phototransformation of selected pharmaceuticals during UV treatment of drinking water. Water Res. 2008, 42, 121–128. [Google Scholar] [CrossRef]
- Im, J.-K.; Cho, I.-H.; Kim, S.-K.; Zoh, K.-D. Optimization of carbamazepine removal in O3/UV/H2O2 system using a response surface methodology with central composite design. Desalination 2012, 285, 306–314. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research. Mutat. Res./Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Agarwal, P.; Gupta, R.; Agarwal, N. A review on enzymatic treatment of phenols in wastewater. J. Biotechnol. Biomater. 2016, 6, 2. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Enzymatic catalysis treatment method of meat industry wastewater using lacasse. J. Environ. Health Sci. Eng. 2015, 13, 86. [Google Scholar] [CrossRef] [Green Version]
- Martinez, A.T.; Ruiz-Duenas, F.J.; Camarero, S.; Serrano, A.; Linde, D.; Lund, H.; Vind, J.; Tovborg, M.; Herold-Majumdar, O.M.; Hofrichter, M. Oxidoreductases on their way to industrial biotransformations. Biotechnol. Adv. 2017, 35, 815–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Geißen, S.-U. In vitro degradation of carbamazepine and diclofenac by crude lignin peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Yang, S. Removal of Micropollutants by a Fungus-Augmented Membrane Bioreactor. Master’s Thesis, University of Wollongong, Wollongong, Australia, 2012. [Google Scholar]
- Zhang, Y.; Geißen, S.-U. Elimination of carbamazepine in a non-sterile fungal bioreactor. Bioresour. Technol. 2012, 112, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Montanez-Hurtado, E.; Pramparo, L. Degradation of a Nonsteroidal Anti-Inflammatory Drug Using Horseradish Peroxidase Enzyme. Res. J. Appl. Sci. 2018, 13, 425–430. [Google Scholar] [CrossRef]
- Xu, R.; Si, Y.; Li, F.; Zhang, B. Enzymatic removal of paracetamol from aqueous phase: Horseradish peroxidase immobilized on nanofibrous membranes. Environ. Sci. Pollut. Res. 2015, 22, 3838–3846. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Pogorilyi, R.P.; Pylypchuk, I.; Melnyk, I.V.; Zub, Y.L.; Seisenbaeva, G.A.; Kessler, V.G. Sol-Gel Derived Adsorbents with Enzymatic and Complexonate Functions for Complex Water Remediation. Nanomaterials 2017, 7, 298. [Google Scholar] [CrossRef]
- Pylypchuk, I.V.; Kessler, V.G.; Seisenbaeva, G.A. Simultaneous removal of acetaminophen, diclofenac, and Cd (II) by Trametes Versicolor lacсase immobilized on Fe3O4/SiO2-DTPA hybrid nanocomposites. ACS Sustain. Chem. Eng. 2018, 6, 9979–9989. [Google Scholar] [CrossRef]
- Lalwani, G.; Xing, W.; Sitharaman, B. Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. J. Mater. Chem. B 2014, 2, 6354–6362. [Google Scholar] [CrossRef] [Green Version]
- Batool, S. Purification, Characterization and Immobilization of Lignin Peroxidase Produced by Ganoderma Lucidum for Industrial Applications. Ph.D. Thesis, University of Agriculture Faisalabad, Faisalabad, Pakistan, 2012. [Google Scholar]
- Braun, S.; Rappoport, S.; Zusman, R.; Avnir, D.; Ottolenghi, M. Biochemically active sol-gel glasses: The trapping of enzymes. Mater. Lett. 1990, 10, 1–5. [Google Scholar] [CrossRef]
- Frenkel-Mullerad, H.; Avnir, D. Sol—Gel materials as efficient enzyme protectors: Preserving the activity of phosphatases under extreme pH conditions. J. Am. Chem. Soc. 2005, 127, 8077–8081. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pymol.org/2/ (accessed on 5 February 2020).
- Wen, X.; Jia, Y.; Li, J.J.C. Degradation of tetracycline and oxytetracycline by crude lignin peroxidase prepared from Phanerochaete chrysosporium–a white rot fungus. Chemosphere 2009, 75, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Eibes, G.; Debernardi, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M.J.B. Oxidation of pharmaceutically active compounds by a ligninolytic fungal peroxidase. Biodegradation 2011, 22, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Santosa, I.; Grossmana, M.J.; Sartorattob, A.; Ponezib, A.N.; Durranta, L.R. Degradation of the recalcitrant pharmaceuticals carbamazepine and 17α-ethinylestradiol by ligninolytic fungi. Chem. Eng. 2012, 27, 169–174. [Google Scholar]
- Ma, Z.Y.; Guan, Y.P.; Liu, H.Z. Superparamagnetic silica nanoparticles with immobilized metal affinity ligands for protein adsorption. J. Magn. Magn. Mater. 2006, 301, 469–477. [Google Scholar] [CrossRef]
- Mel’nik, I.V.; Zub, Y.L.; Alonso, B.; Abramov, N.V.; Gorbik, P.P. Creation of a Functional Polysiloxane Layer on the Surface of Magnetic Nanoparticles Using the Sol-Gel Method. Glass Phys. Chem. 2012, 38, 96–104. [Google Scholar] [CrossRef]
- Liu, J.; Xie, Y.; Peng, C.; Yu, G.; Zhou, J. Molecular Understanding of Laccase Adsorption on Charged Self-Assembled Monolayers. J. Phys. Chem. B 2017, 121, 10610–10617. [Google Scholar] [CrossRef]
- Tuisel, H.; Sinclair, R.; Bumpus, J.A.; Ashbaugh, W.; Brock, B.J.; Aust, S.D. Lignin peroxidase H2 from Phanerochaete chrysosporium: Purification, characterization and stability to temperature and pH. Arch. Biochem. Biophys. 1990, 279, 158–166. [Google Scholar] [CrossRef]
- Bosco, F.; Capolongo, A.; Ruggeri, B. Effect of temperature, pH, ionic strength, and sodium nitrate on activity of LiPs: Implications for bioremediation. Bioremediat. J. 2002, 6, 65–76. [Google Scholar] [CrossRef]
- Lu, Q.; Kim, Y.; Bassim, N.; Raman, N.; Collins, G.E. Catalytic activity and thermal stability of horseradish peroxidase encapsulated in self-assembled organic nanotubes. Analyst 2016, 141, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Besharati Vineh, M.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and activity improvement of horseradish peroxidase by covalent immobilization on functionalized reduced graphene oxide and biodegradation of high phenol concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kwon, O.Y.; Yoon, Y.J.; Ryu, K. Immobilization of horseradish peroxidase on multi-wall carbon nanotubes and its electrochemical properties. Biotechnol. Lett. 2006, 28, 39–43. [Google Scholar] [CrossRef]
- Quintana, J.B.; Weiss, S.; Reemtsma, T. Pathway’s and metabolites of microbial degradation of selected acidic pharmaceutical and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Res. 2005, 39, 2654–2664. [Google Scholar] [CrossRef]
- Kimura, K.; Hara, H.; Watanabe, Y. Elimination of selected acidic pharmaceuticals from municipal wastewater by an activated sludge system and membrane bioreactors. Environ. Sci Technol 2007, 41, 3708–3714. [Google Scholar] [CrossRef]
- Groning, J.; Held, C.; Garten, C.; Claussnitzer, U.; Kaschabek, S.R.; Schlomann, M. Transformation of diclofenac by the indigenous microflora of river sediments and identification of a major intermediate. Chemosphere 2007, 69, 509–516. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Perez-Trujillo, M.; Cruz-Morato, C.; Caminal, G.; Vicent, T. Degradation of the drug sodium diclofenac by Trametes versicolor pellets and identification of some intermediates by NMR. J. Hazard. Mater. 2010, 176, 836–842. [Google Scholar] [CrossRef]
- Forrez, I.; Carballa, M.; Verbeken, K.; Vanhaecke, L.; Ternes, T.; Boon, N.; Verstraete, W. Diclofenac oxidation by biogenic manganese oxides. Environ. Sci. Technol. 2010, 44, 3449–3454. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Biotransformation of carbamazepine by laccase-mediator system: Kinetics, by-products and toxicity assessment. Process. Biochem. 2018, 67, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Uetrecht, J.P. Peroxidase-mediated bioactivation of hydroxylated metabolites of carbamazepine and phenytoin. Drug Metab. Dispos. 2008, 36, 1624–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullrich, R.; Hofrichter, M. Enzymatic hydroxylation of aromatic compounds. Cell. Mol. Life Sci. 2007, 64, 271–293. [Google Scholar] [CrossRef]
- Zhang, Y.; Tsitkov, S.; Hess, H. Proximity does not contribute to activity enhancement in the glucose oxidase–horseradish peroxidase cascade. Nat. Commun. 2016, 7, 13982. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, T.; Manavalan, A.; Heese, K. Characterization of lignocellulolytic enzymes from white-rot fungi. Curr. Microbiol. 2015, 70, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Mester, T.; Tien, M. Oxidation mechanism of ligninolytic enzymes involved in the degradation of environmental pollutants. Int. Biodeterior. Biodegrad. 2000, 46, 51–59. [Google Scholar] [CrossRef]
- De Cassia Pereira, J.; Giese, E.C.; de Souza Moretti, M.M.; dos Santos Gomes, A.C.; Perrone, O.M.; Boscolo, M.; da Silva, R.; Gomes, E.; Martins, D.A.B. Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. In Enzyme Inhibitors and Activators; InTech: Vienna, Austria, 2017. [Google Scholar]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Membr. Sci. 2014, 452, 229–240. [Google Scholar] [CrossRef]
- Ji, C.; Hou, J.; Wang, K.; Zhang, Y.; Chen, V. Biocatalytic degradation of carbamazepine with immobilized laccase-mediator membrane hybrid reactor. J. Membr. Sci. 2016, 502, 11–20. [Google Scholar] [CrossRef]













| pH = 5 | pH = 3 | |||
|---|---|---|---|---|
| Sol–Gel Fe3O4/HRP/SiO2, | Sol–Gel Fe3O4/LiP/SiO2 | Sol–Gel Fe3O4/HRP/SiO2, | Sol–Gel Fe3O4/LiP/SiO2 | |
| Diclofenac | 59% ± 8% | 64% ± 8% | 100% ± 10% | 100% ± 10% |
| Paracetamol | 9% ± 5% | 9% ± 5% | 50 ± 10% | 50 ± 10% |
| Carbamazepine | 60% ± 8% | 68 ± 8% | 100% ± 10% | 100 ± 10% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pylypchuk, I.V.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A. Removal of Diclofenac, Paracetamol, and Carbamazepine from Model Aqueous Solutions by Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites. Nanomaterials 2020, 10, 282. https://doi.org/10.3390/nano10020282
Pylypchuk IV, Daniel G, Kessler VG, Seisenbaeva GA. Removal of Diclofenac, Paracetamol, and Carbamazepine from Model Aqueous Solutions by Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites. Nanomaterials. 2020; 10(2):282. https://doi.org/10.3390/nano10020282
Chicago/Turabian StylePylypchuk, Ievgen V., Geoffrey Daniel, Vadim G. Kessler, and Gulaim A. Seisenbaeva. 2020. "Removal of Diclofenac, Paracetamol, and Carbamazepine from Model Aqueous Solutions by Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites" Nanomaterials 10, no. 2: 282. https://doi.org/10.3390/nano10020282