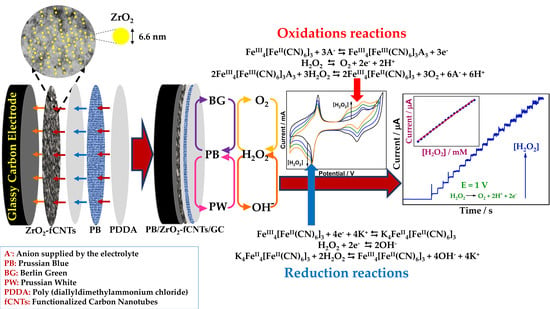
Electrochemical Sensor Based on Prussian Blue Electrochemically Deposited at ZrO2 Doped Carbon Nanotubes Glassy Carbon Modified Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Carbon Nanotubes (CNTs) Functionalization
2.3. Pre-Functionalization of CNTs
2.4. Functionalization of CNTs
2.5. Synthesis of ZrO2-fCNT Nanostructured System
2.6. Material Characterization
2.7. Electrode Modification
2.8. Electrochemical Characterization
2.9. H2O2 Detection
3. Results and Discussion
3.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.2. Raman Spectroscopy
3.3. Surface Area
3.4. Thermal Analysis
3.5. Zeta Potential
3.6. X-Ray Diffraction (XRD) Spectroscopy
3.7. Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM) and Atomic Force Microscopy (AFM)
3.8. Electrochemical Characterization
3.9. Stability of PB Films
3.10. Cyclic Voltammetry Behavior of the PB/ZrO2-fCNTs/GC Modified Electrodes in Presence of Hydrogen Peroxide
3.11. Electrochemical Detection of H2O2 at PB/ZrO2-fCNTs/GC Electrode
3.12. Comparison of Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
- Prussian blue (PB)
- glassy carbon (GC)
- zirconia (ZrO2)
- carbon nanotubes (CNTs)
- pristine carbon nanotubes (pCNTs)
- functionalized carbon nanotubes (fCNTs)
- Berlin green (BG)
- Prussian white (PW)
- poly(diallyldimethylammonium chloride) (PDDA)
- cyclic voltammetry (CV)
- chronoamperometry (CA)
- anion supplied by the electrolyte (A-)
- quantification limit (LQ)
- detection limit (LD)
- thermogravimetric analysis (TGA)
- transmission electron microscopy (TEM)
- field emission scanning electron microscopy (FESEM)
- atomic force microscopy (AFM)
- X-ray diffraction (XRD)
- Fourier transform infrared (FTIR)
- dimethylformamide (DMF)
- Brunauer–Emmett–Teller (BET)
- Barrett–Joyner–Halenda (BJH)
- multi-walled carbon nanotubes (MWCNTs)
- International Union of Pure and Applied Chemistry (IUPAC)
- horseradish peroxidase (HRP)
- surface concentration ()
- geometric area (A)
- current (I)
- current density (j)
- anodic peak current (Ipa)
- cathodic peak current (Ipc)
- half-wave potential (E1/2); peak-to-peak separation potential (∆E)
- peak current (Ip)
- number of electrons involved in the redox process (n)
- F = 96 485 C mol(e)−1
- Scan rate ()
- R = 8.314 J K−1 mol−1 and temperature (T)
- rate constant for the catalytic reaction ()
- time (t)
- double layer capacitance (Cdl).
References
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.X.; Yang, H.B.i.n.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Tung, S. Development and applications of portable biosensors. J. Lab. Autom. 2015, 20, 365–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, S.; Liu, Y.; Shao, Y.; Jiang, D.; Duan, Q. Iron carbonyl compounds with aromatic dithiolate bridges as organometallic mimics of [FeFe] hydrogenases. Coord. Chem. Rev. 2020, 402, 213081. [Google Scholar] [CrossRef]
- Magro, M.; Baratella, D.; Colò, V.; Vallese, F.; Nicoletto, C.; Santagata, S.; Sambo, P.; Molinari, S.; Salviulo, G.; Venerando, A.; et al. Electrocatalytic nanostructured ferric tannate as platform for enzyme conjugation: Electrochemical determination of phenolic compounds. Bioelectrochemistry 2020, 132, 107418. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Rana, S.; Thakur, S.; Pant, D. Bioelectrofuel synthesis by nanoenzymes: Novel alternatives to conventional enzymes. Trends Biotechnol. 2020, 38, 469–473. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, P.; Yang, J.; Fan, Y.; Wang, L.; Jiang, W.; Cheng, X.; Deng, Y.; Luo, W. Mesoporous materials–based electrochemical biosensors from enzymatic to nonenzymatic. Small 2019, 1904022, 1–16. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, M.; Liu, Y.; Wang, C.; Guan, L.; Li, K.; Lin, Y. Fabrication of cobalt nanocomposites as enzyme mimetic with excellent electrocatalytic activity for superoxide oxidation and cellular release Detection. ACS Sustain. Chem. Eng. 2019, 7, 10227–10233. [Google Scholar] [CrossRef]
- Benedetti, T.M.; Andronescu, C.; Cheong, S.; Wilde, P.; Wordsworth, J.; Kientz, M.; Tilley, R.D.; Schuhmann, W.; Gooding, J.J. Electrocatalytic nanoparticles that mimic the three-dimensional geometric architecture of enzymes: Nanozymes. J. Am. Chem. Soc. 2018, 140, 13449–13455. [Google Scholar] [CrossRef]
- Yang, B.; Li, J.; Deng, H.; Zhang, L. Progress of mimetic enzymes and their applications in chemical sensors. Crit. Rev. Anal. Chem. 2016, 46, 469–481. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Tricard, S.; Yu, L.; Fang, J. A sensitive and selective electrochemical sensor based on N, P-Doped molybdenum Carbide@Carbon/Prussian blue/graphite felt composite electrode for the detection of dopamine. Anal. Chim. Acta 2020, 1094, 80–89. [Google Scholar] [CrossRef]
- Matos-Peralta, Y.; Antuch, M. Prussian blue and its analogs as appealing materials for eslectrochemical sensing and biosensing. J. Electrochem. Soc. 2020, 167. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. Amperometric biosensor for glutamate using Prussian Blue-based “artificial peroxidase” as a transducer for hydrogen peroxide. Anal. Chem. 2000, 72, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Jirakunakorn, R.; Khumngern, S.; Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. Uric acid enzyme biosensor based on a screen-printed electrode coated with Prussian blue and modified with chitosan-graphene composite cryogel. Microchem. J. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Farah, A.M.; Shooto, N.D.; Thema, F.T.; Modise, J.S.; Dikio, E.D. Fabrication of prussian blue/multi-walled carbon nanotubes modified glassy carbon electrode for electrochemical detection of hydrogen peroxide. Int. J. Electrochem. Sci. 2012, 7, 4302–4313. [Google Scholar]
- Zhang, Y.; Huang, B.; Yu, F.; Yuan, Q.; Gu, M.; Ji, J.; Zhang, Y.; Li, Y. 3D nitrogen-doped graphite foam@Prussian blue: An electrochemical sensing platform for highly sensitive determination of H2O2 and glucose. Microchim. Acta 2018, 185, 2–9. [Google Scholar] [CrossRef]
- Sheng, Q.; Zhang, D.; Shen, Y.; Zheng, J. Synthesis of hollow Prussian blue cubes as an electrocatalyst for the reduction of hydrogen peroxide. Front. Mater. Sci. 2017, 11, 147–154. [Google Scholar] [CrossRef]
- Shukrullah, S.; Naz, Y.M.; Ali, K.; Sharma, S.K. Carbon nanotubes: Synthesis and application in solar Cells. In Solar Cells: From Materials to Device Technology; Ali, K., Sharma, S.K., Eds.; Springer Nature Switzerland: Basel, Switzerland, 2020; pp. 159–184. [Google Scholar]
- Ali, Z.; Ahmad, R. Nanotechnology for water treatment. In Environmental Nanotechnology Volume 3; Springer International Publishing: Cham, Switzerland, 2020; Volume 32, pp. 143–163. [Google Scholar]
- Ibrahim, I.D.; Kambole, C.; Eze, A.A.; Adeboje, A.O.; Sadiku, E.R.; Kupolati, W.K.; Jamiru, T.; Olagbenro, B.W.; Adekomaya, O. Preparation and properties of nanocomposites for energy applications. In Nanomaterials-Based Composites for Energy Applications; Apple Academic Press: Burlington, ON, Canada, 2019; pp. 3–41. [Google Scholar]
- You, J.M.; Jeong, Y.N.; Ahmed, M.S.; Kim, S.K.; Choi, H.C.; Jeon, S. Reductive determination of hydrogen peroxide with MWCNTs-Pd nanoparticles on a modified glassy carbon electrode. Biosens. Bioelectron. 2011, 26, 2287–2291. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Zhang, K.; Xu, J.; Zhang, L.; Zhuo, X.; Shi, H.; Qin, M.; Wang, C.; Zhang, N. Simple strategy for fabricating a Prussian blue/chitosan/carbon nanotube composite and its application for the sensitive determination of hydrogen peroxide. Micro Nano Lett. 2016, 12, 23–26. [Google Scholar] [CrossRef]
- Dwivedi, R.; Maurya, A.; Verma, A.; Prasad, R.; Bartwal, K.S. Microwave assisted sol-gel synthesis of tetragonal zirconia nanoparticles. J. Alloys Compd. 2011, 509, 6848–6851. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Tiwari, S.; Srivastava, S.; Srivastava, M.; Yadav, B.K.; Kumar, S.; Tran, T.T.; Dewan, A.K.; Mulchandani, A.; et al. Biofunctionalized nanostructured zirconia for biomedical application: A smart approach for oral cancer detection. Adv. Sci. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Zong, S.; Cao, Y.; Zhou, Y.; Ju, H. Zirconia nanoparticles enhanced grafted collagen tri-helix scaffold for unmediated biosensing of hydrogen peroxide. Langmuir 2006, 22, 8915–8919. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Deng, M.; Cui, S.; Chen, H.; Qiu, J. Direct electrochemistry and electrocatalysis of myoglobin immobilized on zirconia/multi-walled carbon nanotube nanocomposite. Mater. Res. Bull. 2010, 45, 1855–1860. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Firoozi, S.; Korani, A.; Soltanian, S. One-pot hydrothermal synthesis of zirconium dioxide nanoparticles decorated reduced graphene oxide composite as high performance electrochemical sensing and biosensing platform. Electrochim. Acta 2014, 143, 196–206. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Wang, L.; Sheng, K.; Zou, L.; Ye, B. Electrochemical behavior of diosmin and its sensitive determination on ZrO2-NPs-coated poly(diallyldimethylammonium chloride)-functionalized graphene modified electrode. Microchem. J. 2018, 143, 430–440. [Google Scholar] [CrossRef]
- Gonzalez, G.; Albano, C.; Hermán, V.; Boyer, I.; Monsalve, A.; Brito, J. Nanocomposites building blocks of TiO2-MWCTf and ZrO2-MWCNTf. Mater. Charact. 2012, 64, 96–106. [Google Scholar] [CrossRef]
- Salam, M.A.; Burk, R. Synthesis and characterization of multi-walled carbon nanotubes modified with octadecylamine and polyethylene glycol. Arab. J. Chem. 2017, 10, S921–S927. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Tyagi, P.; Rai, P.; Misra, D.S. FTIR Spectroscopy of multiwalled carbon nanotubes: A simple approach to study the nitrogen doping. J. Nanosci. Nanotechnol. 2007, 7, 1820–1823. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Na, N.; Liu, Z.; Han, B.; An, G. Synthesis of ZrO2−Carbon nanotube composites and their application as chemiluminescent sensor material for ethanol. J. Phys. Chem. B 2006, 110, 13410–13414. [Google Scholar] [CrossRef]
- Fernández López, E.; Sánchez Escribano, V.; Panizza, M.; Carnasciali, M.M.; Busca, G. Vibrational and electronic spectroscopic properties of zirconia powders. J. Mater. Chem. 2001, 11, 1891–1897. [Google Scholar] [CrossRef]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring oxidation of multiwalled carbon nanotubes by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 697, p. 63. [Google Scholar]
- Kaszuba, M.; Corbett, J.; Watson, F.M.N.; Jones, A. High-concentration zeta potential measurements using light-scattering techniques. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4439–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aqel, A.; El-Nour, K.M.M.A.; Ammar, R.A.A.; Al-Warthan, A. Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 2012, 5, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon N. Y. 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Luo, T.Y.; Liang, T.X.; Li, C.S. Addition of carbon nanotubes during the preparation of zirconia nanoparticles: Influence on structure and phase composition. Powder Technol. 2004, 139, 118–122. [Google Scholar] [CrossRef]
- Drits, V. XRD Measurement of mean crystallite thickness of illite and illite/smectite: Reappraisal of the Kubler Index and the Scherrer equation. Clays Clay Miner. 1997, 45, 461–475. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Ito, S.; Kawazoe, Y.; Wang, J.T. Critical size of the phase transition from cubic to tetragonal in pure zirconia nanoparticles. Nano Lett. 2003, 3, 871–875. [Google Scholar] [CrossRef]
- Nièpce, J.-C.; Pizzagalli, L. Structure and phase transitions in nanocrystals. In Nanomaterials and Nanochemistry; Bréchignac, C., Houdy, P., Lahmani, M., Eds.; Springer Berlin Heidelberg New York: New York, NY, USA, 2007; pp. 35–54. [Google Scholar]
- Luo, T.; Liang, T.; Li, C. Stabilization of cubic zirconia by carbon nanotubes. Mater. Sci. Eng. A 2004, 366, 206–209. [Google Scholar] [CrossRef]
- Graeve, O.A. Zirconia. In Ceramic and Glass Materials: Structure, Properties and Processing; Shackelford, J.F., Doremus, R.H., Eds.; Springer: Boston, MA, USA, 2008; pp. 169–197. [Google Scholar]
- Itaya, K.; Ataka, T.; Toshima, S.; Shinohara, T. Electrochemistry of Prussian blue. An in situ Mössbauer effect measurement. J. Phys. Chem. 1982, 86, 2415–2418. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. On the mechanism of H2O2 reduction at Prussian Blue modified electrodes. Electrochem. commun. 1999, 1, 78–82. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Fernández, L.; González, G.; Montero-Jiménez, M.; Uribe, R.; Díaz Barrios, A.; Espinoza-Montero, P.J. Peroxide electrochemical sensor and biosensor based on nanocomposite of TiO2 nanoparticle/multi-walled carbon nanotube modified glassy carbon electrode. Nanomaterials 2019, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itaya, K.; Shoji, N.; Uchida, I. Catalysis of the reduction of molecular oxygen to water at Prussian Blue modified electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, K.; Sun, D.C.; Xia, X.H.; Chen, H.Y. Ultrathin layers of densely packed Prussian Blue nanoclusters prepared from a ferricyanide solution. Chem. Mater. 2003, 15, 4163–4165. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry; VCH: New York, NY, USA, 1994. [Google Scholar]
- Karyakin, A.A. Prussian Blue and its analogues: Electrochemistry and analytical applications. Electroanalysis 2001, 13, 813–819. [Google Scholar] [CrossRef]
- Du, D.; Wang, M.; Qin, Y.; Lin, Y. One-step electrochemical deposition of Prussian Blue–multiwalled carbon nanotube nanocomposite thin-film: Preparation, characterization and evaluation for H2O2 sensing. J. Mater. Chem. 2010, 20, 1532–1537. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Puganova, E.A.; Budashov, I.A.; Kurochkin, I.N.; Karyakina, E.E.; Levchenko, V.A.; Matveyenko, V.N.; Varfolomeyev, S.D. Prussian Blue based nanoelectrode arrays for H2O2 detection. Anal. Chem. 2004, 76, 474–478. [Google Scholar] [CrossRef]
- Haghighi, B.; Varma, S.; Alizadeh Sh., F.M.; Yigzaw, Y.; Gorton, L. Prussian blue modified glassy carbon electrodes-Study on operational stability and its application as a sucrose biosensor. Talanta 2004, 64, 3–12. [Google Scholar] [CrossRef]
- Lingane, J.J.; Lingane, P.J. Chronopotentiometry of hydrogen peroxide with a platinum wire electrode. J. Electroanal. Chem. 1963, 5, 411–419. [Google Scholar] [CrossRef]
- Galus, Z. Fundamentals of Electrochemical Analysis; Halsted Press: New York, NY, USA, 1976. [Google Scholar]
- Karyakin, A.A.; Karyakina, E.E. Prussian blue-based “artificial peroxidase” as a transducer for hydrogen peroxide detection. Application to biosensors. Sensors Actuators B Chem. 1999, 57, 268–273. [Google Scholar] [CrossRef]
- Haghighi, B.; Hamidi, H.; Gorton, L. Electrochemical behavior and application of Prussian blue nanoparticle modified graphite electrode. Sensors Actuators B. Chem. 2010, 147, 270–276. [Google Scholar] [CrossRef]
- Centeno, D.A.; Solano, X.H.; Castillo, J.J. A new peroxidase from leaves of guinea grass ( Panicum maximum ): A potential biocatalyst to build amperometric biosensors. Bioelectrochemistry 2017, 116, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Y.; Yang, X.; Li, C. Photoelectrochemical Detection of H2O2 Based on Flower-Like CuInS2-Graphene Hybrid. Electroanalysis 2014, 26, 573–580. [Google Scholar] [CrossRef]
- Cao, L.; Liu, Y.; Zhang, B.; Lu, L. In situ Controllable growth of Prussian Blue nanocubes on reduced graphene oxide: Facile synthesis and their application as enhanced nanoelectrocatalyst for H2O2 reduction. ACS Appl. Mater. Interfaces 2010, 2, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Teepoo, S.; Dawan, P.; Barnthip, N. Electrospun chitosan-gelatin biopolymer composite nanofibers for horseradish peroxidase immobilization in a hydrogen peroxide biosensor. Biosensors 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Wang, W.; Yin, G.; Ma, X. Nonenzymatic H2O2 sensor based on Pt nanoflower electrode. J. Clust. Sci. 2012, 23, 1061–1068. [Google Scholar] [CrossRef]












| Sample | Area BET (m2·g−1) | BJH Pore Volume (cm3·g−1) |
|---|---|---|
| fCNTs | 298.4 | 2.52 |
| ZrO2-fCNTs | 92.12 | 0.20 |
| ZrO2 | 249.1 | --- |
| Sample | First Stage | Weight Loss (%) | Second Stage | Weight Loss (%) | Percentage of ZrO2 | ||
|---|---|---|---|---|---|---|---|
| Ti (°C) | T Decomp. (°C) | Ti (°C) | T Decomp. (°C) | ||||
| pCNTs | 426 | 724 | 91 | --- | --- | --- | --- |
| fCNTs | 504 | 707 | 97 | --- | --- | --- | --- |
| ZrO2-fCNTs | 80 | 150 | 10 | --- | --- | --- | 40 |
| 276 | 347 | 2 | 366 | 597 | 40 | ||
| CNT Sample | 2θ (°) | d (Å) | FWHM (°) | Intensity (a.u) |
|---|---|---|---|---|
| Pristine | 25.36 | 3.59 | 3.96 | 384 |
| Functionalized | 25.18 | 3.61 | 3.73 | 885 |
| Reflection Angle (2θ) (°) | Miller Index | |
|---|---|---|
| ZrO2-fCNTs | ZrO2 Cubic Phase | |
| 30.30 | 30.51 | (111) |
| 35.25 | 35.19 | (200) |
| 50.54 | 50.68 | (220) |
| 60.24 | 60.33 | (311) |
| 63.08 | 63.21 | (222) |
| 74.62 | 74.74 | (400) |
| CNT Sample | Diameter (nm) | Distribution of Diameter (nm) |
|---|---|---|
| Pristine | 18 ± 4 | 12–25 |
| Functionalized | 12 ± 2 | 7–16 |
| Modified Electrode | Sensitivity (µA mM−1) | Detection Limit (mM) | Detection Potential (V) | Ref. |
|---|---|---|---|---|
| PB/ZrO2-fCNTs/GC | 91.3 | 0.00359 | +1.0 | this work |
| PB-fCNTs/GC. | 163.01 | 0.015 | 0.00 | [47] |
| HRP-TiO2/fCNTs/GC | 963 | 0.81 | −1.50 | [47] |
| HRP from leaves of Guinea grass/graphene. | 39.93 | 0.15. | −0.65 | [59] |
| CuInS2-graphene/HRP. | 11.2 | 0.047 | −0.2 | [60] |
| Prussian blue nanocubes on reduced graphene oxide. | Not reported | 0.04 | 0.2 | [61] |
| HRP/chitosan-gelatin composite biopolymers nanofibers/graphite electrode. | 44 | 0.05 | −0.30 | [62] |
| Pt/Au | 22.181 | 0.06 | −0.20 | [63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerez-Masaquiza, M.D.; Fernández, L.; González, G.; Montero-Jiménez, M.; Espinoza-Montero, P.J. Electrochemical Sensor Based on Prussian Blue Electrochemically Deposited at ZrO2 Doped Carbon Nanotubes Glassy Carbon Modified Electrode. Nanomaterials 2020, 10, 1328. https://doi.org/10.3390/nano10071328
Jerez-Masaquiza MD, Fernández L, González G, Montero-Jiménez M, Espinoza-Montero PJ. Electrochemical Sensor Based on Prussian Blue Electrochemically Deposited at ZrO2 Doped Carbon Nanotubes Glassy Carbon Modified Electrode. Nanomaterials. 2020; 10(7):1328. https://doi.org/10.3390/nano10071328
Chicago/Turabian StyleJerez-Masaquiza, Marlon Danny, Lenys Fernández, Gema González, Marjorie Montero-Jiménez, and Patricio J. Espinoza-Montero. 2020. "Electrochemical Sensor Based on Prussian Blue Electrochemically Deposited at ZrO2 Doped Carbon Nanotubes Glassy Carbon Modified Electrode" Nanomaterials 10, no. 7: 1328. https://doi.org/10.3390/nano10071328