The Application of Metal–Organic Frameworks and Their Derivatives for Supercapacitors
Abstract
:1. Introduction
2. Pristine MOFs or MOFs Composites Directly Used for SCs
2.1. Modifying MOF Itself
2.2. Constructing MOF/X Composites
2.2.1. X = C
2.2.2. X = Metal and Metal Oxide
2.2.3. X = Others
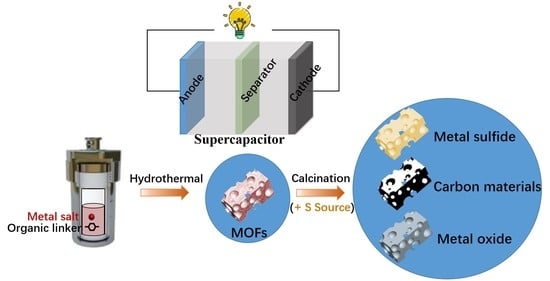
3. MOFs as Precursors for SCs
3.1. MOFs Precursors for Porous Carbon
3.2. MOF Precursors for Metal Oxides/Hydroxides
3.3. MOF Precursors for Metal Sulfide Composites
3.4. MOF Precursors for Metal Phosphide Composites
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Abbreviation | Full Name | Abbreviation | Full Name |
|---|---|---|---|
| NF | Ni foam | CC | carbon cloth |
| AC | activated carbon | CFP | carbon fiber paper |
| NS | nanosheets | HPC | hexahedral porous carbon |
| LDH | layered double hydroxide | PNT | polypyrrole nanotubes |
| CNT | carbon nanotube | NPC | nitrogen-doped carbon |
| NCT | nitrogen-doped carbon tubes | NCP | nitrogen-doped carbon particles |
| GO | graphene oxide | rGO | reduced graphene oxide |
| BTC | 1,3,5-benzenetricarboxylate | PTA | p-benzenedicarboxylic acid |
| 2MI | 2-methylimidazole | PVA | polyvinyl alcohol |
| Tipa | tri(4-imidazolylphenyl)amine | BPDC | 4,4′-biphenyldicarboxylic acid |
| PEDOT | poly(3,4-ethylene dioxythiophene) | HITP | 2,3,6,7,10,11-hexaiminotriphenylene |
| PANI | Polyaniline | CTP-COOH | hexakis(4-carboxylphenoxy)cyclotriphosphazene |
References
- Dang, T.; Zhang, G.; Li, Q.; Cao, Z.; Zhang, G.; Duan, H. Ultrathin hetero-nanosheets assembled hollow Ni-Co-P/C for hybrid supercapacitors with enhanced rate capability and cyclic stability. J. Colloid Interface Sci. 2020, 577, 368–378. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Han, L.; Yuan, R.; Lu, J. NiCo2O4 nanosheets sheathed SiC@CNTs core-shell nanowires for high-performance flexible hybrid supercapacitors. J. Colloid Interface Sci. 2020, 577, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Ren, L.; Hou, Z.; Shao, M. Flexible reduced graphene oxide/prussian blue films for hybrid supercapacitors. Chem. Eng. J. 2020, 397, 125521. [Google Scholar] [CrossRef]
- Wang, P.; Wang, R.; Lang, J.; Zhang, X.; Chen, Z.; Yan, X. Porous niobium nitride as a capacitive anode material for advanced Li-ion hybrid capacitors with superior cycling stability. J. Mater. Chem. A 2016, 4, 9760–9766. [Google Scholar] [CrossRef]
- Lim, E.; Kim, H.; Jo, C.; Chun, J.; Ku, K.; Kim, S.; Lee, H.I.; Nam, I.S.; Yoon, S.; Kang, K.; et al. Advanced hybrid supercapacitor based on a mesoporous niobium pentoxide/carbon as high-performance anode. ACS Nano 2014, 8, 8968–8978. [Google Scholar] [CrossRef]
- Yan, Y.; Li, A.; Lu, C.; Zhai, T.; Lu, S.; Li, W.; Zhou, W. Double-layered yolk-shell microspheres with NiCo2S4-Ni9S8-C hetero-interfaces as advanced battery-type electrode for hybrid supercapacitors. Chem. Eng. J. 2020, 396, 125316. [Google Scholar] [CrossRef]
- Xu, S.; Su, C.; Wang, T.; Ma, Y.; Hu, J.; Hu, J.; Hu, N.; Su, Y.; Zhang, Y.; Yang, Z. One-step electrodeposition of nickel cobalt sulfide nanosheets on Ni nanowire film for hybrid supercapacitor. Electrochim. Acta 2018, 259, 617–625. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Yang, Z.; Wang, T.; Xu, M.; Zhang, L.; Yang, C.; Hu, N.; He, D.; Zhang, Y. A novel Ni@Ni(OH)2 coaxial core-sheath nanowire membrane for electrochemical energy storage electrodes with high volumetric capacity and excellent rate capability. Electrochim. Acta 2015, 182, 464–473. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Yang, Z.; Wang, T.; Jiang, W.; Yang, C.; Wang, S.; Hu, N.; Wei, H.; Zhang, Y. Nanofoaming to Boost the Electrochemical Performance of Ni@Ni(OH)2 Nanowires for Ultrahigh Volumetric Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 27868–27876. [Google Scholar] [CrossRef]
- Hong, M.; Zhou, C.; Xu, S.; Ye, X.; Yang, Z.; Zhang, L.; Zhou, Z.; Hu, N.; Zhang, Y. Bi-metal organic framework nanosheets assembled on nickel wire films for volumetric-energy-dense supercapacitors. J. Power Sources 2019, 423, 80–89. [Google Scholar] [CrossRef]
- Zong, S.; Zhang, Y.; Lu, N.; Ma, P.; Wang, J.; Shi, X.-R. A DFT Screening of M-HKUST-1 MOFs for Nitrogen-Containing Compounds Adsorption. Nanomaterials 2018, 8, 958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, S.; Huang, S.; Shi, X.-R.; Sun, C.; Xu, S.; Ma, P.; Wang, J. Impact of linker functionalization on the adsorption of nitrogen-containing compounds in HKUST-1. Dalton Trans. 2020, 49, 12610–12621. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, W.; Jiang, H.; Lin, R.; Wang, Z.; Wu, J.; He, G.; Shearing, P.R.; Brett, D.J.L. ZIF-8-Derived Hollow Carbon for Efficient Adsorption of Antibiotics. Nanomaterials 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.M.; Yi, J.W.; Li, S.; Jiang, C.; Wei, J.H.; Wu, Y.P.; Zhao, J.; Li, D.S. Stable Bimetal-MOF Ultrathin Nanosheets for Pseudocapacitors with Enhanced Performance. Inorg. Chem. 2019, 58, 9543–9547. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Zhang, H.; Mei, H.; Sun, D. Recent progress in metal-organic framework-based supercapacitor electrode materials. Coord. Chem. Rev. 2020, 420, 213438. [Google Scholar] [CrossRef]
- Zhang, K.; Kirlikovali, K.O.; Le, Q.V.; Jin, Z.; Varma, R.S.; Jang, H.W.; Farha, O.K.; Shokouhimehr, M. Extended Metal-Organic Frameworks on Diverse Supports as Electrode Nanomaterials for Electrochemical Energy Storage. ACS Appl. Nano Mater. 2020, 3, 3964–3990. [Google Scholar] [CrossRef]
- Meng, J.; Liu, X.; Niu, C.; Pang, Q.; Li, J.; Liu, F.; Liu, Z.; Mai, L. Advances in metal-organic framework coatings: Versatile synthesis and broad applications. Chem. Soc. Rev. 2020, 49, 3142–3186. [Google Scholar] [CrossRef]
- Rajak, R.; Kumar, R.; Ansari, S.N.; Saraf, M.; Mobin, S.M. Recent highlights and future prospects on mixed-metal MOFs as emerging contestants for supercapacitors. Dalton Trans. 2020, 49, 11792–11818. [Google Scholar] [CrossRef]
- Jafari, H.; Mohammadnezhad, P.; Khalaj, Z.; Naderi, H.R.; Kohan, E.; Milani Hosseini, M.R.; Shiralizadeh Dezfuli, A. Terbium metal-organic frameworks as capable electrodes for supercapacitors. New J. Chem. 2020, 44, 11615–11621. [Google Scholar] [CrossRef]
- He, H.; Wang, G.; Shen, B.; Wang, Y.; Lu, Z.; Guo, S.; Zhang, J.; Yang, L.; Jiang, Q.; Xiao, Z. Three isostructural Zn/Ni nitro-containing metal-organic frameworks for supercapacitor. J. Solid State Chem. 2020, 288, 121375. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Xiong, Y.; Cheng, D.; Zeng, Y.; Bu, Y. Fabrication of 3D Co-doped Ni-based MOF hierarchical micro-flowers as a high-performance electrode material for supercapacitors. Appl. Surf. Sci. 2019, 483, 1158–1165. [Google Scholar] [CrossRef]
- Ehsani, A.; Bigdeloo, M.; Assefi, F.; Kiamehr, M.; Alizadeh, R. Ternary nanocomposite of conductive polymer/chitosan biopolymer/metal organic framework: Synthesis, characterization and electrochemical performance as effective electrode materials in pseudocapacitors. Inorg. Chem. Commun. 2020, 115, 107885. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bi, R.; Mao, F.; Wang, K.; Wu, H.; Wang, X. A polythreaded MnII-MOF and its super-performances for dye adsorption and supercapacitors. Inorg. Chem. Front. 2020, 7, 718–730. [Google Scholar] [CrossRef]
- Yang, R.X.; Lan, H.M.; Zhu, P.Y.; Yang, L.Z.; Yu, Y.M.; Wang, L.L.; Wang, D.Z. Synthesis, structures, magnetic and electric properties of four new coordination polymers constructed with heterocyclic nitrogen ligands and multidentate organic acid. Inorg. Chim. Acta 2020, 506, 119410. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, X.; Gong, L.; Wang, C.; Wang, C.; Yu, K.; Zhou, B. Coral-like {SiW10Mn2}-based Mn-MOFs: Facile fabrication with high electrochemical capacitor performance. J. Solid State Chem. 2020, 288, 121409. [Google Scholar] [CrossRef]
- Yue, L.; Wang, X.; Sun, T.; Liu, H.; Li, Q.; Wu, N.; Guo, H.; Yang, W. Ni-MOF coating MoS2 structures by hydrothermal intercalation as high-performance electrodes for asymmetric supercapacitors. Chem. Eng. J. 2019, 375, 121959. [Google Scholar] [CrossRef]
- Patterson, N.; Xiao, B.; Ignaszak, A. Polypyrrole decorated metal-organic frameworks for supercapacitor devices. RSC Adv. 2020, 10, 20162–20172. [Google Scholar] [CrossRef]
- Radhika, M.G.; Gopalakrishna, B.; Chaitra, K.; Bhatta, L.K.G.; Venkatesh, K.; Sudha Kamath, M.K.; Kathyayini, N. Electrochemical studies on Ni, Co & Ni/Co-MOFs for high-performance hybrid supercapacitors. Mater. Res. Express 2020, 7, 054003. [Google Scholar] [CrossRef]
- Hong, J.H.; Jung, Y.; Kim, S. Synthesis of Bi-Metallic Organic Frameworks and Their Capacitive Behaviors According to Metal Mixing Ratio. J. Nanosci. Nanotechnol. 2020, 20, 2987–2991. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Ji, X.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; Ren, Y.; He, Y. Nickel/cobalt bimetallic metal-organic frameworks ultrathin nanosheets with enhanced performance for supercapacitors. J. Alloys Compd. 2020, 825, 154069. [Google Scholar] [CrossRef]
- Zhao, S.; Zeng, L.; Cheng, G.; Yu, L.; Zeng, H. Ni/Co-based metal-organic frameworks as electrode material for high performance supercapacitors. Chin. Chem. Lett. 2019, 30, 605–609. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, G.Y.; Qin, J.S.; Yu, J. Discrete nanographene implanted in zirconium metal-organic framework for electrochemical energy storage. J. Solid State Chem. 2020, 287, 121377. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, N.; Yang, S.; Fan, Q.; Lei, D.; Liu, A.; Chen, X. Shape-controlled synthesis of Ni-based metal-organic frameworks with albizia flower-like spheres@nanosheets structure for high performance supercapacitors. J. Colloid Interface Sci. 2020, 575, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Safy, M.E.A.; Haikal, R.R.; Elshazly, B.; Hamdy, A.; Ali, F.; Maarouf, A.A.; Alkordi, M.H. Charge percolation in metal-organic framework (HKUST-1)‒graphene nanocomposites. Appl. Mater. Today 2020, 19, 100604. [Google Scholar] [CrossRef]
- Yang, J.; Li, P.; Wang, L.; Guo, X.; Guo, J.; Liu, S. In-situ synthesis of Ni-MOF@CNT on graphene/Ni foam substrate as a novel self-supporting hybrid structure for all-solid-state supercapacitors with a high energy density. J. Electroanal. Chem. 2019, 848, 113301. [Google Scholar] [CrossRef]
- Xu, S.; Liu, R.; Shi, X.; Ma, Y.; Hong, M.; Chen, X.; Wang, T.; Li, F.; Hu, N.; Yang, Z. A dual CoNi MOF nanosheet/nanotube assembled on carbon cloth for high performance hybrid supercapacitors. Electrochim. Acta 2020, 342, 136124. [Google Scholar] [CrossRef]
- Srimuk, P.; Luanwuthi, S.; Krittayavathananon, A.; Sawangphruk, M. Solid-type supercapacitor of reduced graphene oxide-metal organic framework composite coated on carbon fiber paper. Electrochim. Acta 2015, 157, 69–77. [Google Scholar] [CrossRef]
- Saraf, M.; Rajak, R.; Mobin, S.M. A fascinating multitasking Cu-MOF/rGO hybrid for high performance supercapacitors and highly sensitive and selective electrochemical nitrite sensors. J. Mater. Chem. A 2016, 4, 16432–16445. [Google Scholar] [CrossRef]
- He, F.; Yang, N.; Li, K.; Wang, X.; Cong, S.; Zhang, L.; Xiong, S.; Zhou, A. Hydrothermal synthesis of Ni-based metal organic frameworks/graphene oxide composites as supercapacitor electrode materials. J. Mater. Res. 2020, 35, 1439–1450. [Google Scholar] [CrossRef]
- Rajpurohit, A.S.; Punde, N.S.; Srivastava, A.K. A dual metal organic framework based on copper-iron clusters integrated sulphur doped graphene as a porous material for supercapacitor with remarkable performance characteristics. J. Colloid Interface Sci. 2019, 553, 328–340. [Google Scholar] [CrossRef]
- Kumaraguru, S.; Yesuraj, J.; Mohan, S. Reduced graphene oxide-wrapped micro-rod like Ni/Co organic-inorganic hybrid nanocomposite as an electrode material for high-performance supercapacitor. Compos. Part B Eng. 2020, 185, 107767. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Chen, Y.; Wang, C.; Guo, L. NiCo-MOF nanosheets wrapping polypyrrole nanotubes for high-. Appl. Surf. Sci. 2019, 507, 145089. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wang, H.; Zhao, P.; Hou, H.; Guo, L. Acetylene black enhancing the electrochemical performance of NiCo-MOF nanosheets for supercapacitor electrodes. Appl. Surf. Sci. 2019, 492, 455–463. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yang, S.Y.; Yue, Y.; Bian, S.W. Conductive copper-based metal-organic framework nanowire arrays grown on graphene fibers for flexible all-solid-state supercapacitors. J. Alloys Compd. 2020, 835, 155238. [Google Scholar] [CrossRef]
- Xiong, S.; Jiang, S.; Wang, J.; Lin, H.; Lin, M.; Weng, S.; Liu, S.; Jiao, Y.; Xu, Y.; Chen, J. A high-performance hybrid supercapacitor with NiO derived NiO@Ni-MOF composite electrodes. Electrochim. Acta 2020, 340, 135956. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Q.; Zeng, Y.; Cheng, D.; Xiong, Y.; Bu, Y. Rational construction of triangle-like nickel-cobalt bimetallic metal-organic framework nanosheets arrays as battery-type electrodes for hybrid supercapacitors. J. Colloid Interface Sci. 2019, 555, 42–52. [Google Scholar] [CrossRef]
- Jiang, J.; Sun, Y.; Chen, Y.; Zhou, Q.; Rong, H.; Hu, X.; Chen, H.; Zhu, L.; Han, S. Design and fabrication of metal-organic frameworks nanosheet arrays constructed by interconnected nanohoneycomb-like nickel-cobalt oxide for high energy density asymmetric supercapacitors. Electrochim. Acta 2020, 342, 136077. [Google Scholar] [CrossRef]
- Shinde, P.A.; Seo, Y.; Lee, S.; Kim, H.; Pham, Q.N.; Won, Y.; Chan, J.S. Layered manganese metal-organic framework with high specific and areal capacitance for hybrid supercapacitors. Chem. Eng. J. 2020, 387, 122982. [Google Scholar] [CrossRef]
- Sun, P.P.; Zhang, Y.H.; Yu, X.; Shi, Q.; Tian, B.; Gao, J.; Shi, F.N. Cu powder decorated 3D Mn-MOF with excellent electrochemical properties for supercapacitors. Inorg. Chim. Acta 2020, 508, 119629. [Google Scholar] [CrossRef]
- Shrivastav, V.; Sundriyal, S.; Kaur, A.; Tiwari, U.K.; Mishra, S.; Deep, A. Conductive and porous ZIF-67/PEDOT hybrid composite as superior electrode for all-solid-state symmetrical supercapacitors. J. Alloys Compd. 2020, 843, 155992. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Farid, S.; Afzal, A.M. Co-MOF/polyaniline-based electrode material for high performance supercapattery devices. Electrochim. Acta 2020, 346, 136039. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.-J.; Chung, S.; Kim, S. Preparation and Capacitance of Ni Metal Organic Framework/Reduced Graphene Oxide Composites for Supercapacitors as Nanoarchitectonics. J. Nanosci. Nanotechnol. 2020, 20, 2750–2754. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, N.; Zhang, J.; Yan, R.; Li, J.; Wang, L.; Wang, N.; Lv, M.; Zhang, M. Ultrasensitive aptamer-based protein assays based on one-dimensional core-shell nanozymes. Biosens. Bioelectron. 2020, 150, 111881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, N.; Peng, H.; Li, J.; Yan, R.; Shi, X.; Ma, P.; Lv, M.; Wang, L.; Tang, Z.; et al. Multi-triggered and enzyme-mimicking graphene oxide/polyvinyl alcohol/G-quartet supramolecular hydrogels. Nanoscale 2020, 12, 5186–5195. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Zhang, M.; Ding, L.; Zheng, J.; Zeng, C.; Wen, Y.; Liu, G.; Aldalbahi, A.; Shi, J.; Song, S.; et al. Yolk–shell nanostructured Fe3O4@C magnetic nanoparticles with enhanced peroxidase-like activity for label-free colorimetric detection of H2O2 and glucose. Nanoscale 2017, 9, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Zhao, C.; Luo, D.; Wang, K.; Wang, F. Synthesis of copper benzene-1, 3, 5-tricarboxylate metal organic frameworks with mixed phases as the electrode material for supercapacitor applications. Appl. Surf. Sci. 2018, 460, 33–39. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Zhao, S.; Chen, H.; Tao, K.; Han, L. Solvent-Controlled Morphology of Amino-Functionalized Bimetal Metal–Organic Frameworks for Asymmetric Supercapacitors. Inorg. Chem. 2020, 59, 11385–11395. [Google Scholar] [CrossRef]
- Du, W.; Bai, Y.; Yang, Z.; Li, R.; Zhang, D.; Ma, Z.; Yuan, A.; Xu, J. A conductive anionic Co-MOF cage with zeolite framework for supercapacitors. Chin. Chem. Lett. 2020, 31, 2309–2313. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Ren, Z.; Li, C.; Chu, Y.; Wang, Z.; Zhang, M.; Wu, H.; Zhang, Q. 2D Metal–Organic Frameworks (MOFs) for High-Performance BatCap Hybrid Devices. Small 2020, 16, 2001987. [Google Scholar] [CrossRef]
- Wang, M.; Shi, H.; Zhang, P.; Liao, Z.; Wang, M.; Zhong, H.; Schwotzer, F.; Nia, A.S.; Zschech, E.; Zhou, S.; et al. Phthalocyanine-Based 2D Conjugated Metal-Organic Framework Nanosheets for High-Performance Micro-Supercapacitors. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Deng, T.; Shi, X.; Zhang, W.; Wang, Z.; Zheng, W. In-plane Assembly of Distinctive 2D MOFs with Optimum Supercapacitive Performance. iScience 2020, 23, 101220. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wen, H.; Sun, X.; Guan, X.; Zhang, J.; Tian, W.; Feng, H.; Wang, H.; Yao, Y. Ultrathin Mn doped Ni-MOF nanosheet array for highly capacitive and stable asymmetric supercapacitor. Chem. A Eur. J. 2020, 202003220. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Wu, M.; Wang, S.C.; Chen, C.; Xiong, D.; Yi, F.Y. Morphology control of nanoscale metal-organic frameworks for high-performance supercapacitors. Electrochim. Acta 2020, 343, 135617. [Google Scholar] [CrossRef]
- Li, Q.; Dai, Z.; Wu, J.; Liu, W.; Di, T.; Jiang, R.; Zheng, X.; Wang, W.; Ji, X.; Li, P.; et al. Fabrication of Ordered Macro-Microporous Single-Crystalline MOF and Its Derivative Carbon Material for Supercapacitor. Adv. Energy Mater. 2020, 1903750. [Google Scholar] [CrossRef]
- Yao, M.; Ji, D.; Chen, Y.; Wang, Z.; Dong, J.; Zhang, Q.; Ramakrishna, S.; Zhao, X. Boosting storage properties of reduced graphene oxide fiber modified with MOFs-derived porous carbon through a wet-spinning fiber strategy. Nanotechnology 2020, 31, 395603. [Google Scholar] [CrossRef]
- Zhou, P.; Wan, J.; Wang, X.; Xu, K.; Gong, Y.; Chen, L. Nickel and cobalt metal-organic-frameworks-derived hollow microspheres porous carbon assembled from nanorods and nanospheres for outstanding supercapacitors. J. Colloid Interface Sci. 2020, 575, 96–107. [Google Scholar] [CrossRef]
- Yang, C.; Liu, D.; Huang, S.; Lei, W. Pressure-induced monolithic carbon aerogel from metal-organic framework. Energy Storage Mater. 2020, 28, 393–400. [Google Scholar] [CrossRef]
- Yang, H.X.; Zhao, D.L.; Meng, W.J.; Zhao, M.; Duan, Y.J.; Han, X.Y.; Tian, X.M. Nickel nanoparticles incorporated into N-doped porous carbon derived from N-containing nickel-MOF for high-performance supercapacitors. J. Alloys Compd. 2019, 782, 905–914. [Google Scholar] [CrossRef]
- Zhang, J.; Li, W.; Ahmed, T.; Sun, J.; Jia, C.; Zhao, Y.; Cui, Y. Hierarchical porous carbon foam supported on carbon cloth as high-performance anodes for aqueous supercapacitors. J. Power Sources 2019, 439, 227066. [Google Scholar] [CrossRef]
- Shrivastav, V.; Sundriyal, S.; Kim, K.; Sinha, R.K.; Tiwari, U.K.; Deep, A. Metal-organic frameworks-derived titanium dioxide–carbon nanocomposite for supercapacitor applications. Int. J. Energy Res. 2020, 44, 6269–6284. [Google Scholar] [CrossRef]
- Eswaramoorthi, T.; Ganesan, S.; Marimuthu, M.; Santhosh, K. Thin niobium and iron–graphene oxide composite metal–organic framework electrodes for high performance supercapacitors. New J. Chem. 2020, 44, 12664–12673. [Google Scholar] [CrossRef]
- Xuan, X.; Qian, M.; Han, L.; Wan, L.; Li, Y.; Lu, T.; Pan, L.; Niu, Y.; Gong, S. In-situ growth of hollow NiCo layered double hydroxide on carbon substrate for flexible supercapacitor. Electrochim. Acta 2019, 321, 134710. [Google Scholar] [CrossRef]
- Van Ngo, T.; Moussa, M.; Tung, T.T.; Coghlan, C.; Losic, D. Hybridization of MOFs and graphene: A new strategy for the synthesis of porous 3D carbon composites for high performing supercapacitors. Electrochim. Acta 2020, 329, 135104. [Google Scholar] [CrossRef]
- Govindan, R.; Hong, X.J.; Sathishkumar, P.; Cai, Y.P.; Gu, F.L. Construction of metal-organic framework-derived CeO2/C integrated MoS2 hybrid for high-performance asymmetric supercapacitor. Electrochim. Acta 2020, 353, 136502. [Google Scholar] [CrossRef]
- Wu, J.; Wei, F.; Sui, Y.; Qi, J.; Zhang, X. Interconnected NiS-nanosheets@porous carbon derived from Zeolitic-imidazolate frameworks (ZIFs) as electrode materials for high-performance hybrid supercapacitors. Int. J. Hydrogen Energy 2020, 45, 19237–19245. [Google Scholar] [CrossRef]
- Xu, X.; Yang, T.; Zhang, Q.; Xia, W.; Ding, Z.; Eid, K.; Abdullah, A.M.; Shahriar, A.; Hossain, M.; Zhang, S.; et al. Ultrahigh capacitive deionization performance by 3D interconnected MOF-derived nitrogen-doped carbon tubes. Chem. Eng. J. 2020, 390, 124493. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, L.; Li, L.; Ma, X.; Yang, Y.; Li, Z.; Zhang, Z. Integration of MnO2 and ZIF-Derived nanoporous carbon on nickel foam as an electrode for high-performance supercapacitors. Ceram. Int. 2020, 46, 21033–21038. [Google Scholar] [CrossRef]
- Shi, X.; Yu, J.; Huang, J.; Chen, B.; Fang, L.; Shao, L.; Sun, Z. Metal-organic framework derived high-content N, P and O-codoped Co/C composites as electrode materials for high performance supercapacitors. J. Power Sources 2020, 467, 228304. [Google Scholar] [CrossRef]
- Shi, X.; Yu, J.; Liu, Q.; Shao, L.; Zhang, Y.; Sun, Z.; Huang, H. Metal-Organic-Framework-Derived N-, P-, and O-Codoped Nickel/Carbon Composites Homogeneously Decorated on Reduced Graphene Oxide for Energy Storage. ACS Appl. Nano Mater. 2020, 3, 5625–5636. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, J.; Wang, Y.; Li, S.; Jin, P.; Chen, Y. Facile synthesis of manganese oxide nanostructures with different crystallographic phase and morphology for supercapacitors. J. Alloys Compd. 2020, 830, 154524. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Yang, H.; Sun, Y.; Hu, C.; Huang, Y. Flexible asymmetric micro-supercapacitors based on Bi2O3 and MnO2 nanoflowers: Larger areal mass promises higher energy density. Adv. Energy Mater. 2015, 5, 5. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Sohn, Y.; Shin, W.G. Electrochemical performance of facile developed aqueous asymmetric (Fe,Cr)2O3//MnO2 supercapacitor. Electrochim. Acta 2018, 285, 381–392. [Google Scholar] [CrossRef]
- Ma, Z.; Jing, F.; Fan, Y.; Hou, L.; Su, L.; Fan, L.; Shao, G. High-Stability MnOx Nanowires@C@MnOx Nanosheet Core–Shell Heterostructure Pseudocapacitance Electrode Based on Reversible Phase Transition Mechanism. Small 2019, 15, 1900862. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.S.; Aslam, M.K.; Asim, S.; Batool, S.; Idrees, M.; Hussain, S.; Shah, S.S.A.; Saleem, M.; Mai, W.; Hu, C. High-performance flexible hybrid-supercapacitor enabled by pairing binder-free ultrathin Ni–Co–O nanosheets and metal-organic framework derived N-doped carbon nanosheets. Electrochim. Acta 2020, 349, 136384. [Google Scholar] [CrossRef]
- Yang, K.; Yan, Y.; Chen, W.; Zeng, D.; Ma, C.; Han, Y.; Zhang, W.; Kang, H.; Wen, Y.; Yang, Y. Yolk-shell bimetallic metal-organic frameworks derived multilayer core-shells NiCo2O4/NiO structure spheres for high-performance supercapacitor. J. Electroanal. Chem. 2019, 851, 113445. [Google Scholar] [CrossRef]
- Raphael, E.E.; Dong, L.; Wang, J.; Wang, L.; Yan, W.; Zhang, J. MOF-deviated zinc-nickel–cobalt ZIF-67 electrode material for high-performance symmetrical coin-shaped supercapacitors. J. Colloid Interface Sci. 2020, 574, 140–151. [Google Scholar] [CrossRef]
- Devi, B.; Jain, A.; Roy, B.; Rao, R.B.; Tummuru, N.R.; Halder, A.; Koner, R.R. Cobalt-Embedded N-Doped Carbon Nanostructures for Oxygen Reduction and Supercapacitor Applications. ACS Appl. Nano Mater. 2020, 3, 6354–6366. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, S.; Chen, P.; Cheng, G.; Wu, G.; Li, H.; Liu, Y. Nickel nanoparticles embedded in porous carbon nanofibers and its electrochemical properties. Nanotechnology 2020, 31, 305705. [Google Scholar] [CrossRef]
- Li, J.; Cao, W.; Zhou, N.; Xu, F.; Chen, N.; Liu, Y.; Du, G. Hierarchically nanostructured Ni(OH)2–MnO2@C ternary composites derived from Ni-MOFs grown on nickel foam as high-performance integrated electrodes for hybrid supercapacitors. Electrochim. Acta 2020, 343, 136139. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Z.; Guo, Y.; Guo, D.; Liu, G. Block copolymer derived uniform mesopores enable ultrafast electron and ion transport at high mass loadings. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Yao, W.; Zhang, L.; Wang, M.; Fan, T.; Yang, W.; Yang, W. Spear-shaped Mn/Ni bimetallic hydroxide derived from metal-organic frameworks as electrode materials for aqueous and all-solid-state hybrid supercapacitors. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 601, 125011. [Google Scholar] [CrossRef]
- Luo, X.; Zhong, M.; He, P.; Shao, J.; Wang, Q.; Li, K.; Zhao, W. Transformation of 2D Co-LDH into 3D hierarchical hollow Co3O4 polyhedral arrays with enhanced electrochemical performance for supercapacitors. J. Alloys Compd. 2020, 826, 154241. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Jiang, D.; Xie, F.; Xie, K.; Wang, Y. Sulfur-doped ZIF-derived Co3O4 flower-like microstructures for high stability supercapacitors. J. Alloys Compd. 2020, 831, 154772. [Google Scholar] [CrossRef]
- Mukhiya, T.; Ojha, G.P.; Dahal, B.; Kim, T.; Chhetri, K.; Lee, M.; Chae, S.-H.; Muthurasu, A.; Tiwari, A.P.; Kim, H.Y. Designed Assembly of Porous Cobalt Oxide/Carbon Nanotentacles on Electrospun Hollow Carbon Nanofibers Network for Supercapacitor. ACS Appl. Energy Mater. 2020, 3, 3435–3444. [Google Scholar] [CrossRef]
- Bao, Y.; Deng, Y.; Wang, M.; Xiao, Z.; Wang, M.; Fu, Y.; Guo, Z.; Yang, Y.; Wang, L. A controllable top-down etching and in-situ oxidizing strategy: Metal-organic frameworks derived α-Co/Ni(OH)2@Co3O4 hollow nanocages for enhanced supercapacitor performance. Appl. Surf. Sci. 2020, 504, 144395. [Google Scholar] [CrossRef]
- Gong, L.T.; Xu, M.; Ma, R.P.; Han, Y.P.; Xu, H.B.; Shi, G. High-performance supercapacitor based on MOF derived porous NiCo2O4 nanoparticle. Sci. China Technol. Sci. 2020, 63, 1470–1477. [Google Scholar] [CrossRef]
- Meng, X.X.; Li, J.Y.; Yang, B.L.; Li, Z.X. MOF-derived NiO nanoparticles prilled by controllable explosion of perchlorate ion: Excellent performances and practical applications in supercapacitors. Appl. Surf. Sci. 2020, 507, 145077. [Google Scholar] [CrossRef]
- Lan, M.; Wang, X.; Zhao, R.; Dong, M.; Fang, L.; Wang, L. Metal-organic framework-derived porous MnNi2O4 microflower as an advanced electrode material for high-performance supercapacitors. J. Alloys Compd. 2020, 821, 153546. [Google Scholar] [CrossRef]
- Shen, J.; Wang, P.; Jiang, H.; Wang, H.; Pollet, B.G.; Wang, R.; Ji, S. MOF derived graphitic carbon nitride/oxygen vacancies-rich zinc oxide nanocomposites with enhanced supercapacitive performance. Ionics 2020, 26, 1–11. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, P.; Zhang, J.; Qi, H.; Liu, J.; Li, B.; Sun, X.; Zhang, Q.; Wei, C.; Wang, L. Pillar-Coordinated Strategy to Modulate Phase Transfer of α-Ni(OH)2 for Enhanced Supercapacitor Application. ACS Appl. Energy Mater. 2020, 3, 5628–5636. [Google Scholar] [CrossRef]
- Chu, D.; Li, F.; Song, X.; Ma, H.; Tan, L.; Pang, H.; Wang, X.; Guo, D.; Xiao, B. A novel dual-tasking hollow cube NiFe2O4-NiCo-LDH@rGO hierarchical material for high preformance supercapacitor and glucose sensor. J. Colloid Interface Sci. 2020, 568, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; He, Q.; Wang, Y.; Wang, J.; Xiang, Y.; Blackwood, D.J.; Wu, R.; Chen, J.S. MOF-reinforced Co9S8 self-supported nanowire arrays for highly durable and flexible supercapacitor. Electrochim. Acta 2020, 346, 136201. [Google Scholar] [CrossRef]
- Zheng, L.; Song, J.; Ye, X.; Wang, Y.; Shi, X.; Zheng, H. Construction of self-supported hierarchical NiCo-S nanosheet arrays for supercapacitors with ultrahigh specific capacitance. Nanoscale 2020, 12, 13811–13821. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, J.; Zhang, L.; Cheng, B.; Yu, J. Construction of nickel cobalt sulfide nanosheet arrays on carbon cloth for performance-enhanced supercapacitor. J. Mater. Sci. Technol. 2020, 47, 113–121. [Google Scholar] [CrossRef]
- Xin, N.; Liu, Y.; Niu, H.; Bai, H.; Shi, W. In-situ construction of metal organic frameworks derived Co/Zn–S sandwiched graphene film as free-standing electrodes for ultra-high energy density supercapacitors. J. Power Sources 2020, 451, 227772. [Google Scholar] [CrossRef]
- Jia, H.; Wang, J.; Fu, W.; Hu, J.; Liu, Y. In-situ MOFs-derived hollow Co9S8 polyhedron welding on the top of MnCo2S4 nanoneedles for high performance hybrid supercapacitors. Chem. Eng. J. 2019, 391, 123541. [Google Scholar] [CrossRef]
- Yang, W.; Guo, H.; Yue, L.; Li, Q.; Xu, M.; Zhang, L.; Fan, T.; Yang, W. Metal-organic frameworks derived MMoSx (M = Ni, Co and Ni/Co) composites as electrode materials for supercapacitor. J. Alloys Compd. 2020, 834, 154118. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, J.; Li, Q.; Zhao, S.; Yu, X.; Tao, K.; Hu, Y.; Han, L. MOF-assisted construction of a Co9S8@Ni3S2/ZnS microplate array with ultrahigh areal specific capacity for advanced supercapattery. Dalton Trans. 2020, 49, 10535–10544. [Google Scholar] [CrossRef]
- Ji, F.; Jiang, D.; Chen, X.; Pan, X.; Kuang, L.; Zhang, Y.; Alameh, K.; Ding, B. Simple in-situ growth of layered Ni3S2 thin film electrode for the development of high-performance supercapacitors. Appl. Surf. Sci. 2017, 399, 432–439. [Google Scholar] [CrossRef]
- Hou, S.; Lian, Y.; Bai, Y.; Zhou, Q.; Ban, C.; Wang, Z.; Zhao, J.; Zhang, H. Hollow dodecahedral Co3S4@NiO derived from ZIF-67 for supercapacitor. Electrochim. Acta 2020, 341, 136053. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, S.; Ding, Z.; Zhu, G.; Tang, H.; Hou, Y.; Lu, T.; Pan, L. Carbon wrapped CoP hollow spheres for high performance hybrid supercapacitor. J. Alloys Compd. 2020, 822, 153578. [Google Scholar] [CrossRef]
- Gu, J.; Sun, L.; Zhang, Y.; Zhang, Q.; Li, X.; Si, H.; Shi, Y.; Sun, C.; Gong, Y.; Zhang, Y. MOF-derived Ni-doped CoP@C grown on CNTs for high-performance supercapacitors. Chem. Eng. J. 2020, 385, 123454. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, S.; Weng, S.; Wang, J.; Wang, J.; Lin, H.; Jiao, Y.; Chen, J. Rationally designed Ni2P/Ni/C as a positive electrode for high-performance hybrid supercapacitors. New J. Chem. 2020, 44, 6810–6817. [Google Scholar] [CrossRef]
- Mohammadi, Z.A.; Hosseiny, D.S.S. Ultra-high energy density supercapacitors based on metal-organic framework derived yolk-shell Cu-Co-P hollow nanospheres and CuFeS2 nanosheet arrays. Dalton Trans. 2020, 49, 3353–3364. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, S.; Han, Y.; Wei, Z.; Cheng, Y.; Yin, S.; Cui, W. High-Energy-Density Asymmetric Supercapacitor Based on a Durable and Stable Manganese Molybdate Nanostructure Electrode for Energy Storage Systems. ACS Appl. Energy Mater. 2020, 3, 5393–5404. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. Asymmetric capacitor based on superior porous Ni-Zn-Co oxide/hydroxide and carbon electrodes. J. Power Sources 2010, 195, 3017–3024. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Jiang, H.; Zhang, X.; Xu, Q. Metal-organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Pang, R.; Ren, X.; Li, S. Synergetic role of charge transfer and strain engineering in improving the catalysis of Pd single-atom-thick motifs stabilized on a defect-free MoS2/Ag(Au)(111) heterostructure. J. Mater. Chem. A 2020, 8, 17238–17247. [Google Scholar] [CrossRef]
- Shi, X.-R.; Huang, S.; Huang, Y.; Zhang, Y.; Zong, S.; Xu, S.; Chen, Y.; Ma, P. Atomic structures and electronic properties of Ni or N modified Cu/diamond interface. J. Phys. Condens. Matter. 2020, 32, 225001. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, J.; Wang, C.; Qi, Y. The origin of the two-plateaued or one-plateaued open circuit voltage in Li–S batteries. Nano Energy 2020, 75, 104915. [Google Scholar] [CrossRef]
- Ouyang, R.; Curtarolo, S.; Ahmetcik, E.; Scheffler, M.; Ghiringhelli, L.M. SISSO: A compressed-sensing method for identifying the best low-dimensional descriptor in an immensity of offered candidates. Phys. Rev. Mater. 2018, 2, 083802. [Google Scholar] [CrossRef]
- Ouyang, R.; Ahmetcik, E.; Carbogno, C.; Scheffler, M.; Ghiringhelli, L.M. Simultaneous learning of several materials properties from incomplete databases with multi-task SISSO. J. Phys. Mater. 2019, 2, 024002. [Google Scholar] [CrossRef]
- Bartel, C.J.; Millican, S.L.; Deml, A.M.; Rumptz, J.R.; Tumas, W.; Weimer, A.W.; Lany, S.; Stevanović, V.; Musgrave, C.B.; Holder, A.M. Physical descriptor for the Gibbs energy of inorganic crystalline solids and temperature-dependent materials chemistry. Nat. Commun. 2018, 9, 4168. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Hong, W.; Li, P.; Wang, L.; Chen, G. Metal-organic framework derived Ni/NiO micro-particles with subtle lattice distortions for high-performance electrocatalyst and supercapacitor. Appl. Catal. B Environ. 2019, 244, 732–739. [Google Scholar] [CrossRef]
- Dai, S.; Han, F.; Tang, J.; Tang, W. MOF-derived Co3O4 nanosheets rich in oxygen vacancies for efficient all-solid-state symmetric supercapacitors. Electrochim. Acta 2019, 328, 135103. [Google Scholar] [CrossRef]
- Mofarah, S.S.; Adabifiroozjaei, E.; Yao, Y.; Koshy, P.; Lim, S.; Webster, R.; Liu, X.; Nekouei, R.K.; Cazorla, C.; Liu, Z.; et al. Proton-assisted creation of controllable volumetric oxygen vacancies in ultrathin CeO2−x for pseudocapacitive energy storage applications. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]







| MOFs | Electrolyte | Morphology | Cap. | CD | CR(CN) | Ele. 1 | ED@PD | Reference |
|---|---|---|---|---|---|---|---|---|
| Ni-BTC | 6 M KOH | rod and particles | 847.3 | 1 | [52] | |||
| Ni-BTC/rGO | 6 M KOH | rod and particles | 1154.4 | 1 | 90% (3000) | [52] | ||
| NiCo-BTC (Ni:Co ~ 3:2) | 1 M KOH | rod-like | 565 | 1 | 94% (5000) | [41] | ||
| NiCo-BTC/rGO (Ni:Co ~3:2) | 1 M KOH | rod-like | 958 | 1 | 109% (5000) | [41] | ||
| NiCo-BTC (Ni:Co = 2.75:0.25) | 3 M KOH | PS: 2 | 1067 | 1 | 68.4% (2500) | [31] | ||
| NiO@Ni-BTC/NF | 3 M KOH | cage-shape | 1853 5 | 1 7 | 94% (3000) SC 8 | p//CNT | 39.2@700 | [45] |
| CuFe-BTC/S-GNS | SA: 568.75; PS: 9.71 | 1164.3 | 0.5 | 92.5% (10,000) | [40] | |||
| {SiW10Mn2}@Mn-BTC | 1 M Na2SO4 | SA: 16.44; PS: 3.94 | 211 | 1 | 96% (5000) | p//n | [email protected] | [25] |
| Ni-BTC and TPA | 6 M KOH | SA: 64.8 | 920 | 1 | 80% (3000) | p//AC | [33] | |
| NiCo-PTA (Ni:Co of 2:1) | 2 M KOH | Flower-like SA: 0.254 | 1300 | 1 | 71.0% (3000) | [21] | ||
| CoNi-PTA/CC | 1 M KOH | crystal: 200 nm | 0.846 6 | 1 7 | 96.5% (10,000) | p//gCNT | [email protected] | [36] |
| NiCo-PTA@PNTs | 2 M KOH | SA: 66.5; PS: 2~5 | 1109 | 0.5 | 79.1% (10,000) | p//AC | 41.2@375 | [34] |
| NiCo-PTA/NF (Ni:Co = 3:2) | 6M KOH | SA: 22; PS: 2.2 | 2230 | 0.5 | 75.2% (6000) | p//AC | 34.3@375 | [46] |
| MoS2@Ni-PTA | 3 M KOH | SA: 462; PS: 1.3–1.4 | 1590.2 | 1 | 87.97% (20,000) | p//AC | 72.9@375 | [26] |
| Mn 0.1 Ni-PTA/NF | 6 M KOH | nanoarray SA: 0.0182 | 1178 C g−1 | 0.36 | 80.62% (5000) | p//AC | [email protected] | [62] |
| Ni-BPDC | 6 M KOH | rod-like micelles | ~450 | 1 | [39] | |||
| Ni-BPDC/GO-3 | 6 M KOH | macro-nanostrips | 630 | 1 | 95.7% (10,000) | p//rGO | 16.5@250 (5) 9 | [39] |
| Ni-Co@ZIF-67/NF | 2 M KOH | nano honeycomb | 2697 | 1 | p//AC | 61.4@853 | [47] | |
| ZIF-67/PEDOT Co-2MI | PVA/1 M H2SO4 | SA: 1926 | 106.8 | 1 | 93% (4000) | p//n | 11@200 | [50] |
| Co-MOF/PANI 2 | KOH | SA: 0.016; PS: 25.06 | 271 | 0.4 | p//AC | 23.1@1600 | [51] | |
| Mn-MOF 3-Cu | 6 M KOH | triangular prism | 1606 | 0.5 | 83.73% (10,000) | [49] | ||
| Mn-PTA/NF | 2M KOH | 2D NSs SA: 202 | 10.25 6 | 1 | 81.18% (10,000) | p//rGO | 66@441 | [48] |
| Mn-Tipa and TPA | 6 M KOH | polythreaded | 1357.8 | 1 | 105% (2000) | p//AC | 35.8@750 | [23] |
| NiCo-NH2-H2BDC | 3 M KOH | NSHS SA: 11.66 | 1126.7 | 0.5 | 93% (3000) | p//AC | [email protected] | [57] |
| Ni-MOF 4 | 1 M KOH | pentagonal cone | 1024.4 | 1 | 49.1% (5000) | p//AC | 14.6@400 | [63] |
| Ni-MOF-24/Cu3(HITP)2/CFP | 1 M KOH | 2D SA: 90 | 1424 | 2 | 94.3% (7000) | p//AC | 57@1500 (1) 9 | [61] |
| Product | MOF Pr. | Morp. | Electrolyte | Cap. | CD | CR/CN | Electrode | ED@PD | Reference |
|---|---|---|---|---|---|---|---|---|---|
| L-rGO-C-MOF | Cu-BTC | Film SA: > 600 | 1M NaNO3 | 390 | 5 mV/s | 97.8% (5000) | [73] | ||
| CeO2/C/MoS2 | Ce-BTC | SA: 32.8 | 2 M KOH | 1325.7 | 1 | 92.8% (1000) | p//AC | [email protected] | [74] |
| NiS@C | ZIF-8 | Porous C | 2 M KOH | 1827 | 1 | 72% (5000) | p//HPC | 21.6@400 | [75] |
| Co-NC | Co-BTC | SA: 206 | 6 M KOH | 310 | 0.5 | 87% (1200) | p//AC | [87] | |
| NCT | ZIF-8 tube | SA: 1323.5 | 1 M NaCl | 290 | 1 | [76] | |||
| NCP | ZIF-8 particle | SA: 735.5 | 1 M NaCl | 150 | 1 | [76] | |||
| NPC@CFP | ZIF-8/ZIF-67: 50/50 | ultrathin NSs | PVA/KOH hydrogel | 201 SC | 0.55 SC | 90% (20,000) SC | NiCo2O4//n | 69@840 | [84] |
| MnMC/NF | ZIF-67 | nanoflakes | 1 M Na2SO4 | 531 | 1 | 82% (5000) | p//AC | 38.8@200 | [77] |
| Ni-C | Ni-BDC | nanofiber | 6 M KOH | 672 | 2 | 57% CD: 2–10 | p//n | 17.8@350 | [88] |
| N, P and O co-doped Ni/C | Ni-CTP-COOH | SA: 3.7 | 6 M KOH | ~240 | 8 | [79] | |||
| N, P and O co-doped Ni/C/rGO | Ni-CTP-COOH/GO | SA: 126.4 NS | 6 M KOH | 1258.7 | 8 | 110% (5000) | p//AC | 79.7@1275 | [79] |
| N, P and O co-doped Co/C | Co-CTP-COOH | SA: 16 PS: 5 | 6 M KOH | 739.6 | 1 | 80.6% (5000) | p//AC | 30.4@800 | [78] |
| Product | MOF Pr. | Morp. | Electrolyte | Cap. | CD | CR/CN | Ele. | ED@PD | Reference |
|---|---|---|---|---|---|---|---|---|---|
| δ-MnO2 | Mn-PTA | SA: 240 | 1 M NaOH | 416 | 0.5 | 60.5% (5000) | p//AC | 23.2@425 | [80] |
| MnNiDH | Mn/Ni-MOF-74 | SA: 235 | 3 M KOH | 2498 | 1 | 80.2% (10,000) SC | p//AC | 58.5@800 SC | [91] |
| Co3O4 | MOF-74 1 | SA: 48.9 | 1 M KOH | 181.5 | 0.5 | 86% (3000)@10 | [96] | ||
| Co3O4@CC (−60) | ZIF-67 (Co-2MI) | array SA: 16.23 | 2 M KOH | 806 | 1 | 86.5% (4000) SC@5 A g−1 | p//AC | 25.3@752 | [92] |
| S-Co3O4@NF | ZIF-67 (Co-2MI) | Follower-like | KOH/PVA | 1424 | 1 | 81.5% (8000)@3 A g−1 | p//AC | 29.6@804 | [93] |
| Co3O4/C@HCNFs | ZIF-67 (Co-2MI) | nanotentacles SA: 225.7 | 2 M KOH | 1623 | 1 | 85.2% (7000) | p//NGH | 36.6@471 | [94] |
| α-CoNi(OH)2@Co3O4-70 | ZIF-67 (CoNi-2MI) | SA: 153.6 | 6 M KOH | 1000 | 1 | 72.3% (8000) | p//AC | 23.88@75 | [95] |
| NiO | Ni-MOF 2 | SA: 148.9 PS: 42.5 | 2 M KOH | 1863 | 0.5 | 82% (5000) SC | p//AC | 38.4@400 | [97] |
| NiO | MOF-74 3 | SA: 227.5 PS: 4 | 1 M KOH | 105 | 0.5 | [96] | |||
| NiCo2O4 | MOF-74 | SA: 59.6 PZ: 10 | 1 M KOH | 684 | 0.5 | 86% (3000)@10 A g−1 | [96] | ||
| MnCo2O4/Co3O4 | MnCo-LDH/ZIF-67 | hollow structure | 6 M KOH | ~838 | 1 | [86] | |||
| MnNi2O4 | Ni/Mn-PTA | NS SA: 50.80 | 6 M KOH | 2848 | 1 | 93.25% (5000)@10 A g−1 | p//AC | 142.8@800 | [98] |
| Co3O4, ZnCo2O4 and NiCo2O4 | ZIF-67 (ZnNiCo-2MI) | Polyhedron SA: 65.9 | 6 M KOH | 247 | 0.1 | 99% (5000) | p//n | 27.9@1300 | [86] |
| ZnOx/g-C3N4 | TRD-ZIF-8 (Zn-2MI and CTAB) | SA: 8.59 | 3 M KOH aq | 3000 (680) | 3 | 95.6% (1000) | p//AC | 100.9@1740 | [99] |
| α-Ni(BO2−)-LDH | Ni-PTA | SA: 463.1 PS: 3.5–21 | 6 M KOH | 1760 | 1 | 61.1% (10,000) | p//rGO | 56.5 @111 | [100] |
| Ni(OH)2-MnO2@C/NF | Ni-BTC | SA: 204.1 | 1 M KOH | 965.1 C g−1 | 2 4 | 93.90% (5000) | p//AC | [email protected] | [89] |
| NiFe2O4-NiCo-LDH@rGO | Fe-BTC Ni-2MI | hollow cube SA: 52.8 | 6 M KOH | 750 C g−1 | 0.5 | 93% (3000) SC@3 A g−1 | p//AC | 50@780 | [101] |
| Product | MOF Pr. | Morphology | Electrolyte | Cap. | CD | CR/CN | Ele. | ED@PD | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Co3S4 | ZIF-67 (Co-2MI) | SA: 62.8 | 6 M KOH | 1416 | 1 | 66.1% (10,000)@5 A g−1 | [110] | ||
| Co3S4@NiO | ZIF-67 (Co-2MI) | SA: 132.9 | 6 M KOH | 1878 | 1 | 92.6% (10,000)@5 A g−1 | p//AC | 54.99@780 | [110] |
| Co9S8-Ni3S2 | ZIF-67 (Co-2MI) | powder | 2 M KOH | ~550 | 1 | 73% (3000) | [104] | ||
| Co9S8-Ni3S2/CC | ZIF-67 (Co-2MI) | NSs arrays | 2 M KOH | 1653 | 1 | 84% (3000) | p//AC | 40@379 | [104] |
| Co9S8/Ni3S2 | ZIF-67 (Co-2MI) | Co9S8 NS wrapping around Co9S8 NWs on Ni3S2 | 6 M NaOH | 4.48 1 | 2 2 | 51% (100,000)@25 2 | p//AC | [102] | |
| MnCo2S4/Co9S8 | MnCo-LDH/ZIF-67 | SA: 34.5 PS: ~3.7 | 6 M KOH | 1101 | 1 | 94.80% (5000) | p//AC | 45.8@800 | [106] |
| NiCo-S/NF (NiCo2S4, Co(OH)2 and Ni3S2) | ZIF-67 (Co-2MI) | SA: 136 | 3 M KOH | 3724 | 1 | 90% (3000) SC | p//AC | 44.76@800 | [103] |
| NiCo2S4-Ni9S8-C | Co0.5Ni0.5-BTC | yolk-shell SA: 61.7 PS: 3.6 and 10 | 6 M KOH | 2114 | 1 | 87.3% (5000) | p//rGO gel | [email protected] | [6] |
| Zn0.76Co0.24S@rGO | ZIF (Co/Zn-2MI) | Sandwich | 6 M KOH | 1640 | 1 | 90.3% (8000) | p//AC | 91.8@800 | [105] |
| Co9S8@Ni3S2/ZnS | NiZn-MOF (NiZn-H2BDC) | SA: 48.3 | 2 M KOH | 2427 | 2 | 79.7% (4000) | p//AC | [email protected] 3 | [108] |
| NiCoMoSx | Ni/Co-MOF (Ni/Co-PTA) | layered | 3 M KOH | 2595 | 1 | 91.6% (10,000) | p//AC | 48.2@ 807.2 | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Shi, X.-R.; Sun, C.; Duan, Z.; Ma, P.; Xu, S. The Application of Metal–Organic Frameworks and Their Derivatives for Supercapacitors. Nanomaterials 2020, 10, 2268. https://doi.org/10.3390/nano10112268
Huang S, Shi X-R, Sun C, Duan Z, Ma P, Xu S. The Application of Metal–Organic Frameworks and Their Derivatives for Supercapacitors. Nanomaterials. 2020; 10(11):2268. https://doi.org/10.3390/nano10112268
Chicago/Turabian StyleHuang, Simin, Xue-Rong Shi, Chunyan Sun, Zhichang Duan, Pan Ma, and Shusheng Xu. 2020. "The Application of Metal–Organic Frameworks and Their Derivatives for Supercapacitors" Nanomaterials 10, no. 11: 2268. https://doi.org/10.3390/nano10112268