Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Cellulose Nanofibers (CNFs) and Synthesis of Graphene-CNF/Poly (Vinyl Alcohol) (GN-CNF@PVA) Hybrid Hydrogels
2.3. Characterizations
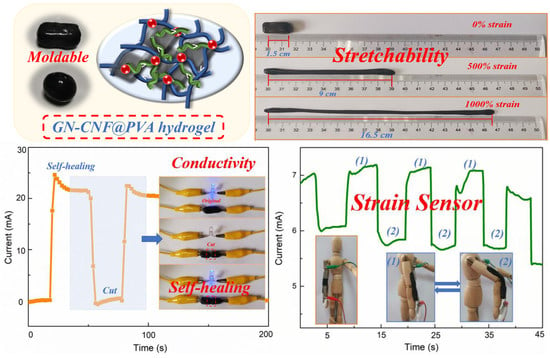
3. Results and Discussion
3.1. Fabrication Process of GN-CNF@PVA Composite Hydrogels
3.2. Morphology of GN-CNF Nanocomplexes
3.3. Chemical Structure of GN-CNF@PVA Composite Hydrogels
3.4. Rheological Behavior of Composite Hydrogels
3.5. Mechanical Properties of GN-CNF@PVA Hybrid Hydrogels
3.6. Self-Healing Properties of GN-CNF@PVA Hybrid Hydrogels
3.7. Electrochemical Performance of Hydrogels
3.8. Strain-Sensitive Performance of GN-CNF@PVA Hydrogel-Based Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Park, H.; Jeong, Y.R.; Yun, J.; Hong, S.Y.; Jin, S.; Lee, S.J.; Ha, J.S. Stretchable array of highly sensitive pressure sensors consisting of polyaniline nanofibers and Au-coated polydimethylsiloxane micropillars. ACS Nano 2015, 9, 9974–9985. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Cao, W.T.; Ma, M.G.; Wan, P. Ultrasensitive Wearable Soft Strain Sensors of Conductive, Self-healing, and Elastic Hydrogels with Synergistic “Soft and Hard” Hybrid Networks. ACS Appl. Mater. Interfaces 2017, 9, 25559–25570. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Zhu, J.; Yeom, B. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature 2013, 500, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Lee, J. A stretchable strain sensor based on a metal nanoparticle thin film for human motion detection. Nanoscale 2014, 6, 11932–11939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, C.; Wang, H. Carbonized Cotton Fabric for High-Performance Wearable Strain Sensor. Adv. Funct. Mater. 2017, 27, 1604795. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Gao, E. Carbonized Silk Fabric for Ultrastretchable, Highly Sensitive, and Wearable Strain Sensors. Adv. Mater. 2016, 28, 6640–66408. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Razmjou, A.; Ebrahimi Warkiani, M. Sensitive and Flexible Polymeric Strain Sensor for Accurate Human Motion Monitoring. Sensors 2018, 18, 418. [Google Scholar] [CrossRef]
- Yuan, C.; Lou, Z.; Wang, W. Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin Clusters and Its Application for Quick and Sensitive Detection of PrPSc through a Sandwich SPR Detection Assay. Int. J. Mol. Sci. 2019, 20, 741. [Google Scholar] [CrossRef]
- Tang, G.; Xiong, R.; Lv, D. Gas-Shearing Fabrication of Multicompartmental Microspheres: A One-Step and Oil-Free Approach. Adv. Sci. 2019, 6, 1802342. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Wang, M.; Meng, L. Mussel-Inspired Cellulose Nanocomposite Tough Hydrogels with Synergistic Self-Healing, Adhesive, and Strain-Sensitive Properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Y.; Han, J. Mechanochemical esterification of waste mulberry wood by wet Ball-milling with tetrabutylammonium fluoride. Bioresour. Technol. 2019, 285, 121354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Lu, H.L.; Yu, J. Preparation of high-strength sustainable lignocellulose gels and their applications for anti-ultraviolet weathering and dye removal. ACS Sustain. Chem. Eng. 2019, 7, 2998–3009. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D. Stable, self-healing hydrogels from nanofibrillated cellulose, poly(vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Kang, H.; Zhao, S.; Zhang, W.; Zhang, S. Polyphenol-induced cellulose nanofibrils anchored graphene oxide as nanohybrids for strong yet tough soy protein nanocomposites. Carbohydr. Polym. 2018, 180, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pint, C.L.; Lee, M.H. Optically-and thermally-responsive programmable materials based on carbon nanotube-hydrogel polymer composites. Nano Lett. 2011, 11, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, J.; Zhang, X.; Zhang, J.; Cai, Z. Synthetic Bio-Graphene Based Nanomaterials through Different Iron Catalysts. Nanomaterials 2018, 8, 840. [Google Scholar] [CrossRef] [PubMed]
- Chuang, W.; Chiu, W.; Tai, H. Temperature-dependent conductive composites: Poly (N-isopropylacrylamide-co-N-methylol acrylamide) and carbon black composite films. J. Mater. Chem. 2012, 22, 20311–20318. [Google Scholar] [CrossRef]
- Tee, B.C.; Wang, C.; Allen, R. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef]
- Choi, S.; Lee, H.; Ghaffari, R. Recent advances in flexible and stretchable bio-electronic devices integrated with nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef]
- Naficy, S.; Razal, J.M.; Spinks, G.M. Electrically Conductive, Tough Hydrogels with pH Sensitivity. Chem. Mater. 2012, 24, 3425–3433. [Google Scholar] [CrossRef] [Green Version]
- Bodenmann, A.K.; Macdonald, A.H. Graphene: Exploring carbon flatland. Phys. Today 2007, 60, 35–41. [Google Scholar] [CrossRef]
- Cai, G.; Wang, J.; Qian, K. Extremely Stretchable Strain Sensors Based on Conductive Self-Healing Dynamic Cross-Links Hydrogels for Human-Motion Detection. Adv. Sci. 2017, 4, 1600190. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zheng, J.; Lu, Y. Swelling and mechanical behaviors of carbon nanotube/poly(vinyl alcohol) hybrid hydrogels. Mater. Lett. 2007, 61, 1704–1706. [Google Scholar] [CrossRef]
- Hur, J.; Im, K.; Kim, S.W. Polypyrrole/agarose-based electronically conductive and reversibly restorable hydrogel. ACS Nano 2014, 8, 10066–10076. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, M.; Ma, C. A Conductive Self-Healing Hybrid Gel Enabled by Metal–Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, J.H.; Zhou, J. Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef]
- Huynh, T.; Haick, H. Self-Healing, Fully Functional, and Multiparametric Flexible Sensing Platform. Adv. Mater. 2016, 28, 138–143. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; Wu, Y. Self-Assembling Behavior of Cellulose Nanoparticles during Freeze-Drying: Effect of Suspension Concentration, Particle Size, Crystal Structure, and Surface Charge. Biomacromolecules 2013, 14, 1529–1540. [Google Scholar] [CrossRef]
- Han, J.; Zhou, C.; French, A.D. Characterization of cellulose II nanoparticles regenerated from 1-butyl-3-methylimidazolium chloride. Carbohydr. Polym. 2013, 94, 773–781. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Wu, X. Cellulose nanocrystals mediated assembly of graphene in rubber composites for chemical sensing applications. Carbohydr. Polym. 2016, 140, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Yu, W.; Yao, X. Nanocellulose-assisted dispersion of graphene to fabricate poly(vinyl alcohol)/graphene nanocomposite for humidity sensing. Compos. Sci. Technol. 2016, 131, 67–76. [Google Scholar] [CrossRef]
- Hajian, A.; Lindström, S.B.; Pettersson, T. Understanding the Dispersive Action of Nanocellulose for Carbon Nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Bossa, N.; Qu, F. Comparison of Hydrophilicity and Mechanical Properties of Nanocomposite Membranes with Cellulose Nanocrystals and Carbon Nanotubes. Environ. Sci. Technol. 2017, 51, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lu, K.; Yue, Y. Nanocellulose-templated assembly of polyaniline in natural rubber-based hybrid elastomers toward flexible electronic conductors. Ind. Crops Prod. 2019, 128, 94–107. [Google Scholar] [CrossRef]
- Han, J.; Yue, Y.; Wu, Q. Effects of nanocellulose on the structure and properties of poly(vinyl alcohol)-borax hybrid foams. Cellulose 2017, 24, 4433–4448. [Google Scholar] [CrossRef]
- Lu, B.; Lin, F.; Jiang, X. One-Pot Assembly of Microfibrillated Cellulose Reinforced PVA–Borax Hydrogels with Self-Healing and pH-Responsive Properties. ACS Sustain. Chem. Eng. 2016, 5, 948–956. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, X.; Yue, Y. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, Y.T.; Xie, X.M. Self-healable, super tough graphene oxide–poly (acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar] [CrossRef]
- Wang, Q.; Mynar, J.L.; Yoshida, M. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef]
- Han, J.; Lei, T.; Wu, Q. Facile preparation of mouldable polyvinyl alcohol-borax hydrogels reinforced by well-dispersed cellulose nanoparticles: Physical, viscoelastic and mechanical properties. Cellulose 2013, 20, 2947–2958. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Impacts of nanowhisker on formation kinetics and properties of all-cellulose composite gels. Carbohydr. Polym. 2011, 83, 1937–1946. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q. A novel polyacrylamide nanocomposite hydrogel reinforced with natural chitosan nanofibers. Coll. Surf. B Biointerfaces 2011, 84, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chang, H.; Wang, M. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater. Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shao, B.; Liu, T. Robust and Mechanically and Electrically Self-Healing Hydrogel for Efficient Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2018, 10, 8245–8257. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Su, G.; Guo, Q. Hierarchically Structured Self-Healing Sensors with Tunable Positive/Negative Piezoresistivity. Adv. Funct. Mater. 2018, 28, 1706658. [Google Scholar] [CrossRef]
- Gui, Z.; Zhu, H.; Gillette, E. Natural cellulose fiber as substrate for supercapacitor. ACS Nano 2013, 7, 6037–6046. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Zhang, P. Freestanding nanocellulose-composite fibre reinforced 3D polypyrrole electrodes for energy storage applications. Nanoscale 2014, 6, 13068–13075. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L. Ultrastretchable and self-healing double-network hydrogel for 3D printing and strain sensor. ACS Appl. Mater. Interfaces 2017, 9, 26429–26437. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.Y.; Lin, Y.J. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Guo, Q.; Luo, Y.; Liu, J. A well-organized graphene nanostructure for versatile strain-sensing application constructed by a covalently bonded graphene/rubber interface. J. Mater. Chem. C 2018, 6, 2139–2147. [Google Scholar] [CrossRef]
- Liu, X.; Lu, C.; Wu, X. Self-healing strain sensors based on nanostructured supramolecular conductive elastomers. J. Mater. Chem. A 2017, 5, 9824–9832. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, J.; Han, Y. Biological phytic acid as a multifunctional curing agent for elastomers: Towards skin-touchable and flame retardant electronic sensors. Green Chem. 2017, 19, 3418–3427. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Ma, C. Detection of non-joint areas tiny strain and anti-interference voice recognition by micro-cracked metal thin film. Nano Energy 2017, 34, 578–585. [Google Scholar] [CrossRef]
- Cao, J.; Lu, C.; Zhuang, J. Multiple Hydrogen Bonding Enables the Self-Healing of Sensors for Human-Machine Interactions. Angew. Chem. Int. Ed. 2017, 56, 8795–8800. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, S.; Lin, Y. Cu–Ag core–shell nanowires for electronic skin with a petal molded microstructure. J. Mater. Chem. C 2015, 3, 9594–9602. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, R.; Chen, S. A compliant, self-adhesive and self-healing wearable hydrogel as epidermal strain sensor. J. Mater. Chem. C 2018, 6, 4183–4190. [Google Scholar] [CrossRef]








| Sample | GN | CNF | PVA |
|---|---|---|---|
| PVA | 0.0% | 0.0% | 2.0% |
| CNF/PVA | 0.0% | 2.0% | 2.0% |
| GN-CNF@PVA-A | 0.3% | 2.0% | 2.0% |
| GN-CNF@PVA-B | 0.5% | 2.0% | 2.0% |
| GN-CNF@PVA-C | 0.7% | 2.0% | 2.0% |
| Parameter | PVA | CNF/PVA | GN-CNF@PVA-A | GN-CNF@PVA-B | GN-CNF@PVA-C |
|---|---|---|---|---|---|
| High-frequency plateau of G′, G′∞(Pa) | 421.1 | 790.1 | 1989.8 | 3700.5 | 973.3 |
| Maximum G″, G″ (Pa) | 141.3 | 237.6 | 471.3 | 770.4 | 252.9 |
| Maximum G*, G* (Pa) | 887.7 | 1051.0 | 3698.0 | 4227.8 | 2032.4 |
| Hydrogels | σe at ε = 75% (KPa) | Ea at ε = 75% (kJ m−3) | σt (KPa) | εt (%) | E (KPa) | Wc (%) | Ρ (g cm−3) |
|---|---|---|---|---|---|---|---|
| PVA | 7.4 ± 0.7 | 0.2 ± 0.1 | 5.9 ± 0.3 | 1264.3 ± 50.2 | 4.1 ± 0.5 | 96.3 ± 0.2 | 1.0 ± 0.1 |
| CNF/PVA | 15.3 ± 1.2 | 0.3 ± 0.1 | 6.6 ± 0.6 | 1177.7 ± 35.7 | 4.9 ± 0.8 | 95.5 ± 0.4 | 1.2 ± 0.3 |
| GN-CNF@PVA-A | 95.0 ± 8.3 | 1.0 ± 0.3 | 7.6 ± 0.5 | 1000.2 ± 36.8 | 6.3 ± 1.0 | 95.2 ± 0.1 | 1.3 ± 0.1 |
| GN-CNF@PVA-B | 148.1 ± 10.4 | 1.8 ± 0.4 | 8.5 ± 0.4 | 936.7 ± 23.5 | 9.4 ± 1.2 | 95.0 ± 0.2 | 1.5 ± 0.2 |
| GN-CNF@PVA-C | 48.0 ± 3.5 | 0.5 ± 0.2 | 7.1 ± 0.3 | 866.6 ± 28.4 | 5.2 ± 0.4 | 94.7 ± 0.3 | 1.6 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, C.; Yue, Y.; Gan, L.; Xu, X.; Mei, C.; Han, J. Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels. Nanomaterials 2019, 9, 937. https://doi.org/10.3390/nano9070937
Zheng C, Yue Y, Gan L, Xu X, Mei C, Han J. Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels. Nanomaterials. 2019; 9(7):937. https://doi.org/10.3390/nano9070937
Chicago/Turabian StyleZheng, Chunxiao, Yiying Yue, Lu Gan, Xinwu Xu, Changtong Mei, and Jingquan Han. 2019. "Highly Stretchable and Self-Healing Strain Sensors Based on Nanocellulose-Supported Graphene Dispersed in Electro-Conductive Hydrogels" Nanomaterials 9, no. 7: 937. https://doi.org/10.3390/nano9070937