Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering
Abstract
:1. Introduction
2. Structural Behavior
3. Scaffold Fabrication
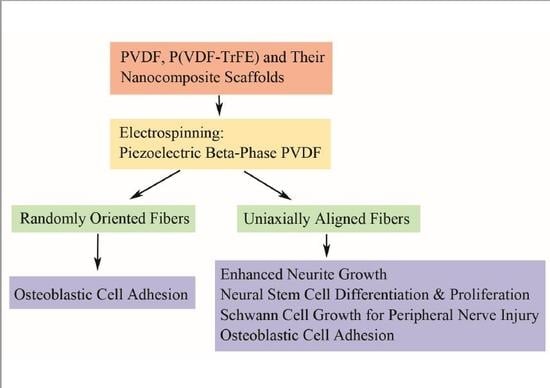
3.1. Electrospinning
3.1.1. Electrospun PVDF Scaffolds
Uniaxially Aligned Nanofibers
PVDF Nanocomposite Scaffolds
3.1.2. Electrospun P(VDF-TrFE) Scaffolds
3.1.3. Melt Electrospinning
4. In Vitro and In Vivo Models
4.1. Bone Tissue Engineering
4.1.1. In Vitro Cell Cultivation
Electrospun Fibrous Scaffolds
4.1.2. In Vivo Models for Bone Tissue Engineering
4.2. Neural Tissue Engineering
4.2.1. In Vitro Model
Fibrous Scaffolds for Neural Tissue Engineering
4.2.2. In Vivo Models for Neural Tissue Engineering
5. Major Challenges
6. Future Direction
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Acosta, M.; Novak, N.; Patel, S.; Vaish, R.; Koruza, J.; Rossetti, R.A.; Rodel, J. BaTiO3-based piezoelectrics: Fundamentals, current status, and perspectives. Appl. Phys. Rev. 2017, 4, 041305. [Google Scholar] [CrossRef]
- Martins, P.; Lopes, A.C.; Lanceros-Méndez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated polymers as smart materials for advanced biomedical applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Soulestin, T.; Ladmiral, V.; Dos Santos, F.D.; Ameduri, B. Vinylidene fluoride- and trifluoroethylene-containing fluorinated electroactive copolymers. How does chemistry impact properties? Prog. Polym. Sci. 2017, 72, 16–60. [Google Scholar] [CrossRef]
- Lee, Y.S.; Collins, G.; Arinzeh, T.L. Neurite extension of primary neurons on electrospun piezoelectric scaffolds. Acta Biomater. 2011, 7, 3877–3886. [Google Scholar] [CrossRef] [PubMed]
- Hitscherich, P.; Wu, S.; Gordan, R.; Xieh, L.H.; Arinzeh, T.; Lee, E.J. The effect of PVDF-TrFE scaffolds on stem cell derived cardiovascular cells. Biotechnol. Bioeng. 2016, 113, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Rouabhia, M.; Zhang, Z. Electrical Stimulation in Tissue Regeneration. In Applied Biomedical Engineering; Gargiulo, G., McEwan, A., Eds.; IntechOpen: London, UK, 2011; Chapter 3; pp. 38–62. [Google Scholar]
- Szewczyk, P.K.; Metwally, S.; Karbowniczek, J.E.; Marzec, M.M.; Stodolak-Zych, E.; Gruszczyński, A.; Bernasik, A.; Stachewic, U. Surface-potential-controlled cell proliferation and collagen mineralization on electrospun polyvinylidene fluoride (PVDF) fiber scaffolds for bone regeneration. ACS Biomater. Sci. Eng. 2019, 5, 582–593. [Google Scholar] [CrossRef]
- Przekora, A. Current trends in fabrication of biomaterials for bone and cartilage regeneration: Materials modifications and biophysical stimulations. Int. J. Mol. Sci. 2019, 20, 435. [Google Scholar] [CrossRef] [PubMed]
- More, N.; Kapusetti, G. Piezoelectric material—A promising approach for bone and cartilage regeneration. Med. Hypotheses 2017, 108, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Parssinen, J.; Sencadas, V.V.; Correia, V.V.; Miettinen, S.; Hytonen, V.P.; Lanceros-Mendez, S.; Pärssinen, J.; Sencadas, V.V.; Correia, V.V.; et al. Dynamic piezoelectric stimulation enhances osteogenic differentiation of human adipose stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.C.; Wianny, F.; Livi, S.; Dehay, C.; Duchet-Rumeau, J.; Gérard, J.F. Effect of polyvinylidene fluoride electrospun fiber orientation on neural stem cell differentiation. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2376–2393. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, A.; Ross, R.; Abagnale, G.; Joussen, S.; Schuster, P.; Arshi, A.; Pallua, N.; Jockenhoevel, S.; Gries, T.; Wagner, W. 3D Non-woven polyvinylidene fluoride scaffolds: Fibre cross section and texturizing patterns have impact on growth of mesenchymal stromal cells. PLoS ONE 2014, 9, e94353. [Google Scholar] [CrossRef] [PubMed]
- Damaraju, S.M.; Wu, S.; Jaffe, M.; Arinzeh, T. Structural changes in PVDF fibers due to electrospinning and its effect on biological function. Biomed. Mater. 2013, 8, 045007. [Google Scholar] [CrossRef]
- Anderson, J.; Eriksson, C. Piezoelectric properties of dry and wet bone. Nature 1970, 227, 491–492. [Google Scholar] [CrossRef]
- Halperin, C.; Mutchnik, S.; Agronin, A.; Molotskii, M.; Urenski, P.; Salai, M.; Rosenman, G. Piezoelectric effect in human bones studied in nanometer scale. Nano Lett. 2004, 4, 1253–1256. [Google Scholar] [CrossRef]
- Reis, J.; Silva, F.C.; Queiroga, C.; Lucena, S.; Potes, J. Bone mechanotranduction: A review. J. Biomed. Bioeng. 2011, 2, 37–44. [Google Scholar]
- Jonsson, A.L.; Roberts, M.A.; Kiappes, J.L.; Scott, K.A. Essential chemistry for biochemists. Essays Biochem. 2017, 61, 401–427. [Google Scholar] [CrossRef]
- Rodriquez, R.; Rangel, D.; Fonseca, G.; Gonzalez, M.; Vargas, S. Piezoelectric properties of synthetic hydroxyapatite-based organic-inorganic hydrated materials. Results Phys. 2016, 925–932. [Google Scholar] [CrossRef]
- Kang, H.; Hou, Z.; Qin, Q.H. Experimental study of time response of bending deformation of bone cantilevers in an electric field. J. Mech. Behav. Biomed. Mater. 2018, 77, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ahn, A.C.; Grodzinsky, A.J. Relevance of collagen piezoelectricity to “Wolff’s law”: A critical review. Med. Eng. Phys. 2009, 31, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.R.; García-Aznar, J.M.; Martínez, R. Piezoelectricity could predict sites of formation/resorption in bone remodelling and modelling. J. Theor. Biol. 2012, 292, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A.; Becker, R.O. Generation of electrical potentials by bone in response to mechanical stress. Science 1962, 137, 1063–1064. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Wang, J.; Zhu, H.; Yang, W.; Cao, R.; Qian, Y.; Feng, M. Quality of life is related to social support in elderly osteoporosis patients in a Chinese population. PLoS ONE 2015, 10, e0127849. [Google Scholar] [CrossRef]
- Albergaria, B.; Chalem, M.; Clark, P.; Messina, O.D.; Pereira, R.M.; Vidal, L.F. Consensus statement: Osteoporosis prevention and treatment in Latin America—Current structure and future directions. Arch. Osteporos. 2018, 13, 90. [Google Scholar] [CrossRef]
- Coleman, R.; Body, J.J.; Aapro, M.; Hadji, P. Bone health in cancer patients: ESMO clinical practice guidelines. Ann. Oncol. 2014, 25, iii124–iii137. [Google Scholar] [CrossRef]
- Poidvin, A.; Carel, J.C.; Ecosse, E.; Levy, D.; Michon, J.; Coste, J. Increased risk of bone tumors after growth hormone treatment in childhood: A population-based cohort study in France. Cancer Med. 2018, 7, 3465–3473. [Google Scholar] [CrossRef]
- Ekegren, C.L.; Beck, B.; Simpson, P.M.; Gabbe, B.J. Ten-year incidence of sport and recreation injuries resulting in major trauma or death in Victoria, Australia, 2005–2015. Orthop J. Sports Med. 2018, 6. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering. Bone Jt. Res. 2018, 7, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Griesbach, G.S.; Masel, B.E.; Helvie, R.E.; Ashley, M.J. The Impact of traumatic brain injury on later life: Effects on normal aging and neurodegenerative diseases. J. Neurotrauma 2018, 35, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Esopenko, C.; Levine, B. Aging, neurodegenerative disease, and traumatic brain injury: The role of neuroimaging. J. Neurotrauma 2015, 32, 209–220. [Google Scholar] [CrossRef] [PubMed]
- McKee, A.C.; daneshvar, D.H. The neuropathology of traumatic brain injury. Handb. Clin. Neurol. 2015, 127, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Gumera, C.; Rauck, B.; Wang, Y. Materials for central nervous system regeneration: Bioactive cues. J. Mater. Chem. 2011, 21, 7033–7051. [Google Scholar] [CrossRef]
- Song, C.G.; Zhang, Y.Z.; Wu, H.N.; Cao, X.L.; Guo, C.J.; Li, Y.Q.; Zheng, M.H.; Han, H. Stem cells: A promising candidate to treat neurological disorders. Neural Regen. Res. 2018, 13, 1294–1304. [Google Scholar] [PubMed]
- Guo, H.F.; Li, Z.S.; Dong, S.W.; Chen, W.J.; Deng, L.; Wang, Y.F.; Ying, D.J. Piezoelectric PU/PVDF electrospun scaffolds for wound healing applications. Colloids Surf. B Biointerfaces 2012, 96, 29–36. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue engineering and cell-based therapies for fractures and bone defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Palama, I.E.; D’Amone, S.; Gigli, G. Influence of electrotaxis on cell behaviour. Integr. Biol. 2014, 6, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Ateh, D.D.; Navsaria, H.A.; Vadgama, P. Polypyrrole-based conducting polymers and interactions with biological tissues. J. R. Soc. Interface 2006, 3, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Hoop, M.; Chen, X.Z.; Ferrari, A.; Mushtaq, F.; Ghazaryan, G.; Tervoort, T.; Poulikakos, D.; Nelson, B.; Panel, S. Ultrasound-mediated piezoelectric differentiation of neuron-like PC12 cells on PVDF membranes. Sci. Rep. 2017, 7, 4028. [Google Scholar] [CrossRef]
- Zayzafoon, M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell Biochem. 2006, 97, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.M.; Ribeiro, S.; Ribeiro, C.; Sencadas, V.; Gomes, A.C.; Gama, F.M.; Lanceros-Méndez, S. Effect of poling state and morphology of piezoelectric poly(vinylidene fluoride) membranes for skeletal muscle tissue engineering. RSC Adv. 2013, 3, 17938–17944. [Google Scholar] [CrossRef] [Green Version]
- Mondal, D.; Gayen, A.L.; Paul, B.K.; Bandyopadhyay, P.; Bera, D.; Bhar, D.S.; Das, K.; Das, N.S. Enhancement of β-phase crystallization and electrical properties of PVDF by impregnating ultra high diluted novel metal derived nanoparticles: Prospect of use as a charge storage device. J. Mater. Sci. Mater. Electron. 2018, 29, 14535. [Google Scholar] [CrossRef]
- Mishra, S.; Unnikrishnan, L.; Nayak, S.K.; Mohanty, S. Advances in piezoelectric polymer composites for energy harvesting applications: A systematic review. Macromol. Mater. Eng. 2019, 304, 1800463. [Google Scholar] [CrossRef]
- Liu, C.; Shen, J.; Liao, C.Z.; Yeung, K.W.K.; Tjong, S.C. Novel electrospun polyvinylidene fluoride-graphene oxide-silver nanocomposite membranes with protein and bacterial antifouling characteristics. Express Polym. Lett. 2018, 12, 365–382. [Google Scholar] [CrossRef]
- Laroche, G.; Marois, Y.; Guidoin, R.; King, M.W.; Martin, L.; How, T.; Douville, Y. Polyvinylidene fluoride (PVDF) as a biomaterial: From polymeric raw material to monofilament vascular suture. J. Biomed. Mater. Res. 1995, 29, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Wan, C.; Bowen, C.R. Multiscale-structuring of polyvinylidene fluoride for energy harvesting: The impact of molecular-, micro- and macro-structure. J. Mater. Chem. A 2017, 5, 3091–3128. [Google Scholar] [CrossRef]
- Yu, Y.J.; McGaughey, A.J.H. Energy barriers for dipole moment flipping in PVDF-related ferroelectric polymers. J. Chem. Phys. 2016, 144, 014901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prest, W.M., Jr.; Luca, D.J. The morphology and thermal response of high-temperature-crystallized poly(vinylidene fluoride). J. Appl. Phys. 1975, 46, 4136–4143. [Google Scholar] [CrossRef]
- Bao, S.P.; Liang, G.D.; Tjong, S.C. Effect of mechanical stretching on electrical conductivity and positive temperature coefficient characteristics of poly(vinylidene fluoride)/carbon nanofiber composites prepared by non-solvent precipitation. Carbon 2011, 49, 1758–1768. [Google Scholar] [CrossRef]
- He, L.X.; Tjong, S.C. Graphene oxide/polyvinylidene fluoride mixture as a precursor for fabricating thermally reduced graphene oxide/polyvinylidene fluoride composites. RSC Adv. 2013, 22981–22987. [Google Scholar] [CrossRef]
- Li, Y.C.; Ge, X.; Wang, L.; Liu, W.; Li, H.; Li, R.K.Y.; Tjong, S.C. Dielectric relaxation behavior of PVDF composites with nanofillers of different conductive nature. Curr. Nanosci. 2013, 9, 679–685. [Google Scholar] [CrossRef]
- Li, Y.C.; Tjong, S.C.; Li, R.K.Y. Dielectric properties of binary polyvinylidene fluoride/barium titanate nanocomposites and their nanographite doped hybrids. Express Polym. Lett. 2011, 5, 526–534. [Google Scholar] [CrossRef]
- Tjong, S.C.; Chen, H. Nanocrystalline materials and coatings. Mater. Sci. Eng. R Rep. 2004, 45, 1–88. [Google Scholar] [CrossRef]
- Tjong, S.C. Polymer nanocomposite bipolar plates reinforced with carbon nanotubes and graphite nanosheets. Energy Environ. Sci. 2011, 44, 605–626. [Google Scholar] [CrossRef]
- He, L.X.; Tjong, S.C. Facile synthesis of silver-decorated reduced graphene oxide as a hybrid filler material for electrically conductive polymer composites. RSC Adv. 2015, 5, 15070–15076. [Google Scholar] [CrossRef]
- He, L.X.; Tjong, S.C. Nanostructured transparent conductive films: Fabrication, characterization and applications. Mater. Sci. Eng. R Rep. 2016, 109, 1–101. [Google Scholar] [CrossRef]
- He, L.X.; Tjong, S.C. Aqueous graphene oxide-dispersed carbon nanotubes as inks for the scalable production of all-carbon transparent conductive films. J. Mater. Chem. C 2016, 4, 7043–7051. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Falsini, S.; Bardi, U.; Abou-Hassan, A.; Ristori, S. Sustainable strategies for large-scale nanotechnology manufacturing in the biomedical field. Green Chem. 2018, 20, 3897–3907. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shen, J.; Yeung, K.W.K.; Tjong, S.C. Development and antibacterial performance of novel polylactic acid-graphene oxide-silver nanoparticle nanocomposite mats prepared by electrospinning. ACS Biomater. Sci. Eng. 2017, 3, 471–486. [Google Scholar] [CrossRef]
- Chan, K.W.; Liao, C.Z.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Preparation of polyetheretherketone composites with nanohydroxyapatite rods and carbon nanofibers having high strength, good biocompatibility and excellent thermal stability. RSC Adv. 2016, 6, 19417–19429. [Google Scholar] [CrossRef]
- Liu, C.; Wong, H.; Yeung, K.; Tjong, S.C. Novel electrospun polylactic acid nanocomposite fiber mats with hybrid graphene oxide and nanohydroxyapatite reinforcements having enhanced biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef]
- Liao, C.Z.; Wong, H.M.; Yeung, K.W.; Tjong, S.C. The development, fabrication and material characterization of polypropylene composites reinforced with carbon nanofiber and hydroxyapatite nanorod hybrid fillers. Int. J. Nanomed. 2014, 9, 1299–1310. [Google Scholar]
- Chen, H.L.; Ju, S.P.; Lin, C.Y.; Pan, C.T. Investigation of microstructure and mechanical properties of polyvinylidene fluoride/carbon nanotube composites after electric field polarization: A molecular dynamics study. Comput. Mater. Sci. 2018, 149, 217–229. [Google Scholar] [CrossRef]
- Kabir, E.; Khatun, M.; Nasrin, L.; Raihan, M.J.; Rahman, M. Pure β-phase formation in polyvinylidene fluoride (PVDF)-carbon nanotube composites. J. Phys. D Appl. Phys. 2017, 50, 163002. [Google Scholar] [CrossRef]
- Ahn, Y.; Lim, J.Y.; Hong, S.M.; Lee, J.; Ha, J.; Choi, H.J.; Seo, Y. Enhanced piezoelectric properties of electrospun poly(vinylidene fluoride)/multiwalled carbon nanotube composites due to high β-phase formation in poly(vinylidene fluoride). J. Phys. Chem. C 2013, 117, 11791–11799. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Yousefi, A.A. Piezoelectric sensor based on electrospun PVDF-MWCNT-Cloisite 30B hybrid nanocomposites. Org. Electron. 2017, 50, 121–129. [Google Scholar] [CrossRef]
- El Achaby, M.; Arrakhiz, F.Z.; Vaudreuil, S.; Essassi, E.M.; Qaiss, A. Piezoelectric β-polymorph formation and properties enhancement in graphene oxide—PVDF nanocomposite films. Appl. Surf. Sci. 2012, 258, 7668–7677. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zheng, G.P.; Zhan, K.; Han, Z.; Yan, J.H. Formation of piezoelectric β-phase crystallites in poly(vinylidene fluoride)-graphene oxide nanocomposites under uniaxial tensions. J. Phys. D Appl. Phys. 2015, 48, 245303. [Google Scholar] [CrossRef]
- Abolhasani, M.M.; Shirvanimoghaddam, K.; Naebe, M. PVDF/graphene composite nanofibers with enhanced piezoelectric performance for development of robust nanogenerators. Compos. Sci. Technol. 2017, 138, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Eom, Y.; Shin, Y.E.; Hwang, S.H.; Ko, H.H.; Chae, H.G. Effect of interfacial interaction on the conformational variation of poly(vinylidene fluoride) (PVDF) chains in PVDF/graphene oxide (GO) nanocomposite fibers and corresponding mechanical properties. ACS Appl. Mater. Interfaces 2019, 11, 13665–13675. [Google Scholar] [CrossRef]
- Abzan, N.; Kharazhiha, N.; Labbaf, S. Development of three-dimensional piezoelectric polyvinylidene fluoride-graphene oxide scaffold by non-solvent induced phase separation method for nerve tissue engineering. Mater. Des. 2019, 167, 107636. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Ghaderi, S.; Amini, M.; Ahmad-Ramazani, S.A. Mechanical and piezoelectric characterizations of electrospun PVDF-nanosilica fibrous scaffolds for biomedical applications. Mater. Today Proc. 2018, 5, 15710–15716. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Sundaray, B.; Bossard, F.; Lati, P.; Orgéas, L.; Sanchez, J.Y.; Lepretre, J.C. Unusual process-induced curl and shrinkage of electrospun PVDF membranes. Polymer 2013, 54, 4588–4593. [Google Scholar] [CrossRef]
- Zheng, J.; He, A.; Li, J.; Han, C.C. Polymorphism control of poly(vinylidene fluoride) through electrospinning. Macromol. Rapid Commun. 2007, 28, 2159–2162. [Google Scholar] [CrossRef]
- Li, M.; Wondergem, H.J.; Spijkman, M.J.; Asadi, K.; Katsouras, I.; Blom, P.W.; de Leeuw, D.M. Revisiting the δ-phase of poly(vinylidene fluoride) for solution-processed ferroelectric thin films. Nat. Mater. 2013, 12, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, S.; Litt, M.H.; Lando, J.B. The crystal structure of the γ phase of poly(vinylidene fluoride). Macromolecules 1980, 13, 5095–5099. [Google Scholar] [CrossRef]
- Koga, K.; Ohigashi, H. Piezoelectricity and related properties of vinylidene fluoride and trifluoroethylene copolymers. J. Appl. Phys. 1986, 59, 2142–2150. [Google Scholar] [CrossRef]
- Koga, K.; Nakano, N.; Hattori, T.; Ohigashi, H. Crystallization, field-induced phase transformation, thermally induced phase transition, and piezoelectric activity in P(vinylidene fluoride-TrFE) copolymers with high molar content of vinylidene fluoride. J. Appl. Phys. 1990, 67, 965–974. [Google Scholar] [CrossRef]
- Xia, W.; Xu, Z.; Zhang, Q.; Zhang, Z.; Chen, Y. Dependence of dielectric, ferroelectric, and piezoelectric properties on crystalline properties of p(VDFco-TrFE) copolymers. J. Polym. Sci. B Polym. Phys. 2012, 50, 1271–1276. [Google Scholar] [CrossRef]
- Sun, F.C.; Dongare, A.M.; Asandei, A.D.; Alpay, S.P.; Nakhmanson, S. Temperature dependent structural, elastic, and polar properties of ferroelectric polyvinylidene fluoride (PVDF) and trifluoroethylene (TrFE) copolymers. J. Mater. Chem. C 2015, 3, 8389–8396. [Google Scholar] [CrossRef]
- Issa, A.A.; Al-Maadeed, M.A.; Luyt, A.S.; Ponnamma, D.; Hassan, M.K. Physico-mechanical, dielectric, and piezoelectric properties of PVDF electrospun mats containing silver nanoparticles. Carbon 2017, 3, 30. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Webster, T.J. The investigation of ZnO/poly(vinylidene fluoride) nanocomposites with improved mechanical, piezoelectric, and antimicrobial properties for orthopedic applications. J. Biomed. Nanotechnol. 2018, 14, 536545. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Sultana, A.; Mandal, D. Biomechanical and acoustic energy harvesting from TiO2 nanoparticle modulated PVDF nanofiber made high performance nanogenerator. ACS Appl. Energy Mater. 2018, 1, 3103–3112. [Google Scholar] [CrossRef]
- Singh, H.H.; Singh, S.; Khare, N. Enhanced β-phase in PVDF polymer nanocomposite and its application for nanogenerator. Adv. Polym. Technol. 2018, 29, 143–150. [Google Scholar] [CrossRef]
- Ke, K.; Potschke, P.; Jehnichen, D.; Fischer, D.; Voit, B. Achieving β-phase poly(vinylidene fluoride) from melt cooling: Effect of surface functionalized carbon nanotubes. Polymer 2014, 55, 611–619. [Google Scholar] [CrossRef]
- Bormashenko, Y.; Pogreb, R.; Stanevsky, O.; Bormashenko, E. Vibrational spectrum of PVDF and its interpretation. Polym. Test. 2004, 23, 791–796. [Google Scholar] [CrossRef]
- Gregorio, R., Jr.; Cestari, M. Effect of crystallization temperature on the crystalline phase content and morphology of poly(vinylidene fluoride). J. Polym. Sci. B Polym. Phys. 1994, 32, 859–870. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Song, S.; Xu, H.; Pan, M.; Zhong, G.J. How chain intermixing dictates the polymorphism of PVDF in poly(vinylidene fluoride)/polymethylmethacrylate binary system during recrystallization: A comparative study on core–shell particles and latex blend. Polymers 2017, 9, 448. [Google Scholar] [CrossRef]
- Ribeiro, S.; Ribeiro, T.; Ribeiro, C.; Correia, D.M.; Farinha, J.P.; Gomes, A.C.; Baleizão, C.; Lanceros-Méndez, S. Multifunctional platform based on electroactive polymers and silica nanoparticles for tissue engineering applications. Nanomaterials 2018, 8, 933. [Google Scholar] [CrossRef]
- Nooeid, P.; Salih, V.; Beier, J.; Bocaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. B 2017, 105, 431–459. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive poly(vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Ribeiro, C.; Sencadas, V.; Vikingsson, L.; Gasch, M.O.; Ribelles, J.L.; Botelho, G.; Lanceros-Méndez, S. Strategies for the development of three dimensional scaffolds from piezoelectric poly(vinylidene fluoride). Mater. Des. 2016, 92, 674–681. [Google Scholar] [CrossRef]
- Abzan, N.; Kharaziha, M.; Labbaf, S.; Saeidi, N. Modulation of the mechanical, physical and chemical properties of polyvinylidene fluoride scaffold via non-solvent induced phase separation process for nerve tissue engineering applications. Eur. Polym. J. 2018, 104, 115–127. [Google Scholar] [CrossRef]
- Hu, N.; Xiao, T.; Cai, X.; Ding, L.; Fu, Y.; Yang, X. Preparation and characterization hydrophilically modified PVDF membranes by a novel nonsolvent thermally induced phase separation method. Membranes 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, M.Y. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Braschier, T.M.; Renaud, P. Advances in the design of macroporous polymer scaffolds for potential applications in dentistry. J. Periodontal Implant Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef]
- Idris, A.; Man, Z.; Maulud, A.S.; Khan, M.S. Effects of phase separation behavior on morphology and performance of polycarbonate membranes. Membranes 2017, 7, 21. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Botelho, G.; Lanceros-Méndez, S. Nonsolvent induced phase separation preparation of poly(vinylidene fluoride-co-chlorotrifluoroethylene) membranes with tailored morphology, piezoelectric phase content and mechanical properties. Mater. Des. 2015, 88, 390–397. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M. Preparation and characterization of membranes formed by nonsolvent induced phase separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Eigler, S.; Hirch, A. Chemistry with graphene and graphene oxide—Challenges for synthetic chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Manu, M.J.; Unnikrishnan, B.S.; Shiji, R.; Sreelekha, T.T. Biomedical applications of natural polymer based nanofibrous scaffolds. Int. J. Med. Nano Res. 2015, 2, 2. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. A 2017, 105, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; Bhaw-Luximon, A.; Jhurry, D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance. Acta Mater. 2017, 50, 41–55. [Google Scholar] [CrossRef]
- Schwinté, P.; Keller, L.; Lemoine, S.; Gottenberg, J.E.; Benkirane-Jessel, N.; Vanleen, M. Nano-engineered scaffold for osteoarticular regenerative medicine. J. Nanomed. Nanotechnol. 2015, 6, 258. [Google Scholar] [CrossRef]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthcare Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Xie, J.; MacEwan, M.R.; Ray, W.Z.; Liu, W.; Siewe, D.Y.; Xia, Y. Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano 2010, 4, 5027–5036. [Google Scholar] [CrossRef]
- Cozza, E.; Monticelli, O.; Marsano, E.; Cebe, P. On the electrospinning of PVDF: Influence of the experimental conditions on the nanofiber properties. Polym. Int. 2013, 62, 41–48. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Ribelles, J.L.; Lanceros-Méndez, S. Influence of processing conditions on polymorphism and nanofiber morphology of electroactive poly(vinylidene fluoride) electrospun membranes. Soft. Mater. 2010, 8, 274–287. [Google Scholar] [CrossRef]
- Ghafari, E.; Jiang, X.; Lu, N. Surface morphology and beta-phase formation of single polyvinylidene fluoride (PVDF) composite nanofibers. Adv. Compos. Hybrid Mater. 2018, 1, 332–340. [Google Scholar] [CrossRef]
- Motamedi, A.S.; Mirzadeh, H.; Hajiesmaeilbaigi, F.; Bagheri-Khoulenjan, S.; Shokrgozar, M. Effect of electrospinning parameters on morphological properties of PVDF nanofibrous scaffolds. Prog. Biomater. 2017, 6, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Yee, W.A.; Kotaki, M.; Liu, Y.; Lu, X. Morphology, polymorphism behavior and molecular orientation of electrospun poly(vinylidene fluoride) fibers. Polymer 2007, 48, 512–521. [Google Scholar] [CrossRef]
- Matabola, K.P.; Moutioali, R. The influence of electrospinning parameters on the morphology and diameter of poly(vinyledene fluoride) nanofibers—Effect of sodium chloride. J. Mater. Sci. 2013, 48, 5475–5482. [Google Scholar] [CrossRef]
- Pillay, V.; Dotte, C.; Choonara, Y.E.; Tyagi, C.; Tomar, l.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M. A Review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanometer. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Shao, H.; Fang, J.; Wang, H.; Lin, T. Effect of electrospinning parameters and polymer concentrations on mechanical-to-electrical energy conversion of randomly-oriented electrospun poly(vinylidene fluoride) nanofiber mats. RSC Adv. 2015, 5, 14345–14350. [Google Scholar] [CrossRef]
- Sengupta, D.; Kottapalli, A.G.; Chen, S.H.; Miao, J.M.; Kwok, C.Y.; Triantafyllou, M.S.; Warkian, M.E.; Asadnia, M. Characterization of single polyvinylidene fluoride (PVDF) nanofiber for flow sensing applications. AIP Adv. 2017, 7, 105205. [Google Scholar] [CrossRef]
- Yang, D.; Lu, B.; Zhao, Y.; Jiang, X. Fabrication of aligned fibrous arrays by magnetic electrospinning. Adv. Mater. 2007, 19, 3702–3706. [Google Scholar] [CrossRef]
- Yu, K.; Wang, S.; Li, Y.; Chen, D.; Liang, C.; Lei, T.; Sun, D.; Zhao, Y. Piezoelectric performance of aligned PVDF nanofibers fabricated by electrospinning and mechanical spinning. In Proceedings of the 13th IEEE Conference Nanotechnology (IEEE-Nano 2013), Beijing, China, 5–8 August 2013. [Google Scholar] [CrossRef]
- Wang, S.H.; Wan, Y.; Sun, B.; Liu, L.Z.; Xi, W. Mechanical and electrical properties of electrospun PVDF/MWCNT ultrafine fibers using rotating collector. Nanoscale Res. Lett. 2014, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Chou, M.H.; Zeng, W.Y. Piezoelectric response of aligned electrospun polyvinylidene fluoride/carbon nanotube nanofibrous membranes. Nanomaterials 2018, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Shehata, N.; Elnabawy, E.; Abdelkader, M.; Hassanin, A.H.; Salah, M.; Nair, R.; Bhat, S.A. Static-aligned piezoelectric poly (vinylidene fluoride) electrospun nanofibers/MWCNT composite membrane: Facile method. Polymers 2018, 10, 965. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.; Bhaduri, S. Biomedical applications of electrospun nanofibers: Drug and nanoparticle delivery. Pharmaceutics 2019, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.S.; Qi, S.J. Realising the potential of graphene-based materials for biosurfaces—A future perspective. Biosurf. Biotribol. 2015, 1, 229–248. [Google Scholar] [CrossRef]
- Cardenas, L.; MacLeod, J.; Lipton-Duffin, J.; Seifu, D.G.; Popescu, F.; Siaj, M.; Mantovani, D.; Rosei, F. Reduced graphene oxide growth on 316L stainless steel for medical applications. Nanoscale 2014, 6, 8664–8670. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Issa, A.A.; Al-Maadeed, M.A.; Mrlik, M.; Luyt, A.S. Electrospun PVDF graphene oxide composite fibre mats with tunable physical properties. J. Polym. Res. 2016, 23, 232. [Google Scholar] [CrossRef]
- Moraldi, R.; Karimi-Sabet, J.; Shariaty-Niassar, M.; Koochaki, M.A. Preparation and characterization of polyvinylidene fluoride/graphene superhydrophobic fibrous films. Polymers 2015, 7, 1444–1463. [Google Scholar] [CrossRef]
- Damjanovic, D. Ferroelectric, dielectric and piezoelectric properties of ferroelectric thin films and ceramics. Rep. Prog. Phys. 1998, 61, 1267–1324. [Google Scholar] [CrossRef] [Green Version]
- Fulay, P.; Lee, J.K. Electronic, Magnetic and Optical Materials; CRC Press: Boca Raton, FL, USA, 2010; pp. 299–362. ISBN 9781498701693. [Google Scholar]
- Katsouras, I.; Asadi, K.; Li, M.; van Driel, T.B.; Kjaer, K.S.; Zhao, D.; Lenz, T.; Gu, Y.; Blom, P.W.; Damjanovic, D.; et al. The negative piezoelectric effect of the ferroelectric polymer poly(vinylidene fluoride). Nat. Mater. 2016, 15, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Srinivas, V.; Madras, G.; Bose, S. Outstanding dielectric constant and piezoelectric coefficient in electrospun nanofiber mats of PVDF containing silver decorated multiwall carbon nanotubes: Assessing through piezoresponse force microscopy. RSC Adv. 2016, 6, 6251–6258. [Google Scholar] [CrossRef]
- Gebrekrstos, A.; Madras, G.; Bose, S. Piezoelectric response in electrospun poly(vinylidene fluoride) fibers containing fluoro-doped graphene derivatives. ACS Omega 2018, 3, 5317–5326. [Google Scholar] [CrossRef]
- Mahdi, R.I.; Gan, W.C.; Majid, W.H. Hot plate annealing at a low temperature of a thin ferroelectric P(VDF-TrFE) Film with an improved crystalline structure for sensors and actuators. Sensors 2014, 14, 19115–19127. [Google Scholar] [CrossRef] [PubMed]
- Hintzer, K.; Zipplies, T.; Carlso, D.P.; Schmiegel, W. Fluoropolymers, Organic. In Ullmann’s Polymers and Plastics: Products and Processes; Wiley-VCH: Weinheim, Germany, 2016; Volume 2, pp. 604–656. ISBN 978-3-527-33823-8. [Google Scholar]
- Pesano, L.; Dagdevire, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef] [PubMed]
- Ico, G.; Showater, A.; Bosze, W.; Gott, S.C.; Kim, B.S.; Rao, M.P.; Myung, N.V.; Nam, J. Size-dependent piezoelectric and mechanical properties of electrospun P(VDF-TrFE) nanofibers for enhanced energy harvesting. J. Mater. Chem. A 2016, 4, 2293–2304. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Gong, L.; Hu, X.; Zhao, Y.; Chen, H.; Feng, L.; Zhang, D. Aligned P(VDF-TrFE) nanofibers for enhanced piezoelectric directional strain sensing. Polymers 2018, 10, 364. [Google Scholar] [CrossRef]
- Denning, D.; Guyonnet, J.; Rodriguez, B.J. Applications of piezoresponse force microscopy in materials research: From inorganic ferroelectrics to biopiezoelectrics and beyond. Int. Mater. Rev. 2016, 61, 46–70. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, X.; He, H.W.; Yu, M.; Ning, X.; Long, Y.Z. Solvent-free electrospinning: Opportunities and challenges. Polym. Chem. 2017, 8, 333–352. [Google Scholar] [CrossRef]
- Bubakir, M.; Li, H.; Barhoum, A.; Yang, Y. Advances in Melt Electrospinning Technique. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.H., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–30. [Google Scholar]
- Mota, C.; Puppi, D.; Gazzarri, M.; Bartolo, P.; Chiellini, F. Melt electrospinning writing of three-dimensional star poly(ε-caprolactone) scaffolds. Polym. Int. 2013, 62, 893–900. [Google Scholar] [CrossRef]
- Zaiss, S.; Brown, T.D.; Reichert, J.C.; Berner, A. Poly(ε-caprolactone) scaffolds Fabricated by melt electrospinning for bone tissue engineering. Materials 2016, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Meng, Z. Melt electrospinning vs. solution electrospinning: A comparative study of drug-loaded poly (ε-caprolactone) fibres. Mater. Sci. Eng. C 2017, 74, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Kikuchi, M.; Shimada, N.; Nakane, K. Effect of melt and solution electrospinning on the formation and structure of poly(vinylidene fluoride) fibres. RSC Adv. 2017, 7, 17593. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; He, T.; Qian, L.; Tan, G.; Ning, C. Polarization of an electroactive functional film on titanium for inducing osteogenic differentiation. Sci. Rep. 2016, 6, 35512. [Google Scholar] [CrossRef] [PubMed]
- Rutkovskiy, A.; Stenslokken, A.; Vaage, A.J. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Fukada, E.; Yasuda, I. On the piezoelectric effect of bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Eischen-Loges, M.; Oliveira, K.M.; Bhavsar, M.B.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, e4959. [Google Scholar] [CrossRef]
- Mobini, S.; Leppik, L.; Parameswaran, V.T.; Barker, J.H. In vitro effect of direct current electrical stimulation on rat mesenchymal stem cells. PeerJ 2017, 5, e2821. [Google Scholar] [CrossRef]
- Leppik, L.; Han, Z.; Mobini, S.; Parameswaran, V.T.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef] [PubMed]
- Kulterer, B.; Friedl, G.; Jandrositz, A.; Sanchez-Cabo, F.; Prokesch, A.; Paar, C.; Scheideler, M.; Windhager, R.; Windhager, R.; Preisegger, K.; et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genom. 2007, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Panadero, J.A.; Sencadas, V.; Lanceros-Mendez, S.; Tamano, M.N.; Moratal, D.; Salmeron-Sanchez, M.; Ribelles, J.L. Fibronectin adsorption and cell response on electroactive poly(vinylidene fluoride) films. Biomed. Mater. 2012, 7, 035004. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Pereira, J.; Ribeiro, S.; Ribeiro, C.; Gomblek, C.J.; Gama, F.M.; Gomes, A.C.; Patterso, D.A.; Lanceros-Mendez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym. Test. 2014, 44, 234–241. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, J.; Guo, H.; Liu, S.; He, W. The effects of surface properties of nanostructured bone repair materials on their performances. J. Nanomater. 2015, 2015, 893545. [Google Scholar] [CrossRef]
- Pärssinen, H.; Hammarén, H.; Rahikainen, R.; Sencadas, V.; Ribeiro, C.; Vanhatupa, S.; Miettinen, S.; Lanceros-Méndez, S.; Hytönen, V.P. Enhancement of adhesion and promotion of osteogenic differentiation of human adipose stem cells by poled electroactive poly(vinylidene fluoride). J. Biomed. Mater. Res. A 2015, 103, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, A.H.; Jaffe, M.; Arinzeh, T.L. Piezoelectric materials for tissue regeneration: A review. Acta Biomater. 2015, 24, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Arinzeh, T.L.; Collins, G.; Lee, Y.S. Method of Tissue Repair Using a Piezoelectric Scaffold. U.S. Patent 9,476,026 B2, 25 October 2016. [Google Scholar]
- Kitsara, M.; Blanquer, A.; Murillo, G.; Humblot, V.; Vieira, S.; Nojues, C.; Ibanez, E.; Esteve, J.; Barrios, L. Permanently hydrophilic, piezoelectric PVDF nanofibrous scaffolds promoting unaided electromechanical stimulation on osteoblasts. Nanoscale 2019, 11, 8906–8917. [Google Scholar] [CrossRef]
- Wang, A.; Hu, M.; Zhou, L.; Qiang, X. Self-powered well-aligned P(VDF-TrFE) piezoelectric nanofiber nanogenerator for modulating an exact electrical stimulation and enhancing the proliferation of preosteoblasts. Nanomaterials 2019, 9, 349. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Lin, Y.; Hu, P.; Shen, Y.; Wang, K.; Meng, S.; Chai, Y.; Dai, X.; Liu, X.; et al. Nanocomposite membranes enhance bone regeneration through restoring physiological electric microenvironment. ACS Nano 2016, 10, 7279–7286. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Saburi, E.; Islami, M.; Hosseinzadeh, S.; Moghadam, A.S.; Mansour, R.N.; Azadian, E.; Joneidi, Z.; Nikpoor, A.R.; Ghadiani, M.H.; Khodaii, Z.; et al. In vitro osteogenic differentiation potential of the human induced pluripotent stem cells augment when grown on graphene oxide-modified nanofibers. Gene 2019, 696, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Correia, D.M.; Rodrigues, I.; Guardao, L.; Guimareas, S.; Soares, R.; Lanceros-Méndez, S. In-vivo demonstration of the suitability of piezoelectric stimuli for bone reparation. Mater. Lett. 2017, 209, 118–121. [Google Scholar] [CrossRef]
- Wang, A.; Liu, Z.; Hu, M.; Wang, C.; Zhang, X.; Shi, B.; Fang, Y.; Cui, Y.; Li, Z.; Ren, K. Piezoelectric nanofibrous scaffolds as in vivo energy harvesters for modifying fibroblast alignment and proliferation in wound healing. Nano Energy 2018, 43, 63–71. [Google Scholar] [CrossRef]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.G.; Ceseracciu, L.; Marino, A.; Labardi, M.; Marras, S.; Pignatelli, F.; Bruschini, L.; Mattoli, V.; Ciofani, G. P(VDF-TrFE)/BaTiO3 nanoparticle composite films mediate piezoelectric stimulation and promote differentiation of SH-SY5Y neuroblastoma cells. Adv. Healthc. Mater. 2016, 5, 1808–1820. [Google Scholar] [CrossRef]
- Swindle-Reilly, K.E.; Paranjape, C.S.; Miller, C.A. Electrospun poly(caprolactone)-elastin scaffolds for peripheral nerve regeneration. Prog. Biomater. 2014, 3, 20. [Google Scholar] [CrossRef]
- Xu, J.; Wu, T.; Xia, Y. Perspective: Aligned arrays of electrospun nanofibers for directing cell migration. APL Mater. 2018, 6, 120902. [Google Scholar] [CrossRef] [Green Version]
- Corey, J.M.; Lin, D.Y.; Mycek, K.B.; Chen, Q.; Samuel, S.; Feldman, E.L.; Martin, D.C. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J. Biomed. Mater. Res. A 2007, 83, 636–645. [Google Scholar] [CrossRef]
- Koppes, A.N.; Zaccor, N.W.; Rivet, C.J.; Williams, L.A.; Piselli, J.M.; Gilbert, R.J.; Thompsom, D.M. Neurite outgrowth on electrospun PLLA fibers is enhanced by exogenous electrical stimulation. J. Neural Eng. 2014, 11, 046002. [Google Scholar] [CrossRef]
- Yoshida, M.; Onogi, T.; Onishi, K.; Inagaki, T.; Tajitsu, Y. High piezoelectric performance of poly(lactic acid) film manufactured by solid-state extrusion. Jpn. J. Appl. Phys. 2014, 53. [Google Scholar] [CrossRef]
- Lee, Y.S.; Arinzeh, T.L. The Influence of piezoelectric scaffolds on neural differentiation of human neural stem/progenitor cells. Tissue Eng. A 2012, 18, 2063–2072. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Z.; Hay, A.S.; Jian, X.G.; Tjong, S.C. Synthesis and properties of poly (aryl ether sulfone)s containing the phthalazinone moiety. J. Appl. Polym. Sci. 1998, 68, 137–143. [Google Scholar] [CrossRef]
- Tjong, S.C.; Meng, Y.Z. Morphology and mechanical characteristics of compatibilized polyamide 6-liquid crystalline polymer composites. Polymer 1997, 38, 4609–4615. [Google Scholar] [CrossRef]
- Meng, Y.Z.; Tjong, S.C. Rheology and morphology of compatibilized polyamide 6 blends containing liquid crystalline copolyesters. Polymer 1998, 39, 99–107. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, L.; Song, Z.B.; Zhang, X.Z.; Qin, J.Q. The influence of the alignment of electrospun fibrous scaffolds on the biological behavior of RSC96 cells. J. Biomater. Tissue Eng. 2014, 4, 488–491. [Google Scholar] [CrossRef]
- Kim, S.; Maynard, J.C.; Strickland, A.; Burlingame, A.L.; Milbrandt, J. Schwann cell O-GlcNAcylation promotes peripheral nerve remyelination via attenuation of the AP-1 transcription factor JUN. Proc. Natl. Acad. Sci. USA 2018, 115, 8019–8024. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Lee, Y.; Bunge, M.B.; Arinzeh, T.L. Investigating Schwann cell growth and neurite extension on PVDF-TrFE scaffolds in vitro. In Frontiers in Bioengineering and Biotechnology Conference Abstract, Proceedings of the 10th World Biomaterials Congress, Montréal, QC, Canada, 17–22 May 2016; Frontiers: Lausanne, Switzerland, 2016. [Google Scholar] [CrossRef]
- Wu, S.; Chen, M.S.; Maurel, P.; Lee, Y.; Bunge, M.B.; Arinzeh, T.L. Aligned fibrous PVDF-TrFE scaffolds 1554 with Schwann cells support neurite extension and myelination in vitro. J. Neural Eng. 2018, 15, 056010. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Ferrari, F. Design and criteria of electrospun fibrous scaffolds for the treatment of spinal cord injury. Neural Regen. Res. 2017, 12, 1786–1790. [Google Scholar] [CrossRef]
- Frost, H.K.; Andersson, T.; Johansson, S.; Englund-Johansson, U.; Ekstrom, P.; Dahlin, L.B.; Johansson, F. Electrospun nerve guide conduits have the potential to bridge peripheral nerve injuries in vivo. Sci. Rep. 2018, 8, 16716. [Google Scholar] [CrossRef]
- Aebischer, P.; Valentini, R.F.; Dario, P.; Domenici, C.; Galletti, P.M. Piezoelectric guidance channels enhance regeneration in the mouse sciatic nerve after axotomy. Brain Res. 1987, 436, 165–168. [Google Scholar] [CrossRef]
- Lee, Y.; Wu, S.; Arinzeh, T.L.; Bunge, M.B. Enhanced noradrenergic axon regeneration into schwann cell-filled PVDF-TrFE conduits after complete spinal cord transection. Biotechnol. Bioeng. 2017, 114, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hong, Y. Enhancing cell infiltration of electrospun fibrous scaffolds in tissue regeneration. Bioact. Mater. 2016, 1, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef] [PubMed]
- McCann, J.T.; Marquez, M.; Xia, Y. Highly porous fibers by electrospinning into a cryogenic liquid. J. Am. Chem. Soc. 2006, 128, 1436–1437. [Google Scholar] [CrossRef] [PubMed]
- Rnjak-Kovacina, J.; Weiss, A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B 2011, 17, 365–372. [Google Scholar] [CrossRef]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef]
- Sun, D.; Chang, C.; Li, S.; Lin, L. Near-field electrospinning. Nano Lett. 2006, 6, 839–842. [Google Scholar] [CrossRef]
- Middleton, R.; Li, X.; Shepherd, J.; Li, Z.; Wang, W.; Best, S.M.; Cameron, R.E.; Huang, Y.Y. Near-field electrospinning patterning polycaprolactone and polycaprolactone/collagen interconnected fiber membrane. Macromol. Mater. Eng. 2018, 303, 1700463. [Google Scholar] [CrossRef]
- Chen, H.; Malheiro, A.B.; van Blitterswijk, C.; Mota, C.; Wieringa, P.A.; Moroni, L. Direct writing electrospinning of scaffolds with multidimensional fiber architecture for hierarchical tissue engineering. ACS Appl. Mater. Interfaces 2017, 9, 38187–38200. [Google Scholar] [CrossRef]
- Liu, Z.H.; Pan, C.T.; Lin, L.W.; Lai, H.W. Piezoelectric properties of PVDF/MWCNT nanofiber using near-field electrospinning. Sens. Actuators A Phys. 2013, 193, 13–24. [Google Scholar] [CrossRef]
- Lee, T.H.; Chen, C.Y.; Tsai, C.Y.; Fuh, Y.K. Near-field electrospun piezoelectric fibers as sound-sensing elements. Polymers 2018, 10, 692. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.L. Toxicology of dimethyl and monomethyl derivatives of acetamide and formamide: A second update. Crit. Rev. Toxicol. 2012, 42, 793–826. [Google Scholar] [CrossRef] [PubMed]
- Hochleitner, G.; Jungst, T.; Brown, T.D.; Hahn, K.; Moseke, C.; Jakob, F.; Dalton, P.D.; Groll, J. Additive manufacturing of scaffolds with sub-micron filaments via melt electrospinning writing. Biofabrication 2015, 7, 035002. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Edin, F.; Detta, N.; Skelton, A.D.; Hutmacher, D.W.; Dalton, P.D. Melt electrospinning of poly(ε-caprolactone) scaffolds: Phenomenological observations associated with collection and direct writing. Mater. Sci. Eng. C 2014, 45, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.T.; Garcia, A.J. Scaffold-based anti-infection strategies in bone repair. Ann. Biomed. Eng. 2015, 43, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, G.; Liu, G.; Liu, Y.; Chen, Q.; Ren, L.; Chen, C. A biodegradable antibiotic-eluting PLGA nanofiber-loaded deproteinized bone for treatment of infected rabbit bone defects. J. Biomater. Appl. 2016, 31, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Ausbaugh, A.G.; Jiang, X.; Zheng, G.; Tsai, A.S.; Kim, W.S.; Thompsom, J.M.; Miller, R.J.; Shahbazian, J.H.; Wang, Y.; Dillen, C.A.; et al. Polymeric nanofiber coating with tunable combinatorial antibiotic delivery prevents biofilm-associated infection in vivo. Proc. Natl. Acad. Sci. USA 2016, 113, 6919–6928. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic nanosilver against multidrug-resistant bacteria (MDRB). Antibiotics 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Medina, C.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomedic. 2014, 9, 1677–1687. [Google Scholar] [CrossRef]
- Zielinska, E.; Tukaj, C.; Radomski, M.W.; Inkielewicz-Stepniak, I. Molecular mechanism of silver nanoparticles-induced human osteoblast cell death: Protective effect of inducible nitric oxide synthase inhibitor. PLoS ONE 2016, 11, e0164137. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Grifin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Models Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]



































| Materials | β-Phase Content | Fiber Diameter, nm | d33 Value, pm/V | d33 Value, pC/N | Ref. |
|---|---|---|---|---|---|
| PVDF random fibers | NA | 1410 | — | 24.90 | [39] |
| PVDF random fibers | 79 ± 3 | 155 ± 17 | — | 16.8 ± 1.4 | [134] |
| PVDF aligned fibers | 88 ± 1 | 118 ± 23 | — | 27.4 ± 1.5 | [134] |
| PVDF/MWCNT aligned fibers | 89 ± 2 | 116 ± 21 | 31.3 ± 2.1 | 31.3 ± 2.1 | [134] |
| PVDF random fibers | 78 | 1500 | 30 | — | [147] |
| PVDF/1% MWCNT random fibers | 84 | 300 | 35 | — | [147] |
| PVDF/1% (MWCNT-AgNP) random fibers | 89 | 800 | 54 | — | [147] |
| PVDF/1% GO random fibers | 70 | 623 | 40 | — | [148] |
| PVDF/1% CGO random fibers | 79 | 622 | 46 | — | [148] |
| PVDF/1% FGO random fibers | 89 | 619 | 63 | — | [148] |
| Human bones | — | — | — | 7–8 | [26] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liao, C.; Tjong, S.C. Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering. Nanomaterials 2019, 9, 952. https://doi.org/10.3390/nano9070952
Li Y, Liao C, Tjong SC. Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering. Nanomaterials. 2019; 9(7):952. https://doi.org/10.3390/nano9070952
Chicago/Turabian StyleLi, Yuchao, Chengzhu Liao, and Sie Chin Tjong. 2019. "Electrospun Polyvinylidene Fluoride-Based Fibrous Scaffolds with Piezoelectric Characteristics for Bone and Neural Tissue Engineering" Nanomaterials 9, no. 7: 952. https://doi.org/10.3390/nano9070952