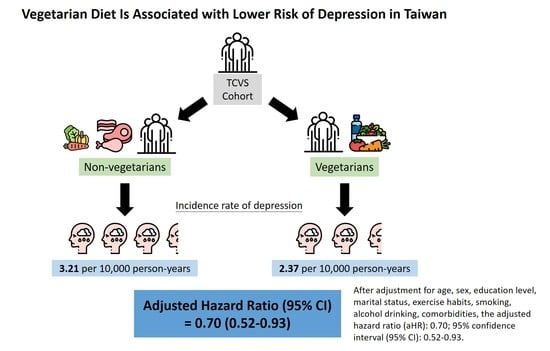
Vegetarian Diet Is Associated with Lower Risk of Depression in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Tzu Chi Vegetarian Study (TCVS)
2.2. Exclusion Criteria
2.3. Case Ascertainment
2.4. Assessment of Diet and Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Vascular
4.2. Inflammatory
4.3. Degenerative
4.4. Neurotransmitters
4.5. Gut Microbiota
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization (WHO): Geneva, Switzerland, 2017. [Google Scholar]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, M.M.; Bland, R.C.; Canino, G.J.; Faravelli, C.; Greenwald, S.; Hwu, H.G.; Joyce, P.R.; Karam, E.G.; Lee, C.K.; Lellouch, J.; et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996, 276, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.C.; Chen, W.J.; Lee, M.B.; Lung, F.W.; Lai, T.J.; Liu, C.Y.; Lin, C.Y.; Yang, M.J.; Chen, C.C. Low prevalence of major depressive disorder in Taiwanese adults: Possible explanations and implications. Psychol. Med. 2012, 42, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.B.; Williams, J.V.; Lavorato, D.H.; Modgill, G.; Jette, N.; Eliasziw, M. Major depression as a risk factor for chronic disease incidence: Longitudinal analyses in a general population cohort. Gen. Hosp. Psychiatry 2008, 30, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.M.; Musliner, K.L.; Benros, M.E.; Vestergaard, M.; Munk-Olsen, T. Mortality and life expectancy in persons with severe unipolar depression. J. Affect. Disord. 2016, 193, 203–207. [Google Scholar] [CrossRef]
- Halaris, A. Inflammation-Associated Co-morbidity Between Depression and Cardiovascular Disease. Curr. Top. Behav. Neurosci. 2017, 31, 45–70. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Opie, R.S.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The impact of whole-of-diet interventions on depression and anxiety: A systematic review of randomised controlled trials. Public Health Nutr. 2015, 18, 2074–2093. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, A.; Quirk, S.E.; Housden, S.; Brennan, S.L.; Williams, L.J.; Pasco, J.A.; Berk, M.; Jacka, F.N. Relationship between diet and mental health in children and adolescents: A systematic review. Am. J. Public Health 2014, 104, e31–e42. [Google Scholar] [CrossRef]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, C.L.; McGuire, M.T.; Neumark-Sztainer, D.; Story, M. Characteristics of vegetarian adolescents in a multiethnic urban population. J. Adolesc. Health 2001, 29, 406–416. [Google Scholar] [CrossRef]
- Baines, S.; Powers, J.; Brown, W.J. How does the health and well-being of young Australian vegetarian and semi-vegetarian women compare with non-vegetarians? Public Health Nutr. 2007, 10, 436–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, J.; Zhang, X.C.; Jacobi, F. Vegetarian diet and mental disorders: Results from a representative community survey. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 67. [Google Scholar] [CrossRef] [Green Version]
- Hibbeln, J.R.; Northstone, K.; Evans, J.; Golding, J. Vegetarian diets and depressive symptoms among men. J. Affect. Disord. 2018, 225, 13–17. [Google Scholar] [CrossRef]
- Askari, M.; Daneshzad, E.; Darooghegi Mofrad, M.; Bellissimo, N.; Suitor, K.; Azadbakht, L. Vegetarian diet and the risk of depression, anxiety, and stress symptoms: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2020, 1–11. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and veganism compared with mental health and cognitive outcomes: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef]
- Lavallee, K.; Zhang, X.C.; Michalak, J.; Schneider, S.; Margraf, J. Vegetarian diet and mental health: Cross-sectional and longitudinal analyses in culturally diverse samples. J. Affect. Disord. 2019, 248, 147–154. [Google Scholar] [CrossRef]
- Beezhold, B.L.; Johnston, C.S.; Daigle, D.R. Vegetarian diets are associated with healthy mood states: A cross-sectional study in seventh day adventist adults. Nutr. J. 2010, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beezhold, B.L.; Johnston, C.S. Restriction of meat, fish, and poultry in omnivores improves mood: A pilot randomized controlled trial. Nutr. J. 2012, 11, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahleova, H.; Hrachovinova, T.; Hill, M.; Pelikanova, T. Vegetarian diet in type 2 diabetes--improvement in quality of life, mood and eating behaviour. Diabet. Med. 2013, 30, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Ho Chan, W.S. Taiwan’s healthcare report 2010. EPMA J. 2010, 1, 563–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Health Insurance Research Database. Available online: https://nhird.nhri.org.tw/en/index.html (accessed on 5 February 2021).
- Chiu, T.H.; Huang, H.Y.; Chen, K.J.; Wu, Y.R.; Chiu, J.P.; Li, Y.H.; Chiu, B.C.; Lin, C.L.; Lin, M.N. Relative validity and reproducibility of a quantitative FFQ for assessing nutrient intakes of vegetarians in Taiwan. Public Health Nutr. 2014, 17, 1459–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Villegas, A.; Henríquez-Sánchez, P.; Ruiz-Canela, M.; Lahortiga, F.; Molero, P.; Toledo, E.; Martínez-González, M.A. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. 2015, 13, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Kandula, N.R.; Kanaya, A.M.; Talegawkar, S.A. Vegetarian diet is inversely associated with prevalence of depression in middle-older aged South Asians in the United States. Ethn. Health 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cengotitabengoa, M.; Carrascón, L.; O’Brien, J.T.; Díaz-Gutiérrez, M.J.; Bermúdez-Ampudia, C.; Sanada, K.; Arrasate, M.; González-Pinto, A. Peripheral Inflammatory Parameters in Late-Life Depression: A Systematic Review. Int. J. Mol. Sci. 2016, 17, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Sloten, T.T.; Sigurdsson, S.; van Buchem, M.A.; Phillips, C.L.; Jonsson, P.V.; Ding, J.; Schram, M.T.; Harris, T.B.; Gudnason, V.; Launer, L.J. Cerebral Small Vessel Disease and Association with Higher Incidence of Depressive Symptoms in a General Elderly Population: The AGES-Reykjavik Study. Am. J. Psychiatry 2015, 172, 570–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Agtmaal, M.J.M.; Houben, A.; Pouwer, F.; Stehouwer, C.D.A.; Schram, M.T. Association of Microvascular Dysfunction With Late-Life Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2017, 74, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Lin, Y.L.; Lin, T.K.; Lin, C.T.; Chen, B.C.; Lin, C.L. Total cardiovascular risk profile of Taiwanese vegetarians. Eur. J. Clin. Nutr. 2008, 62, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Fraser, G.; Katuli, S.; Anousheh, R.; Knutsen, S.; Herring, P.; Fan, J. Vegetarian diets and cardiovascular risk factors in black members of the Adventist Health Study-2. Public Health Nutr. 2015, 18, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.L.; Fang, T.C.; Gueng, M.K. Vascular dilatory functions of ovo-lactovegetarians compared with omnivores. Atherosclerosis 2001, 158, 247–251. [Google Scholar] [CrossRef]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40–53. [Google Scholar] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of inflammation in brain: A matter of fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef]
- Fisher, M.; Levine, P.H.; Weiner, B.; Ockene, I.S.; Johnson, B.; Johnson, M.H.; Natale, A.M.; Vaudreuil, C.H.; Hoogasian, J. The effect of vegetarian diets on plasma lipid and platelet levels. Arch. Intern. Med. 1986, 146, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ball, M.; Bartlett, M.; Sinclair, A. Lipoprotein(a), essential fatty acid status and lipoprotein lipids in female Australian vegetarians. Clin. Sci. (Lond.) 1999, 97, 175–181. [Google Scholar] [CrossRef]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Cho, S.W.; Park, Y.K. Long-term vegetarians have low oxidative stress, body fat, and cholesterol levels. Nutr. Res. Pract. 2012, 6, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Vavakova, M.; Durackova, Z.; Trebaticka, J. Markers of Oxidative Stress and Neuroprogression in Depression Disorder. Oxidative Med. Cell. Longev. 2015, 2015, 898393. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Kazimirova, A.; Barancokova, M.; Krajcovicova-Kudlackova, M.; Volkovova, K.; Staruchova, M.; Valachovicova, M.; Paukova, V.; Blazicek, P.; Wsolova, L.; Dusinska, M. The relationship between micronuclei in human lymphocytes and selected micronutrients in vegetarians and non-vegetarians. Mutat. Res. 2006, 611, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Rowland, I.R.; Barnett, Y.A.; Bradbury, I.; Robson, P.J.; Powell, J.; Fletcher, J. Influence of habitual diet on antioxidant status: A study in a population of vegetarians and omnivores. Eur. J. Clin. Nutr. 2007, 61, 1011–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauma, A.L.; Mykkanen, H. Antioxidant status in vegetarians versus omnivores. Nutrition 2000, 16, 111–119. [Google Scholar] [CrossRef]
- Johnston, C.S. 28-Vegetarian Diet and Possible Mechanisms for Impact on Mood. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Mariotti, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 493–509. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Adeva, M.M.; Calviño, J.; Souto, G.; Donapetry, C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012, 43, 171–181. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Ngo, A.L.; Simopoulos, T.; Kaye, A.D.; Colontonio, M.M.; et al. Gut Microbiome Dysbiosis and Depression: A Comprehensive Review. Curr. Pain Headache Rep. 2020, 24, 36. [Google Scholar] [CrossRef] [PubMed]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Łoniewski, I.; Misera, A.; Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Kaźmierczak-Siedlecka, K.; Misiak, B.; Marlicz, W.; Samochowiec, J. Major Depressive Disorder and gut microbiota-Association not causation. A scoping review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110111. [Google Scholar] [CrossRef] [PubMed]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Brunet, A.; Turecki, G.; Liu, A.; D’Arcy, C.; Caron, J. Risk factor modifications and depression incidence: A 4-year longitudinal Canadian cohort of the Montreal Catchment Area Study. BMJ Open 2017, 7, e015156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendler, K.S.; Gardner, C.O. Depressive vulnerability, stressful life events and episode onset of major depression: A longitudinal model. Psychol. Med. 2016, 46, 1865–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscatell, K.A.; Slavich, G.M.; Monroe, S.M.; Gotlib, I.H. Stressful life events, chronic difficulties, and the symptoms of clinical depression. J. Nerv. Ment. Dis. 2009, 197, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables (n, %) | Non-Vegetarians | Vegetarians | p-Value |
|---|---|---|---|
| (n = 7006, 66.2%) | (n = 3571, 33.8%) | ||
| Sociodemographic characteristics | |||
| Age, mean ± SD years | 50.4 (9.7) | 51.5 (9.5) | <0.01 |
| Sex | <0.01 | ||
| Male | 2740 (39.1%) | 928 (26.0%) | |
| Female | 4266 (61.9%) | 2643 (74.0%) | |
| Education level | <0.01 | ||
| ≤Elementary school | 1455 (20.8%) | 908 (25.4%) | |
| Middle and high school | 3786 (54.0%) | 1801 (50.5%) | |
| Higher education | 1765 (25.2%) | 862 (24.1%) | |
| Marital status a | <0.01 | ||
| Married | 6089 (90.3%) | 3037 (88.0%) | |
| Single | 387 (5.7%) | 242 (7.0%) | |
| Divorce or widowed | 271 (4.0%) | 173 (5.0%) | |
| Life style characteristics | |||
| Regular exercise habit a | <0.01 | ||
| Regular | 2366 (35.3%) | 1070 (31.3%) | |
| Irregular | 4343 (64.7%) | 2354 (68.7%) | |
| Smoking a | <0.01 | ||
| Smoking | 1135 (16.9%) | 367 (10.7%) | |
| Non-smoking | 5570 (83.1%) | 3066 (89.3%) | |
| Alcohol drinking a | <0.01 | ||
| Drinking | 1065 (16.0%) | 386 (11.3%) | |
| Non-drinking | 5573 (84.0%) | 3018 (88.7%) | |
| Physical Comorbidities | |||
| Hypertension | 2801 (40.0%) | 1265 (35.4%) | <0.01 |
| Diabetes mellitus | 1536 (21.9%) | 622 (17.4%) | <0.01 |
| Hyperlipidemia | 2717 (38.8%) | 1117 (31.3%) | <0.01 |
| Depression No. | Person-Years | IR | Model 1 | Model 2 a | |
|---|---|---|---|---|---|
| Crude HR (95%CI) | Adjusted HR (95%CI) | ||||
| Vegetarian | |||||
| Vegetarian | 78 | 32,898.3 | 2.37 | 0.74 (0.57, 0.96) | 0.70 (0.52, 0.93) |
| Non-vegetarian | 206 | 64,234.0 | 3.21 | 1.00 (Reference) | 1.00 (Reference) |
| Age | |||||
| <50 | 127 | 46,983.0 | 2.70 | 0.86 (0.68, 1.09) | 1.20 (0.90, 1.61) |
| ≥50 | 157 | 50,149.3 | 3.13 | 1.00 (Reference) | 1.00 (Reference) |
| Sex | |||||
| Male | 68 | 63,487.8 | 1.07 | 0.59 (0.45, 0.78) | 0.58 (0.41, 0.83) |
| Female | 216 | 33,644.5 | 6.42 | 1.00 (Reference) | 1.00 (Reference) |
| Education level | |||||
| ≤Elementary school | 84 | 21,489.6 | 3.91 | 1.94 (1.36, 2.76) | 1.59 (1.07, 2.38) |
| Secondary school | 151 | 51,362.1 | 2.94 | 1.46 (1.06, 2.01) | 1.28 (0.91, 1.81) |
| College or higher | 49 | 24,280.6 | 2.02 | 1.00 (Reference) | 1.00 (Reference) |
| Marital status | |||||
| Single | 11 | 5835.2 | 1.89 | 0.66 (0.36, 1.20) | 0.80 (0.42, 1.53) |
| Divorce or widowed | 19 | 4010.7 | 4.74 | 1.65 (1.03, 2.63) | 1.54 (0.95, 2.50) |
| Married | 241 | 83,814.9 | 2.88 | 1.00 (Reference) | 1.00 (Reference) |
| Regular exercise | |||||
| Irregular | 181 | 61,589.6 | 2.94 | 1.00 (0.78, 1.29) | 1.03 (0.78, 1.35) |
| Regular | 92 | 31,475.0 | 2.92 | 1.00 (Reference) | 1.00 (Reference) |
| Smoking | |||||
| Smoking | 33 | 13,731.4 | 2.40 | 0.81 (0.56, 1.17) | 1.13 (0.69, 1.85) |
| Non-Smoking | 235 | 79,400.1 | 2.96 | 1.00 (Reference) | 1.00 (Reference) |
| Alcohol drinking | |||||
| Drinking | 31 | 13,287.2 | 2.33 | 0.78 (0.54, 1.14) | 0.98 (0.61, 1.59) |
| Non-drinking | 236 | 78,943.2 | 2.99 | 1.00 (Reference) | 1.00 (Reference) |
| Comorbidities | |||||
| Hypertension | 143 | 37,070.5 | 3.86 | 1.64 (1.30, 2.08) | 1.40 (1.05, 1.86) |
| Diabetes mellitus | 92 | 19,544.3 | 4.71 | 1.90 (1.49, 2.44) | 1.49 (1.10, 2.00) |
| Hyperlipidemia | 140 | 32,898.3 | 4.26 | 1.71 (1.36, 2.16) | 1.34 (1.00, 1.78) |
| Non-Vegetarians | Vegetarians | Adjusted HR (95%CI) | Pinteraction | |||
|---|---|---|---|---|---|---|
| Case No. | Person-Years | Case No. | Person-Years | |||
| Age of baseline, years | 0.97 | |||||
| <50 | 96 | 31,912.6 | 31 | 15,070.4 | 0.58 (0.37, 0.90) | |
| ≥50 | 110 | 32,321.4 | 47 | 17,827.9 | 0.82 (0.56, 1.19) | |
| Gender | 0.94 | |||||
| Male | 55 | 39,118.0 | 13 | 24,369.8 | 0.72 (0.37, 1.39) | |
| Female | 151 | 25,116.0 | 65 | 8528.5 | 0.73 (0.53, 1.01) | |
| Education level | 0.15 | |||||
| ≤Elementary school | 61 | 13,162.9 | 23 | 8326.7 | 0.66 (0.39, 1.11) | |
| Secondary school | 101 | 34,814.8 | 50 | 16,547.2 | 1.02 (0.70, 1.48) | |
| College or higher | 44 | 16,256.3 | 5 | 8024.4 | 0.15 (0.05, 0.49) | |
| Marital status | 0.57 | |||||
| Married | 173 | 55,838.5 | 68 | 27,976.4 | 0.72 (0.53, 0.97) | |
| Single | 8 | 3595.3 | 3 | 2239.9 | 0.42 (0.08, 2.12) | |
| Divorce or widowed | 13 | 2432.5 | 6 | 1578.2 | 0.86 (0.31, 2.37) | |
| Regular exercise | 0.44 | |||||
| Regular | 72 | 21,631.2 | 20 | 9843.8 | 0.62 (0.37, 1.06) | |
| Irregular | 128 | 39,880.1 | 53 | 21,709.5 | 0.77 (0.54, 1.08) | |
| Smoking | 0.77 | |||||
| Non-Smoking | 169 | 51,132.5 | 66 | 28,267.6 | 0.73 (0.54, 0.99) | |
| Smoking | 28 | 10,355.7 | 5 | 3375.7 | 0.68 (0.26, 1.80) | |
| Alcohol drinking | 0.14 | |||||
| Non-drinking | 176 | 51,108.9 | 60 | 27,834.3 | 0.68 (0.50, 0.92) | |
| Drinking | 21 | 9745.4 | 10 | 3541.8 | 1.12 (0.49, 2.53) | |
| Hypertension | 0.90 | |||||
| No | 100 | 38,783.4 | 41 | 21,278.3 | 0.70 (0.47, 1.05) | |
| Yes | 106 | 25,450.6 | 37 | 11,619.9 | 0.73 (0.48, 1.09) | |
| Diabetes mellitus | 0.44 | |||||
| No | 133 | 50,352.0 | 59 | 27,235.9 | 0.77 (0.55, 1.08) | |
| Yes | 73 | 13,882.0 | 19 | 5662.4 | 0.59 (0.34, 1.02) | |
| Hyperlipidemia | 0.76 | |||||
| No | 100 | 39,335.4 | 44 | 24,898.6 | 0.75 (0.51, 1.11) | |
| Yes | 106 | 22,626.8 | 34 | 10,271.5 | 0.67 (0.44, 1.03) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.-C.; Chang, C.-E.; Lin, M.-N.; Lin, C.-L. Vegetarian Diet Is Associated with Lower Risk of Depression in Taiwan. Nutrients 2021, 13, 1059. https://doi.org/10.3390/nu13041059
Shen Y-C, Chang C-E, Lin M-N, Lin C-L. Vegetarian Diet Is Associated with Lower Risk of Depression in Taiwan. Nutrients. 2021; 13(4):1059. https://doi.org/10.3390/nu13041059
Chicago/Turabian StyleShen, Yu-Chih, Chiao-Erh Chang, Ming-Nan Lin, and Chin-Lon Lin. 2021. "Vegetarian Diet Is Associated with Lower Risk of Depression in Taiwan" Nutrients 13, no. 4: 1059. https://doi.org/10.3390/nu13041059