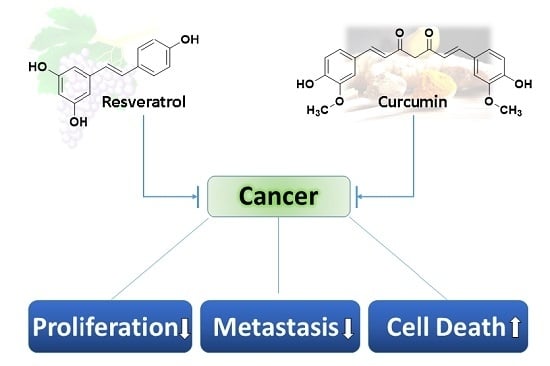
Unraveling the Anticancer Effect of Curcumin and Resveratrol
Abstract
:1. Introduction
2. Cell Proliferation
2.1. Transcription Factors
2.1.1. NF-κB
2.1.2. AP-1
2.1.3. EGR—Early Growth Response
2.1.4. β-Catenin
2.2. Protein Kinases
2.2.1. EGFR—Epidermal Growth Factor Receptor
2.2.2. Polo-Like Kinase (PLK)
2.2.3. Phosphatidylinositol 3-Kinase (PI3K) Pathway
2.2.4. MAPK (p38 e ERK)
2.3. Phosphodiesterases (PDEs)
2.4. Angiogenesis
HIF-1/VEGF/bFGF
2.5. Cell Cycle Regulators
2.6. SIRT
2.7. Others Targets
3. Metastasis
3.1. NF-κB Signaling Pathway
3.2. Matrix Metalloproteinase (MMP)
3.3. E-Cadherin
3.4. Protein Kinases
3.5. Vascular Endothelial Growth Factor (VEGF)
3.6. Hedgehog Signaling Pathway
3.7. STAT-3 Signaling Pathway
3.8. Others
4. Cellular Death
4.1. Apoptosis
4.1.1. ROS
4.1.2. Calcium Homeostasis
4.1.3. Bcl-2 Family
4.1.4. p53 Family
4.1.5. Extrinsic Pathway (Receptor-Mediated Pathway)
Endoplasmatic Reticulum (ER) Stress
4.1.6. NF-κβ
4.1.7. PI3K, Akt/mTOR
4.1.8. Telomerase
4.1.9. JAK/STAT
4.1.10. miRNA
4.2. Autophagy
5. Perspectives
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cimino, S.; Sortino, G.; Favilla, V.; Castelli, T.; Madonia, M.; Sansalone, S.; Russo, G.I.; Morgia, G. Polyphenols: Key issues involved in chemoprevention of prostate cancer. Oxid. Med. Cell. Longev. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.L.; Estrela, J.M.; Ortega, Á.L. Carcinogenesis & mutagenesis natural polyphenols and apoptosis Induction in cancer therapy. J. Carcinog. Mutagen. 2013, 6, 1–10. [Google Scholar]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Burkard, M.; Pfeiffer, M.M.; Lauer, U.M.; Busch, C.; Venturelli, S. Nutritional immunology: Function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutr. J. 2016, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kumar, A.; Butt, N.A.; Zhang, L.; Williams, R.; Rimando, A.M.; Biswas, P.K.; Levenson, A.S. Molecular insight into the differential anti-androgenic activity of resveratrol and its natural analogs: In silico approach to understand biological actions. Mol. BioSyst. 2016, 12, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol compounds and PKC signaling. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zebib, B.; Mouloungui, Z.; Noirot, V. Stabilization of curcumin by complexation with divalent cations in glycerol/water system. Bioinorg. Chem. Appl. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivasan, K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010, 131, 682–691. [Google Scholar] [PubMed]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-M.; Jan, W.-C.; Chien, C.-F.; Lee, W.-C.; Lin, L.-C.; Tsai, T.-H. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011, 127, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Poly(β-cyclodextrin)/curcumin self-assembly: A novel approach to improve curcumin delivery and its therapeutic efficacy in prostate cancer cells. Macromol. Biosci. 2010, 10, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; Mckinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, A. Highly bioavailable curcumin (Theracurmin): Its development and clinical application. PharmaNutrition 2015, 3, 123–130. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Katanasaka, Y.; Hasegawa, K.; Morimoto, T. Clinical applications of curcumin. PharmaNutrition 2015, 3, 131–135. [Google Scholar] [CrossRef]
- Smoliga, J.M.; Blanchard, O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules 2014, 19, 17154–17172. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Pageni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Sessa, M.; Tsao, R.; Liu, R.; Ferrari, G.; Donsì, F. Evaluation of the stability and antioxidant activity of nanoencapsulated resveratrol during in vitro digestion. J. Agric. Food Chem. 2011, 59, 12352–12360. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro characterisation, stability, cytotoxicity and permeation study. AAPS Pharm. Sci. Tech. 2011, 12, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, X.; Wang, Q.; Cheng, S.; Zhang, S.; Zhang, M. Pharmacokinetics, tissue distribution and excretion study of resveratrol and its prodrug 3,5,4’-tri-O-acetylresveratrol in rats. Phytomedicine 2013, 20, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Cottart, C.-H.; Nivet-Antoine, V.; Beaudeux, J.-L. Review of recent data on the metabolism, biologicaleffects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014, 58, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Dose Response 2010, 8, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Dutra, L.A.; de Melo, T.R.F. The paradigma of the interference in assays for natural products. Biochem. Pharmacol. 2016, 5, 1000e183. [Google Scholar] [CrossRef]
- Dos Santos, J.L.; Chin, C.M. Pan-assay interference compounds (PAINS): Warning signs in biochemical-pharmacological evaluations. Biochem. Pharmacol. 2015, 4, 1000e173. [Google Scholar] [CrossRef]
- Ingólfsson, H.I.; Thakur, P.; Herold, K.F.; Hobart, E.A.; Ramsey, N.B.; Periole, X.; de Jong, D.H.; Zwama, M.; Yilmaz, D.; Hall, K.; et al. Phytochemicals perturb membranes and promiscuously alter protein function. ACS Chem. Biol. 2014, 9, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 444. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-kappaB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar] [PubMed]
- Aggarwal, B.B. Nuclear factor-kappaB: The enemy within. Cancer Cell 2004, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.S. Series introduction: The transcription factor NF-kappaB and human disease. J. Clin. Investig. 2001, 107, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Hu, Z.; Zeng, X.; Li, Y.; Su, Y.; Zhang, C.; Ye, Z. Anti-tumor activity of curcumin against androgen-independent prostate cancer cells via inhibition of NF-κB and AP-1 pathway in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Siwak, D.R.; Shishodia, S.; Aggarwal, B.B.; Kurzrock, R. Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B-Raf/mitogen-activated/extracellular signal-regulated protein ki. Cancer 2005, 104, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xia, L.; Zhou, H.; Wu, B.; Mu, Y.; Wu, Y.; Yan, J. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCα and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumor Biol. 2013, 34, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Eckert, R.L. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J. Biol. Chem. 2007, 282, 6707–6715. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, K.M.; Goncharova, E.I.; Young, M.R.; Colburn, N.H.; McMahon, J.B.; Henrich, C.J. A high-throughput cell-based assay to identify specific inhibitors of transcription factor AP-1. J. Biomol. Screen. 2006, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jiao, D.; Wang, L.; Wang, L.; Hu, H.; Song, J.; Yan, J.; Wu, L.; Shi, J. Curcumin inhibits proliferation-migration of NSCLC by steering crosstalk between a Wnt signaling pathway and an adherens junction via EGR-1. Mol. Biosyst. 2015, 11, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Byeong, H.C.; Chang, G.K.; Bae, Y.S.; Lim, Y.; Young, H.L.; Soon, Y.S. p21Waf1/Cip1 expression by curcumin in U-87MG human glioma cells: Role of early growth response-1 expression. Cancer Res. 2008, 68, 1369–1377. [Google Scholar]
- Chen, A.; Xu, J.; Johnson, A. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene 2006, 25, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Lee, G.; Choi, H.Y. Effect of curcumin on the interaction between androgen receptor and Wnt/β-catenin in LNCaP xenografts. Korean J. Urol. 2015, 56, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Lim, J.E.; Hong, J.H. Curcumin interrupts the interaction between the androgen receptor and Wnt/beta-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Y.; Zhang, L.; Li, L.; Shen, Y.; Lin, L.; Zheng, W.; Chen, L.; Bian, X.; Ng, H.K.; Tang, L. Curcumin suppresses cell proliferation through inhibition of the Wnt/β-catenin signaling pathway in medulloblastoma. Oncol. Rep. 2014, 32, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. A thousand and one protein kinases. Cell 1987, 50, 823–829. [Google Scholar] [CrossRef]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. 2015, 100, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The role of protein phosphorylation in human health and disease. Eur. J. Biochem. 2001, 268, 5001–5010. [Google Scholar] [CrossRef] [PubMed]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Lahiry, P.; Torkamani, A.; Schork, N.J.; Hegele, R. A kinase mutations in human disease: Interpreting genotype-phenotype relationships. Nat. Rev. Genet. 2010, 11, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, D.; Cowan-Jacob, S.W.; Moebitz, H. Ten things you should know about protein kinases: IUPHAR Review 14. Br. J. Pharmacol. 2015, 172, 2675–2700. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Huang, C.-Z. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J. Gastroenterol. 2015, 21, 11673–11679. [Google Scholar] [CrossRef] [PubMed]
- Yesilkanal, A.E.; Rosner, M.R. Raf kinase inhibitory protein (RKIP) as a metastasis suppressor: Regulation of signaling networks in cancer. Crit. Rev. Oncog. 2014, 19, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Almhanna, K.; Strosberg, J.; Malafa, M. Targeting AKT protein kinase in gastric cancer. Anticancer Res. 2011, 31, 4387–4392. [Google Scholar] [PubMed]
- Zhou, H.; Huang, S. Role of mTOR signaling in tumor cell motility, invasion and metastasis. Curr. Protein Pept. Sci. 2011, 12, 30–42. [Google Scholar] [PubMed]
- Rattanasinchai, C.; Gallo, K. MLK3 signaling in cancer invasion. Cancers 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Katsiampoura, A.D.; Araujo, J.C.; Gallick, G.E.; Corn, P.G. Src signaling pathways in prostate cancer. Cancer Metastasis Rev. 2014, 33, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Durand, N.; Borges, S.; Storz, P. Functional and therapeutic significance of protein kinase D enzymes in invasive breast cancer. Cell. Mol. Life Sci. 2015, 72, 4369–4382. [Google Scholar] [CrossRef] [PubMed]
- Igea, A.; Nebreda, A.R. The stress kinase p38α as a target for cancer therapy. Cancer Res. 2015, 75, 3997–4002. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Huang, D.; Lu, N.; Luo, L. Role of the LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells (Review). Oncol. Rep. 2015, 34, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Starok, M.; Preira, P.; Vayssade, M.; Haupt, K.; Salomé, L.; Rossi, C. EGFR inhibition by curcumin in cancer cells: A dual mode of action. Biomacromolecules 2015, 16, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Chadalapaka, G.; Jutooru, I.; Burghardt, R.; Safe, S. Drugs that target specificity proteins downregulate epidermal growth factor receptor in bladder cancer cells. Mol. Cancer Res. 2010, 8, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Amani, V.; Prince, E.W.; Alimova, I.; Balakrishnan, I.; Birks, D.; Donson, A.M.; Harris, P.; Levy, J.M.M.; Handler, M.; Foreman, N.K.; et al. Polo-like Kinase 1 as a potential therapeutic target in Diffuse Intrinsic Pontine Glioma. BMC Cancer 2016, 16, 647. [Google Scholar] [CrossRef] [PubMed]
- Van Erk, M.J.; Teuling, E.; Staal, Y.C.; Huybers, S.; Van Bladeren, P.J.; Aarts, J.M.; Van Ommen, B. Time- and dose-dependent effects of curcumin on gene expression in human colon cancer cells. J. Carcinog. 2004, 3, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosieniak, G.; Sliwinska, M.A.; Przybylska, D.; Grabowska, W.; Sunderland, P.; Bielak-Zmijewska, A.; Sikora, E. Curcumin-treated cancer cells show mitotic disturbances leading to growth arrest and induction of senescence phenotype. Int. J. Biochem. Cell Biol. 2016, 74, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. PI 3-kinase, Akt and cell survival. Semin. Cell Dev. Biol. 2004, 15, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Minet, E.; Michel, G.; Mottet, D.; Raes, M.; Michiels, C. Transduction pathways involved in hypoxia-inducible factor-1 phosphorylation and activation. Free Radic. Biol. Med. 2001, 31, 847–855. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Lu, Q.Y.; Zhang, Z.F.; Brown, J.; Le, A.D. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1α and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol. Cancer Ther. 2005, 4, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.C.; Dufort, F.J.; Blair, D.; Wagner, D.; Roberts, M.F.; Chiles, T.C. Inhibition of phosphatidylinositol 3-kinase-mediated glucose metabolism coincides with resveratrol-induced cell cycle arrest in human diffuse large B-cell lymphomas. Biochem. Pharmacol. 2006, 72, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.A.; Pozo-Guisado, E.; Clementi, M.; Castellón, E.A.; Fernandez-Salguero, P.M. Non-genomic action of resveratrol on androgen and oestrogen receptors in prostate cancer: Modulation of the phosphoinositide 3-kinase pathway. Br. J. Cancer 2007, 96, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Benitez, D.A.; Hermoso, M.A.; Pozo-Guisado, E.; Fernández-Salguero, P.M.; Castellón, E.A. Regulation of cell survival by resveratrol involves inhibition of NFκβ-regulated gene expression in prostate cancer cells. Prostate 2009, 69, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.C.; Wong, C.; Bennett, D.J.; Wu, J.M. Regulation of p53 and cell proliferation by resveratrol and its derivatives in breast cancer cells: An in silico and biochemical approach targeting integrin αvβ3. Int. J. Cancer 2011, 129, 2732–2743. [Google Scholar] [CrossRef] [PubMed]
- De Amicis, F.; Giordano, F.; Vivacqua, A.; Pellegrino, M.; Panno, M.L.; Tramontano, D.; Fuqua, S.A.W.; Ando, S. Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J. 2011, 25, 3695–3707. [Google Scholar] [CrossRef] [PubMed]
- Abusnina, A.; Keravis, T.; Yougbaré, I.; Bronner, C.; Lugnier, C. Anti-proliferative effect of curcumin on melanoma cells is mediated by PDE1A inhibition that regulates the epigenetic integrator UHRF1. Mol. Nutr. Food Res. 2011, 55, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Abusnina, A.; Keravis, T.; Zhou, Q.; Justiniano, H.; Lobstein, A.; Lugnier, C. Tumour growth inhibition and anti-angiogenic effects using curcumin correspond to combined PDE2 and PDE4 inhibition. Thromb. Haemost. 2015, 113, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. Targeting angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, F. Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer Lett. 2012, 320, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.K.; Kim, S.H.; Jeong, J.W.; Lee, Y.M.; Kim, H.S.; Kim, S.R.; Yun, I.; Bae, S.K.; Kim, K.W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Schaaf, C.; Schmidt, A.; Lucia, K.; Buchfelder, M.; Losa, M.; Kuhlen, D.; Kreutzer, J.; Perone, M.J.; Arzt, E.; et al. Curcumin suppresses HIF1A synthesis and VEGFA release in pituitary adenomas. J. Endocrinol. 2012, 214, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, X.; Guan, S.; Yan, Y.; Lin, H.; Hua, Z.-C. Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget 2015, 6, 19469–19482. [Google Scholar] [CrossRef] [PubMed]
- Dann, J.M.; Sykes, P.H.; Mason, D.R.; Evans, J.J. Regulation of vascular endothelial growth factor in endometrial tumour cells by resveratrol and EGCG. Gynecol. Oncol. 2009, 113, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, C.; Huang, J.; Hong, L.; Zhang, L.; Chu, Z. Antimyeloma effects of resveratrol through inhibition of angiogenesis. Chin. Med. J. 2007, 120, 1672–1677. [Google Scholar] [PubMed]
- Liu, Z.; Li, Y.; Yang, R. Effects of resveratrol on vascular endothelial growth factor expression in osteosarcoma cells and cell proliferation. Oncol. Lett. 2012, 4, 837–839. [Google Scholar] [PubMed]
- Yang, R.; Zhang, H.; Zhu, L. Inhibitory effect of resveratrol on the expression of the VEGF gene and proliferation in renal cancer cells. Mol. Med. Rep. 2011, 4, 981–983. [Google Scholar] [PubMed]
- Trapp, V.; Parmakhtiar, B.; Papazian, V.; Willmott, L.; Fruehauf, J.P. Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis 2010, 13, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.S.; Mousa, S.S.; Mousa, S.A. Effect of resveratrol on angiogenesis and platelet/fibrin-accelerated tumor growth in the chick chorioallantoic membrane model. Nutr. Cancer 2005, 52, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.L.; Liu, Y.Y.; Ma, Y.G.; Xue, Y.X.; Liu, D.G.; Ren, Y.; Liu, X.B.; Li, Y.; Li, Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through janus kinase-STAT3 signalling pathway. PLoS ONE 2012, 7, e37960. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-G.; Lee, S.-Y.; Huang, Z.; Lim, D.Y.; Chen, H.; Jung, S.K.; Bode, A.M.; Lee, K.W.; Dong, Z. Curcumin suppresses proliferation of colon cancer cells by targeting CDK2. Cancer Prev. Res. 2014, 7, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Hudson, T.S.; Hartle, D.K.; Hursting, S.D.; Nunez, N.P.; Wang, T.T.Y.; Young, H.A.; Arany, P.; Green, J.E. Inhibition of prostate cancer growth by muscadine grape skin extract and resveratrol through distinct mechanisms. Cancer Res. 2007, 67, 8396–8405. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, Z.; Chu, M.; Wang, Z.; Zhang, W.; Wang, L.; Li, C.; Wang, J. Resveratrol inhibited GH3 cell growth and decreased prolactin level via estrogen receptors. Clin. Neurol. Neurosurg. 2012, 114, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.L.; Zhu, Y.; Zhu, H.; Han, L.; Kopelovich, L.; Bickers, D.R.; Athar, M. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp. Dermatol. 2006, 15, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, Y.; Xia, J.; Liu, B.; Zhang, Q.; Liu, J.; Luo, L.; Peng, Z.; Song, Z.; Zhu, R. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol. Med. Rep. 2015, 11, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Fukui, M.; Choi, H.J.; Zhu, B.T. Induction of a reversible, non-cytotoxic S-phase delay by resveratrol: Implications for a mechanism of lifespan prolongation and cancer protection. Br. J. Pharmacol. 2009, 158, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-D.; Yang, J.; Zhang, W.-L.; Liu, D.-X. Resveratrol inhibits oral squamous cell carcinoma through induction of apoptosis and G2/M phase cell cycle arrest. Tumor Biol. 2016, 37, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Carafa, V.; Nebbioso, A.; Altucci, L. Sirtuins and disease: The road ahead. Front. Pharmacol. 2012, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, B.; Zang, W.; Wang, X.; Liu, Z.; Li, W.; Jia, J. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS ONE 2013, 8, e70627. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-N.; Lin, V.C.-H.; Rau, K.-M.; Shieh, P.-C.; Kuo, D.-H.; Shieh, J.-C.; Chen, W.-J.; Tsai, S.-C.; Way, T.-D. Resveratrol modulates tumor cell proliferation and protein translation via SIRT1-dependent AMPK activation. J. Agric. Food Chem. 2010, 58, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, W.; Li, J.; Liu, M.; Wei, M. Resveratrol suppresses the STAT3 signaling pathway and inhibits proliferation of high glucose-exposed HepG2 cells partly through SIRT1. Oncol. Rep. 2013, 30, 2820–2828. [Google Scholar] [PubMed]
- Chang, Y.J.; Huang, C.Y.; Hung, C.S.; Chen, W.Y.; Wei, P.L. GRP78 mediates the therapeutic efficacy of curcumin on colon cancer. Tumor Biol. 2015, 36, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.X.; He, Y.J.; Zhao, S.Z.; Wu, J.G.; Wang, J.T.; Zhu, L.M.; Lin, T.T.; Sun, B.C.; Li, X.R. Inhibition of tumor growth and vasculogenic mimicry by cucumin through downregulation of the EphA2/PI3K/MMP pathway in a murine choroidal melanoma model. Cancer Biol. Ther. 2011, 11, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Q.; Yu, K.; Yan, Q.X.; Xing, C.Y.; Chen, Y.; Yan, Z.; Shi, Y.F.; Zhao, K.W.; Gao, S.M. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class I histone deacetylases. Carcinogenesis 2013, 34, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, Y.; Wang, Y.; Rao, J.; Jiang, X.; Xu, Z. Curcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expression. J. Steroid Biochem. Mol. Biol. 2014, 143, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, J.; Chen, C.; Chen, J.; Ye, L.; Wang, L.; Wu, J.; Xing, C.; Yu, K. Pure curcumin decreases the expression of WT1 by upregulation of miR-15a and miR-16-1 in leukemic cells. J. Exp. Clin. Cancer Res. 2012, 31, 27. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shu, L.; Zhang, C.; Su, Z.-Y.; Kong, A.-N.T. Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1. Biochem. Pharmacol. 2015, 94, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, X.; Cai, X.; Su, J.; Ma, R.; Yin, X.; Zhou, X.; Li, H.; Wang, Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cells. Oncotarget 2015, 6, 18027–18037. [Google Scholar] [CrossRef] [PubMed]
- Atten, M.J.; Godoy-romero, E.; Attar, B.M.; Milson, T.; Zopel, M.; Holian, O. Resveratrol regulates cellular PKC α and δ to inhibit growth and induce apoptosis in gastric cancer cells. Investig. New Drugs 2005, 23, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Choi, B.Y.; Kundu, J.K.; Shin, Y.K.; Na, H.-K.; Surh, Y.-J. Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: Eukaryotic elongation factor 1A2 as a potential target. Cancer Res. 2009, 69, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- Vyas, S.; Asmerom, Y.; De León, D.D. Resveratrol regulates insulin-like growth factor-II in breast cancer cells. Endocrinology 2005, 146, 4224–4233. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.A.; Sinden, M.R.; Eng, C. Phytoestrogen exposure elevates PTEN levels. Hum. Mol. Genet. 2005, 14, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Golkar, L.; Ding, X.Z.; Ujiki, M.B.; Salabat, M.R.; Kelly, D.L.; Scholtens, D.; Fought, A.J.; Bentrem, D.J.; Talamonti, M.S.; Bell, R.H.; Adrian, T.E. Resveratrol inhibits pancreatic cancer cell proliferation through transcriptional induction of Macrophage Inhibitory Cytokine-1. J. Surg. Res. 2007, 138, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.S.; Zancan, P.; Marcondes, M.C.; Ramos-Santos, L.; Meyer-Fernandes, J.R.; Sola-Penna, M.; Da Silva, D. Resveratrol decreases breast cancer cell viability and glucose metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie 2013, 95, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsiao, W.C.; Wright, D.E.; Hsu, C.L.; Lo, Y.C.; Wang Hsu, G.S.; Kao, C.F. Resveratrol activates the histone H2B ubiquitin ligase, RNF20, in MDA-MB-231 breast cancer cells. J. Funct. Foods 2013, 5, 790–800. [Google Scholar] [CrossRef]
- Luo, H.; Yang, A.; Schulte, B.A.; Wargovich, M.J.; Wang, G.Y. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS ONE 2013, 8, e60065. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, M.S.; Barnett, T.L.; Xu, C.W. Resveratrol induces cellular senescence with attenuated mono-ubiquitination of histone H2B in glioma cells. Biochem. Biophys. Res. Commun. 2011, 407, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.A.; Welch, D.R. Metastasis: Recent discoveries and novel treatment strategies. Lancet 2007, 369, 1742–1757. [Google Scholar] [CrossRef]
- Wan, L.; Pantel, K.; Kang, Y. Tumor metastasis: Moving new biological insights into the clinic. Nat. Med. 2013, 19, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.; Theodorescu, D. Metastasis: A therapeutic target for cancer. Nat. Clin. Pract. Oncol. 2008, 5, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Sleeman, J.; Steeg, P.S. Metastasis: A therapeutic target for cancer. Eur. J. Cancer 2010, 46, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zou, L.; Huang, C.; Lei, Y. Redox regulation of cancer metastasis: Molecular signaling and therapeutic opportunities. Drug Dev. Res. 2014, 75, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, L. The emerging molecular machinery and therapeutic targets of metastasis. Trends Pharmacol. Sci. 2015, 36, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L. The molecular genetics of progression and metastasis. Cancer Metastasis Rev. 2000, 19, 327–344. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Tan, B.K.H.; Kumar, A.P.; Sethi, G. The Multifaceted Role of Curcumin in Cancer Prevention and Treatment. Molecules 2015, 20, 2728–2769. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Lee, J.; Lee, Y.S. Curcumin in various cancers. BioFactors 2013, 39, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (Diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα Kinase and Akt activation. Mol. Pharmacol. 2006, 69, 195–206. [Google Scholar] [PubMed]
- Bachmeier, B.E.; Mohrenz, I.V.; Mirisola, V.; Schleicher, E.; Romeo, F.; Höhneke, C.; Jochum, M.; Nerlich, A.G.; Pfeffer, U. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFκB. Carcinogenesis 2008, 29, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massague, J. Genes that mediate breast cancer metastasis to lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Ammanamanchi, S. Curcumin blocks RON tyrosine kinase-mediated invasion of breast carcinoma cells. Cancer Res. 2008, 68, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Thangasamy, A.; Rogge, J.; Ammanamanchi, S. Recepteur d’Origine nantais tyrosine kinase is a direct target of hypoxia-inducible factor-1α-mediated invasion of breast carcinoma cells. J. Biol. Chem. 2009, 284, 14001–14010. [Google Scholar] [CrossRef] [PubMed]
- Zong, H.; Wang, F.; Fan, Q.-X.; Wang, L.-X. Curcumin inhibits metastatic progression of breast cancer cell through suppression of urokinase-type plasminogen activator by NF-kappa B signaling pathways. Mol. Biol. Rep. 2012, 39, 4803–4808. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.S.; Jung, S.H.; Kim, S.Y.; Hyun, J.W.; Ko, K.H.; Kim, W.K.; Kim, H.S. Curcumin suppresses phorbol ester-induced matrix metalloproteinase-9 expression by inhibiting the PKC to MAPK signaling pathways in human astroglioma cells. Biochem. Biophys. Res. Commun. 2005, 335, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Ganapathy, S.; Chen, Q.; Srivastava, R.K. Curcumin sensitizes TRAIL-resistant xenografts: Molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol. Cancer 2008, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Chang, W.-W.; Kuan, Y.-D.; Lin, S.-T.; Hsu, H.-C.; Lee, C.-H. Resveratrol inhibits LPS-induced epithelial-mesenchymal transition in mouse melanoma model. Innate Immun. 2012, 18, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sull, J.W.; Sung, H.J. Suppressing effect of resveratrol on the migration and invasion of human metastatic lung and cervical cancer cells. Mol. Biol. Rep. 2012, 39, 8709–8716. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.L.; Tsai, J.R.; Charles, A.L.; Hwang, J.J.; Chou, S.H.; Ping, Y.H.; Lin, F.Y.; Chen, Y.L.; Hung, C.Y.; Chen, W.C.; et al. Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factor-κB pathway and subsequently downregulating expression of matrix metalloproteinases. Mol. Nutr. Food Res. 2010, 54, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Łoboda, A.; Zagórska, A.; Józkowicz, A. Complex role of heme oxygenase-1 in angiogenesis. Antioxid. Redox Signal. 2004, 6, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Pan, C.; Zhao, S.; Wang, Z.; Zhang, H.; Wu, W. Resveratrol inhibits tumor necrosis factor-alpha-mediated matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. Biomed. Pharmacother. 2008, 62, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ku, B.M.; Lee, Y.K.; Jeong, J.Y.; Kang, S.; Choi, J.; Yang, Y.; Lee, D.H.; Roh, G.S.; Kim, H.J.; et al. Resveratrol reduces TNF-alpha-induced U373MG human glioma cell invasion through regulating NF-kappaB activation and uPA/uPAR expression. Anticancer Res. 2011, 31, 4223–4230. [Google Scholar] [PubMed]
- Behrens, J. The role of cell adhesion molecules in cancer invasion and metastasis. Breast Cancer Res. Treat. 1993, 24, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Reymond, N.; D’Água, B.B.; Ridley, A.J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 2013, 13, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Okegawa, T.; Pong, R.-C.; Li, Y.; Hsieh, J.-T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 2004, 51, 445–457. [Google Scholar] [PubMed]
- Park, J.S.; Kim, K.M.; Kim, M.H.; Chang, H.J.; Baek, M.K.; Kim, S.M.; Jung, Y. Do resveratrol inhibits tumor cell adhesion to endothelial cells by blocking ICAM-1 expression. Anticancer Res. 2009, 29, 355–362. [Google Scholar] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S.K. Curcumin: A potential candidate for matrix metalloproteinase inhibitors. Expert Opin. Ther. Targets 2012, 16, 959–972. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.T.; Murray, G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015, 237, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Park, J.K.; Jouben-Steele, L.; Kremen, T.J.; Liau, L.M.; Vinters, H.V.; Cloughesy, T.F.; Mischel, P.S. Active matrix metalloproteinase 9 expression is associated with primary glioblastoma subtype. Clin. Cancer Res. 2002, 8, 2894–2901. [Google Scholar] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol. Rep. 2009, 21, 1323–1333. [Google Scholar] [PubMed]
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 15, 32–40. [Google Scholar]
- Xu, X.; Wang, Y.; Chen, Z.; Sternlicht, M.D.; Hidalgo, M.; Steffensen, B. Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res. 2005, 65, 130–136. [Google Scholar] [PubMed]
- Chen, Q.; Gao, Q.; Chen, K.; Wang, Y.; Chen, L.; Li, X. Curcumin suppresses migration and invasion of human endometrial carcinoma cells. Oncol. Lett. 2015, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Lakka, S.S.; Jasti, S.L.; Gondi, C.; Boyd, D.; Chandrasekar, N.; Dinh, D.H.; Olivero, W.C.; Gujrati, M.; Rao, J.S. Downregulation of MMP-9 in ERK-mutated stable transfectants inhibits glioma invasion in vitro. Oncogene 2002, 21, 5601–5608. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.S.; Chen, W.C.; Lin, Y.Y.; Tsai, C.H.; Liao, C.I.; Shyu, H.Y.; Ko, C.J.; Tzeng, S.F.; Huang, C.Y.; Yang, P.C.; et al. Curcumin-targeting pericellular serine protease matriptase role in suppression of prostate cancer cell invasion, tumor growth, and metastasis. Cancer Prev. Res. 2013, 6, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Duan, X.; Cai, H.; Wang, L.; Li, M.; Qu, J.; Li, W.; Wang, Y.; Wang, J. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCα/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-Y.; Zheng, Y.; Jiao, D.-M.; Chen, F.-Y.; Hu, H.-Z.; Wu, Y.-Q.; Song, J.; Yan, J.; Wu, L.-J.; Lv, G.-Y. Curcumin inhibits lung cancer cell migration and invasion through Rac1-dependent signaling pathway. J. Nutr. Biochem. 2014, 25, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Banerji, A.; Chakrabarti, J.; Mitra, A.; Chatterjee, A. Effect of curcumin on gelatinase a (MMP-2) activity in B16F10 melanoma cells. Cancer Lett. 2004, 211, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kurschat, P.; Zigrino, P.; Nischt, R.; Breitkopf, K.; Steurer, P.; Klein, C.E.; Krieg, T.; Mauch, C. Tissue inhibitor of matrix metalloproteinase-2 regulates matrix metalloproteinase-2 activation by modulation of membrane-type 1 matrix metalloproteinase activity in high and low invasive melanoma cell lines. J. Biol. Chem. 1999, 274, 21056–21062. [Google Scholar] [CrossRef] [PubMed]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [PubMed]
- Mitra, A.; Chakrabarti, J.; Banerji, A.; Chatterjee, A.; Das, B.R. Curcumin, a potential inhibitor of MMP-2 in human laryngeal squamous carcinoma cells HEp2. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Wang, Z.; Deng, Z.; Ren, H.; Li, X. Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1-MMP/MMP2 pathway. Int. J. Clin. Exp. Med. 2015, 8, 8948–8957. [Google Scholar] [PubMed]
- Gagliano, N.; Moscheni, C.; Torri, C.; Magnani, I.; Bertelli, A.A.; Gioia, M. Effect of resveratrol on matrix metalloproteinase-2 (MMP-2) and Secreted Protein Acidic and Rich in Cysteine (SPARC) on human cultured glioblastoma cells. Biomed. Pharmacother. 2005, 59, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S.; Ruhe, C.; Derikito, M.G.; Bose, G.; Sauer, H.; Wartenberg, M. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer Lett. 2007, 250, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Ha, A.W.; Kim, W.K. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr. Res. Pract. 2012, 6, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, Y.; Guo, T.; Wang, H.; Zhang, X.; He, W.; Tan, H. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol. Sin. 2006, 27, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.J.; Wu, C.F.; Huang, H.W.; Wu, C.H.; Ho, C.T.; Yen, G.C. Evaluation of anti-invasion effect of resveratrol and related methoxy analogues on human hepatocarcinoma cells. J. Agric. Food Chem. 2010, 58, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Antos, C.; Butz, S.; Huber, O.; Delmas, V.; Dominis, M.; Kemler, R. A role for cadherins in tissue formation. Development 1996, 122, 3185–3194. [Google Scholar] [PubMed]
- Zhang, B.; Zhang, H.; Shen, G. Metastasis-associated protein 2 (MTA2) promotes the metastasis of non-small-cell lung cancer through the inhibition of the cell adhesion molecule Ep-CAM and E-cadherin. Jpn. J. Clin. Oncol. 2015, 45, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Galván, J.A.; Zlobec, I.; Wartenberg, M.; Lugli, A.; Gloor, B.; Perren, A.; Karamitopoulou, E. Expression of E-cadherin repressors SNAIL, ZEB1 and ZEB2 by tumour and stromal cells influences tumour-budding phenotype and suggests heterogeneity of stromal cells in pancreatic cancer. Br. J. Cancer 2015, 112, 1944–1950. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-L.; Xie, Y.-G.; Li, Z.; Ma, J.-H.; Xu, X. E-cadherin expression and prognosis of oral cancer: A meta-analysis. Tumour Biol. 2014, 35, 5533–5537. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Hiltwein, F.; Grill, J.; Blum, H.; Krebs, S.; Klanner, A.; Bauersachs, S.; Bruns, C.; Longerich, T.; Horst, D.; et al. Evidence for a role of E-cadherin in suppressing liver carcinogenesis in mice and men. Carcinogenesis 2014, 35, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, K.-M.; Liu, X.; Chu, K.-M. E-cadherin and gastric cancer: Cause, consequence, and applications. Biomed. Res. Int. 2014, 2014, 637308. [Google Scholar] [CrossRef] [PubMed]
- Barber, A.G.; Castillo-Martin, M.; Bonal, D.M.; Jia, A.J.; Rybicki, B.A.; Christiano, A.M.; Cordon-Cardo, C. PI3K/AKT pathway regulates E-cadherin and Desmoglein 2 in aggressive prostate cancer. Cancer Med. 2015, 4, 1258–1271. [Google Scholar] [CrossRef] [PubMed]
- Trillsch, F.; Kuerti, S.; Eulenburg, C.; Burandt, E.; Woelber, L.; Prieske, K.; Eylmann, K.; Oliveira-Ferrer, L.; Milde-Langosch, K.; Mahner, S. E-Cadherin fragments as potential mediators for peritoneal metastasis in advanced epithelial ovarian cancer. Br. J. Cancer 2016, 114, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Latorre, D.; Cascione, F.; Leporatti, S.; Trerotola, M.; Giudetti, A.M.; Capobianco, L.; Lunetti, P.; Rizzello, A.; et al. Proteomics analysis of E-cadherin knockdown in epithelial breast cancer cells. J. Biotechnol. 2015, 202, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kimelman, D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J. Cell Sci. 2007, 120, 3337–3344. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Lee, J.-Y.; Huang, J.-Y.; Wang, C.-C.; Chen, W.-J.; Su, S.-F.; Huang, C.-W.; Ho, C.-C.; Chen, J.J.W.; Tsai, M.-F.; et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008, 68, 7428–7438. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Sureshbabul, M.; Chen, H.W.; Lin, Y.S.; Lee, J.Y.; Hong, Q.S.; Yang, Y.C.; Yu, S.L. Curcumin suppresses metastasis via Sp-1, FAK inhibition, and E-cadherin upregulation in colorectal cancer. Evid. Based Complement. Altern. Med. 2013, 2013, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Zhang, L.; Cheng, X.; Lin, X.-F.F.; Lu, R.-R.R.; Bao, J.-D.D.; Yu, H.-X.X. Curcumin inhibits hypoxia-induced migration in K1 papillary thyroid cancer cells. Exp. Biol. Med. 2015, 240, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Zhang, L.; Yu, H.-X.; Bao, J.-D.; Lu, R.-R. Curcumin inhibits the metastasis of K1 papillary thyroid cancer cells via modulating E-cadherin and matrix metalloproteinase-9 expression. Biotechnol. Lett. 2013, 35, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, X.; Gao, Y.; Zhang, C.; Bao, J.; Guan, H.; Yu, H.; Lu, R.; Xu, Q.; Sun, Y. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Exp. Cell Res. 2016, 341, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Xu, C.; Song, L.; Huang, L.; Lai, Y.; Wang, Y.; Chen, H.; Gu, D.; Ren, L.; Yao, Q. Curcumin inhibits tumor epithelial-mesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol. Rep. 2016, 35, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Long, Q.; Zhang, L.; Shi, Y.; Liu, X.; Li, X.; Guan, B.; Tian, Y.; Wang, X.; Li, L.; He, D. Curcumin inhibits cancer-associated fibroblast-driven prostate cancer invasion through MAOA/mTOR/HIF-1α signaling. Int. J. Oncol. 2015, 47, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Ding, Y.; Zhang, Y.; Zhou, Y.; Li, M.; Wang, C. Curcumin suppresses proliferation and migration of MDA-MB-231 breast cancer cells through autophagy-dependent Akt degradation. PLoS ONE 2016, 11, e0146553. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Tian, T.; Nan, K.; Wang, W. p57: A multifunctional protein in cancer (Review). Int. J. Oncol. 2010, 36, 1321–1329. [Google Scholar] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeong, K.J.; Lee, J.; Yoon, D.S.; Choi, W.S.; Kim, Y.K.; Han, J.W.; Kim, Y.M.; Kim, B.K.; Lee, H.Y. Hypoxia enhances LPA-induced HIF-1α and VEGF expression: Their inhibition by resveratrol. Cancer Lett. 2007, 258, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Baribeau, S.; Chaudhry, P.; Parent, S.; Asselin, E. Resveratrol inhibits cisplatin-induced epithelial-to- mesenchymal transition in ovarian cancer cell lines. PLoS ONE 2014, 9, e86987. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Hsieh, Y.; Yang, S.; Chen, C.; Tang, C.; Chuang, Y.; Lin, C.; Chen, M. Resveratrol suppresses TPA-induced matrix metalloproteinase-9 expression through the inhibition of MAPK pathways in oral cancer cells. J. Oral Pathol. Med. 2015, 44, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Chen, Q.Y.; Wu, L.J.; Yao, X.M.; Sun, X.J. Antitumor and antimetastatic activities of grape skin polyphenols in a murine model of breast cancer. Food Chem. Toxicol. 2012, 50, 3462–3467. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Chiang, E.I.; Sun, Y. Resveratrol inhibits heregulin-β 1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells. J. Nutr. Biochem. 2008, 19, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Gweon, E.J.; Kim, S.J. Resveratrol induces MMP-9 and cell migration via the p38 kinase and PI-3K pathways in HT1080 human fbrosarcoma cells. Oncol. Rep. 2013, 29, 826–834. [Google Scholar] [PubMed]
- Yeh, C.B.; Hsieh, M.J.; Lin, C.W.; Chiou, H.L.; Lin, P.Y.; Chen, T.Y.; Yang, S.F. The antimetastatic effects of resveratrol on hepatocellular carcinoma through the downregulation of a metastasis-associated protease by SP-1 modulation. PLoS ONE 2013, 8, e56661. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lee, W.; Tan, P.; Tang, C.; Hsiao, M.; Hsieh, F.; Chien, M. Upregulation of miR-328 and inhibition of CREB-DNA-binding activity are critical for resveratrol-mediated suppression of matrix metalloproteinase-2 and subsequent metastatic ability in human osteosarcomas. Oncotarget 2015, 6, 2736–2753. [Google Scholar] [CrossRef] [PubMed]
- Vara, J.Á.F.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; Testa, J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005, 24, 7455–7464. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Darjatmoko, S.R.; Polans, A.S. Resveratrol modulates the malignant properties of cutaneous melanoma through changes in the activation and attenuation of the antiapoptotic protooncogenic protein Akt/PKB. Melanoma Res. 2011, 21, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Li, H.; Liu, Y.; Guo, A.; Xu, X.; Qu, X.; Wang, S.; Zhao, J.; Li, Y.; Cao, Y. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients 2015, 7, 4383–4402. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, J.; Ma, Q.; Li, B.; Han, L.; Liu, J.; Xu, Q.; Duan, W.; Yu, S.; Wang, F.; Wu, E. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr. Med. Chem. 2013, 20, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Su, Y.C.; Chen, N.C.; Hsieh, H.S.; Chen, K.S. Resveratrol inhibits migration and invasion of human breast-cancer cells. Mol. Nutr. Food Res. 2008, 52, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, T.; Sel, S.; Hutten, H.; Ropke, M.; Roessner, A.; Nass, N. Curcumin blocks interleukin-1 signaling in chondrosarcoma cells. PLoS ONE 2014, 9, e99296. [Google Scholar] [CrossRef] [PubMed]
- Da, W.; Zhu, J.; Wang, L.; Sun, Q. Curcumin suppresses lymphatic vessel density in an in vivo human gastric cancer model. Tumour Biol. 2015, 36, 5215–5223. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.L.; de Sauvage, F.J. Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 2006, 5, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Amakye, D.; Jagani, Z.; Dorsch, M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 2013, 19, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Takebe, N.; LoRusso, P. Targeting the Hedgehog pathway in cancer. Ther. Adv. Med. Oncol. 2010, 2, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chong, T.; Wang, Z.; Chen, H.; Li, H.; Cao, J.; Zhang, P.; Li, H. A novel anti-cancer effect of resveratrol: Reversal of epithelial-mesenchymal transition in prostate cancer cells. Mol. Med. Rep. 2014, 10, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Matise, M.P.; Joyner, A.L. Gli genes in development and cancer. Oncogene 1999, 18, 7852–7859. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yuan, Y.; Gan, H.; Peng, Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol. Lett. 2015, 9, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, L.; Chen, X.; Lei, J.; Ma, Q. Resveratrol inhibits hypoxia-driven ROS-induced invasive and migratory ability of pancreatic cancer cells via suppression of the Hedgehog signaling pathway. Oncol. Rep. 2016, 35, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 17, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Sikka, S.; Surana, R.; Daia, X.; Zhang, J.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Targeting the STAT-3 signaling pathway in cancer: Role of synthetic and natural inhibitors. Biochim. Biophys. Acta 2014, 1845, 136–154. [Google Scholar] [PubMed]
- Eswaran, D.; Huang, S. STAT-3 as a central regulator of tumor metastases. Curr. Mol. Med. 2009, 9, 626–633. [Google Scholar]
- Lee-Chang, C.; Bodogai, M.; Martin-Montalvo, A.; Wejksza, K.; Sanghvi, M.; Moaddel, R.; de Cabo, R.; Biragyn, A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol. 2013, 191, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Olkhanud, P.B.; Damdinsuren, B.; Bodogai, M.; Gress, R.E.; Sen, R.; Wejksza, K.; Malchinkhuu, E.; Wersto, R.P.; Biragyn, A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011, 71, 3505–3515. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Sumiyoshi, M. Resveratrol prevents tumor growth and metastasis by inhibiting lymphangiogenesis and M2 macrophage activation and differentiation in tumor-associated macrophages. Nutr. Cancer 2016, 68, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.; Michel, J.; Schönhaar, K.; Goerdt, S.; Schledzewski, K. Differentiation and gene expression profile of tumor-associated macrophages. Semin. Cancer Biol. 2012, 22, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, H.; Zhang, Y. Resveratrol inhibits hypoxia-induced glioma cell migration and invasion by the p-STAT-3/miR-34a axis. Neoplasma 2016, 63, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Di Martino, M.T.; De Rosa, G.; Farooqi, A.A.; Lombardi, A.; Campani, V.; Zarone, M.R.; Gullà, A.; Tagliaferri, P.; Tassone, P.; et al. Mir-34: A New Weapon Against Cancer? Mol. Ther. Nucleic Acids 2014, 3, e194. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Jiao, D.E.M.; Yao, Q.H.; Yan, J.; Song, J.; Chen, F.Y.; Lu, G.H.; Zhou, J.Y. Expression analysis of Cdc42 in lung cancer and modulation of its expression by curcumin in lung cancer cell lines. Int. J. Oncol. 2012, 40, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Marshall, C.J. RHO-GTPases and cancer. Nat. Rev. Cancer 2002, 2, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dong, Z.; Zhang, Y.; Miao, J. The roles of integrin β4 in vascular endothelial cells. J. Cell. Physiol. 2012, 227, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.T.; Soung, Y.H.; Surh, Y.J.; Cardelli, J.A.; Chung, J. Curcumin prevents palmitoylation of integrin β4 in breast cancer cells. PLoS ONE 2015, 10, e0125399. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Diouri, J.; O’Shea, O.; Doty, S.B. Curcumin inhibits prostate cancer bone metastasis by up-regulating bone morphogenic protein-7 in vivo. J. Cancer Ther. 2014, 5, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Buijs, J.T.; Rentsch, C.A.; van der Horst, G.; van Overveld, P.G.M.; Wetterwald, A.; Schwaninger, R.; Henriquez, N.V.; Ten Dijke, P.; Borovecki, F.; Markwalder, R.; et al. BMP7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am. J. Pathol. 2007, 171, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; George-William, J.N.; Muppala, S.; Asangani, I.A.; Kumarswamy, R.; Nelson, L.D.; Allgayer, H. Curcumin regulates miR-21 expression and inhibits invasion and metastasis in colorectal cancer. Biosci. Rep. 2011, 31, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Selcuklu, S.D.; Donoghue, M.T.; Spillane, C. miR-21 as a key regulator of oncogenic processes. Biochem. Soc. Trans. 2009, 37, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.; Lin, H.; Huang, C.; Huang, B. Migration-prone glioma cells show curcumin resistance associated with enhanced expression of miR-21 and invasion/anti-apoptosis-related proteins. Oncotarget 2015, 6, 37770–37781. [Google Scholar] [PubMed]
- Bessette, D.C.; Wong, P.C.W.; Pallen, C.J. PRL-3: A metastasis-associated phosphatase in search of a function. Cells Tissues Organs 2007, 185, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, C.; Roy, A.; St. Martin, T.; Dufault, M.R.; Boutin, P.; Liu, D.; Zhang, M.; Puorro-Radzwill, K.; Rulli, L.; Reczek, D.; et al. Protein tyrosine phosphatase PRL-3 in malignant cells and endothelial cells: Expression and function. Mol. Cancer Ther. 2006, 5, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, Y.; Song, R.; Sun, Y.; Xu, J.; Xu, Q. An anticancer effect of curcumin mediated by down-regulating phosphatase of regenerating liver-3 expression on highly metastatic melanoma cells. Mol. Pharmacol. 2009, 76, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Li, K.; Tzivion, G.; Levenson, A.S. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.J.; Cho, K.H.; Panupinthu, N.; Kim, H.; Kang, J.; Park, C.G.; Mills, G.B.; Lee, H.Y. EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: Inhibition by resveratrol. Mol. Oncol. 2013, 7, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Fu, X.; Zhang, L.; Sui, H.; Zhou, L.; Sun, J.; Cai, J.; Qin, J.; Ren, J.; Li, Q. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS ONE 2013, 8, e78700. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Liu, X.; Han, Z.; Zhou, L.; Sui, H.; Yan, L.; Jiang, H.; Ren, J.; Cai, J.; Li, Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer 2015, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Mikula-Pietrasik, J.; Sosinska, P.; Ksiazek, K. Resveratrol inhibits ovarian cancer cell adhesion to peritoneal mesothelium in vitro by modulating the production of α5β1 integrins and hyaluronic acid. Gynecol. Oncol. 2014, 134, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Rodrigue, C.M.; Porteu, F.; Navarro, N.; Bruyneel, E.; Bracke, M.; Romeo, P.-H.; Gespach, C.; Garel, M.-C. The cancer chemopreventive agent resveratrol induces tensin, a cell–matrix adhesion protein with signaling and antitumor activities. Oncogene 2005, 24, 3274–3284. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Zykova, T.A.; Zhu, F.; Zhai, X.; Ma, W.Y.; Ermakova, S.P.; Ki, W.L.; Bode, A.M.; Dong, Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008, 47, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Salado, C.; Olaso, E.; Gallot, N.; Valcarcel, M.; Egilegor, E.; Mendoza, L.; Vidal-Vanaclocha, F. Resveratrol prevents inflammation-dependent hepatic melanoma metastasis by inhibiting the secretion and effects of interleukin-18. J. Transl. Med. 2011, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Henry-Mowatt, J.; Dive, C.; Martinou, J.; James, D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene 2004, 23, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Henzel, W.J.; Liu, X.; Lutschg, A.; Wang, X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c dependent activation of caspace 3. Cell 1997, 90, 405–413. [Google Scholar] [CrossRef]
- Adrain, C.; Slee, E.A.; Harte, M.T.; Martin, S.J. Regulation of apoptotic protease activating factor-1 oligomerization and apoptosis by the WD-40 repeat region. J. Biol. Chem. 1999, 274, 20855–20860. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; Walker, G.; Srinivasula, S.M.; Sun, X.M.; Butterworth, M.; Alnemri, E.S.; Cohen, G.M. Recruitment, activation and retention of caspases-9 and-3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 2001, 20, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Debatin, K.-M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Susin, S.A.; Zamzami, N.; Castedo, M.; Hirsch, T.; Marchetti, P.; Macho, A.; Daugas, E.; Geuskens, M.; Kroemer, G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J. Exp. Med. 1996, 184, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, G.; Huo, W.Q.; Zhang, Y.; Jin, F.S. Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. Int. J. Urol. 2012, 19, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.J.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, M.; El-Mowafy, A.; Renno, W.; Rachid, O.; Ali, A.; Al-Attyiah, R. Resveratrol-induced apoptosis in human breast cancer cells is mediated primarily through the caspase-3-dependent pathway. Arch. Med. Res. 2008, 39, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fei, Z.; Zhen, H.-N.; Zhang, J.-N.; Zhang, X. Resveratrol inhibits cell growth and induces apoptosis of rat C6 glioma cells. J. Neurooncol. 2007, 81, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Chen, T. Resveratrol induces mitochondria-mediated AIF and to a lesser extent caspase-9-dependent apoptosis in human lung adenocarcinoma ASTC-a-1 cells. Mol. Cell. Biochem. 2011, 354, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Chen, T. Resveratrol induces apoptosis via a Bak-mediated intrinsic pathway in human lung adenocarcinoma cells. Cell. Signal. 2012, 24, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.I.; Naqvi, S.T.Q.; Muhammad, S.A. Curcumin: A polyphenol with molecular targets for cancer control. Asian Pac. J. Cancer Prev. 2016, 17, 2735–2739. [Google Scholar] [PubMed]
- Song, F.; Zhang, L.; Yu, H.-X.; Lu, R.-R.; Bao, J.-D.; Tan, C.; Sun, Z. The mechanism underlying proliferation-inhibitory and apoptosis-inducing effects of curcumin on papillary thyroid cancer cells. Food Chem. 2012, 132, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Gahlot, S.; Majumdar, S. Oxidative stress induced by curcumin promotes the death of cutaneous T-cell lymphoma (HuT-78) by disrupting the function of several molecular targets. Mol. Cancer Ther. 2012, 11, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Kaushik, T.; Yadav, S.K.; Sharma, S.K.; Ranawat, P.; Khanduja, K.L.; Pathak, C.M. Curcumin sensitizes lung adenocarcinoma cells to apoptosis via intracellular redox status mediated pathway. Indian J. Exp. Biol. 2012, 50, 853–861. [Google Scholar] [PubMed]
- Hussain, A.R.; Uddin, S.; Bu, R.; Khan, O.S.; Ahmed, S.O.; Ahmed, M.; Al-Kuraya, K.S. Resveratrol suppresses constitutive activation of AKT via generation of ROS and induces apoptosis in diffuse large B cell lymphoma cell lines. PLoS ONE 2011, 6, e24703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, W.; Meng, X.; Jia, B. Resveratrol induces gastric cancer cell apoptosis via reactive oxygen species, but independent of sirtuin1. Clin. Exp. Pharmacol. Physiol. 2012, 39, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Lin, J.G.; Li, T.M.; Chung, J.G.; Yang, J.S.; Ip, S.W.; Lin, W.C.; Chen, G.W. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006, 26, 4379–4389. [Google Scholar] [PubMed]
- Tan, T.-W.; Tsai, H.-R.; Lu, H.-F.; Lin, H.-L.; Tsou, M.-F.; Lin, Y.-T.; Tsai, H.-Y.; Chen, Y.-F.; Chung, J.-G. Curcumin-induced cell cycle arrest and apoptosis in human acute promyelocytic leukemia HL-60 cells via MMP changes and caspase-3 activation. Anticancer Res. 2006, 26, 4361–4371. [Google Scholar] [PubMed]
- Ibrahim, A.; El-Meligy, A.; Lungu, G.; Fetaih, H.; Dessouki, A.; Stoica, G.; Barhoumi, R. Curcumin induces apoptosis in a murine mammary gland adenocarcinoma cell line through the mitochondrial pathway. Eur. J. Pharmacol. 2011, 668, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chiang, I.; Ding, K.; Chung, J.-G.; Lin, W.-J.; Lin, S.-S.; Hwang, J.-J. Curcumin-induced apoptosis in human hepatocellular carcinoma J5 cells: Critical role of Ca+2-dependent pathway. Evid. Based Complement. Altern. Med. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Ribulla, S.; Magnelli, V.; Patrone, M.; Burlando, B. Resveratrol induces intracellular Ca2+ rise via T-type Ca2+ channels in a mesothelioma cell line. Life Sci. 2016, 148, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Skommer, J.; Wlodkowic, D.; Pelkonen, J. Cellular foundation of curcumin-induced apoptosis in follicular lymphoma cell lines. Exp. Hematol. 2006, 34, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, X.; Hu, Y.; Yu, Z.; Wang, D.; Liu, J. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phyther. Res. 2013, 27, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Tsai, T.H.; Hsu, C.W.; Hsu, Y.C. Curcuminoids suppress the growth and induce apoptosis through caspase-3-dependent pathways in glioblastoma multiforme (GBM) 8401 cells. J. Agric. Food Chem. 2010, 58, 10639–10645. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Srivastava, R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007, 30, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shah, D.M. Curcumin down-regulates Ets-1 and Bcl-2 expression in human endometrial carcinoma HEC-1-A cells. Gynecol. Oncol. 2007, 106, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ning, J.; Peng, L.; He, D. Effect of curcumin on Bcl-2 and Bax expression in nude mice prostate cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9272–9278. [Google Scholar] [PubMed]
- Huang, T.; Lin, H.; Chen, C.; Lu, C.; Wei, C.; Wu, T.; Liu, F.; Lai, H. Resveratrol induces apoptosis of human nasopharyngeal carcinoma cells via activation of multiple apoptotic pathways. J. Cell. Physiol. 2010, 226, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.; Gong, Z. Resveratrol induces apoptosis in K562 cells via the regulation of mitochondrial signaling pathways. Int. J. Clin. Exp. Med. 2015, 8, 16926–16933. [Google Scholar] [PubMed]
- Cui, J.; Sun, R.; Yu, Y.; Gou, S.; Zhao, G.; Wang, C. Antiproliferative effect of resveratrol in pancreatic cancer cells. Phyther. Res. 2010, 24, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pérez, F.; Belchí-Navarro, S.; Almagro, L.; Bru, R.; Pedreño, M.A.; Gómez-Ros, L.V. Cytotoxic effect of natural trans-resveratrol obtained from elicited vitis vinifera cell cultures on three cancer cell lines. Plant Foods Hum. Nutr. 2012, 67, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Chen, Y.; Cheng, X.; Zhang, X.; He, Q. Potentiation of resveratrol-induced apoptosis by matrine in human hepatoma HepG2 cells. Oncol. Rep. 2014, 32, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, S. P53-dependent pathways of apoptosis. Cell Death Differ. 2001, 8, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Eifes, S.; Dicato, M.; Aggarwal, B.B.; Diederich, M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem. Pharmacol. 2008, 76, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Alkhalaf, M. Resveratrol-induced apoptosis is associated with activation of p53 and inhibition of protein translation in T47D human breast cancer cells. Pharmacology 2007, 80, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ellisen, L.W.; Bird, J.; West, D.C.; Soreng, A.L.; Reynolds, T.C.; Smith, S.D.; Sklar, J. TAN-l, the human homolog of the drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991, 66, 649–661. [Google Scholar] [CrossRef]
- Lin, H.; Xiong, W.; Zhang, X.; Liu, B.; Zhang, W.; Zhang, Y.; Cheng, J.; Huang, H. Notch-1 activation-dependent p53 restoration contributes to resveratrol-induced apoptosis in glioblastoma cells. Oncol. Rep. 2011, 26, 925–930. [Google Scholar] [PubMed]
- Ozaki, T.; Nakagawara, A. P73, a sophisticated P53 family member in the cancer world. Cancer Sci. 2005, 96, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Moll, U.M.; Slade, N. P63 and P73: Roles in development and tumor formation. Mol. Cancer Res. 2004, 2, 371–386. [Google Scholar] [PubMed]
- Wang, J.; Xie, H.; Gao, F.; Zhao, T.; Yang, H.; Kang, B. Curcumin induces apoptosis in p53-null Hep3B cells through a TAp73/DNp73-dependent pathway. Tumor Biol. 2016, 37, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Harper, N.; Hughes, M.; MacFarlane, M.; Cohen, G.M. Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis. J. Biol. Chem. 2003, 278, 25534–25541. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Chen, Q.; Siddiqui, I.; Sarva, K.; Srivastava, R.K. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3,4′,5 tri-hydroxystilbene): Molecular mechanisms and therapeutic potential. J. Mol. Signal. 2007, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Chen, Q.; Sarva, K.; Siddiqui, I.; Srivastava, R.K. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: Molecular mechanisms of apoptosis, migration and angiogenesis. J. Mol. Signal. 2007, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Li, T.; Tsao, J.; Fong, Y.; Tang, C. Curcumin induces cell apoptosis in human chondrosarcoma through extrinsic death receptor pathway. Int. Immunopharmacol. 2012, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.; Chang, C.; Chien, H.; Wu, C.; Lin, L. Resveratrol enhances the expression of death receptor Fas/CD95 and induces differentiation and apoptosis in anaplastic large-cell lymphoma cells. Cancer Lett. 2011, 309, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Reis-Sobreiro, M.; Gajate, C.; Mollinedo, F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene 2009, 28, 3221–3234. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Hang, L.W.; Yang, J.S.; Chen, H.Y.; Lin, H.Y.; Chiang, J.H.; Lu, C.C.; Yang, J.L.; Lai, T.Y.; Ko, Y.C.; et al. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Res. 2010, 30, 2125–2133. [Google Scholar] [PubMed]
- Li, L.; Aggarwal, B.B.; Shishodia, S.; Abbruzzese, J.; Kurzrock, R. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 2004, 101, 2351–2362. [Google Scholar] [CrossRef] [PubMed]
- Zanotto-Filho, A.; Braganhol, E.; Schroder, R.; De Souza, L.H.T.; Dalmolin, R.J.S.; Pasquali, M.A.B.; Gelain, D.P.; Battastini, A.M.O.; Moreira, J.C.F. NFκB inhibitors induce cell death in glioblastomas. Biochem. Pharmacol. 2011, 81, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, Y.; Liu, X.; Wu, T.; Wang, Y.; He, W.; Wei, W. Resveratrol downregulates the constitutional activation of nuclear factor-κΒ in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet. Cytogenet. 2006, 165, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pozo-guisado, E.; Merino, J.M.; Mulero-navarro, S.; Jesús, M.; Centeno, F.; Alvarez-barrientos, A.; Salguero, P.M.F. Resveratrol-induced apoptosis in MCF-7 human breast cancer cells involves a caspase-independent mechanism with downregulation of Bcl-2 and NF-κB. Int. J. Cancer 2005, 115, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Hardwaj, A.; Sethi, G.; Vadhan-Raj, S.; Bueso-Ramos, C.; Takada, Y.; Gaur, U.; Nair, A.S.; Shishodia, S.; Aggarwal, B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT-3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007, 109, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Kizhakkayil, J.; Thayyullathil, F.; Chathoth, S.; Hago, A.; Patel, M.; Galadari, S. Modulation of curcumin-induced Akt phosphorylation and apoptosis by PI3K inhibitor in MCF-7 cells. Biochem. Biophys. Res. Commun. 2010, 394, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.R.M.R.; Haque, A.; Rahman, M.A.; Chen, Z.G.; Khuri, F.R.; Shin, D.M. Curcumin induces apoptosis of upper aerodigestive tract cancer cells by targeting multiple pathways. PLoS ONE 2015, 10, e0124218. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.R.; Al-Rasheed, M.; Manogaran, P.S.; Al-Hussein, K.A.; Platanias, L.C.; Al Kuraya, K.; Uddin, S. Curcumin induces apoptosis via inhibition of PI3-kinase/AKT pathway in acute T cell leukemias. Apoptosis 2006, 11, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, C.; Xi, H.; Gao, Y.; Xu, D. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway. Mol. Med. Rep. 2015, 12, 5415–5422. [Google Scholar] [PubMed]
- Wong, T.F.; Takeda, T.; Li, B.; Tsuiji, K.; Kitamura, M.; Kondo, A.; Yaegashi, N. Curcumin disrupts uterine leiomyosarcoma cells through AKT-mTOR pathway inhibition. Gynecol. Oncol. 2011, 122, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.F.; Takeda, T.; Li, B.; Tsuiji, K.; Kondo, A.; Tadakawa, M.; Nagase, S.; Yaegashi, N. Curcumin targets the AKT-mTOR pathway for uterine leiomyosarcoma tumor growth suppression. Int. J. Clin. Oncol. 2014, 19, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; Liu, X.; Xing, K.; Wang, Y.; Li, F.; Yao, L. Resveratrol-induced cell inhibition of growth and apoptosis in MCF7 human breast cancer cells are associated with modulation of phosphorylated akt and caspase-9. Appl. Biochem. Biotechnol. 2006, 135, 181–192. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Mustafi, S.B.; Ganguly, S.; Chatterjee, M.; Raha, S. Resveratrol induces apoptosis in K562 (chronic myelogenous leukemia) cells by targeting a key survival protein, heat shock protein 70. Cancer Sci. 2008, 99, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Mustafi, S.B.; Chakraborty, P.K.; Raha, S. Modulation of AKT and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS ONE 2010, 5, e8719. [Google Scholar]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Jajoo, S.; Kaur, T.; Mukherjea, D.; Sheehan, K.; Rybak, L.P.; Ramkumar, V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE 2012, 7, e51655. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lei, P.; Xie, J.; Hu, Y. Antitumor effect of resveratrol on chondrosarcoma cells via phosphoinositide 3-kinase/AKT and p38 mitogen-activated protein kinase pathways. Mol. Med. Rep. 2015, 12, 3151–3155. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.E.; Armando, R.G.; Farina, H.G.; Menna, P.L.; Cerrudo, C.S.; Ghiringhelli, P.D.; Alonso, D.F. Telomere structure and telomerase in health and disease (Review). Int. J. Oncol. 2012, 41, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Roy, M. Inhibition of telomerase activity and induction of apoptosis by curcumin in K-562 cells. Mutat. Res. 2006, 596, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.M.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Dey, S.; Roy, M. Curcumin-induced apoptosis in human leukemia cell HL-60 is associated with inhibition of telomerase activity. Mol. Cell. Biochem. 2007, 297, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Khaw, A.K.; Hande, M.P.; Kalthur, G.; Hande, M.P. Curcumin inhibits telomerase and induces telomere shortening and apoptosis in brain tumour cells. J. Cell. Biochem. 2013, 114, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Lanzilli, G.; Fuggetta, M.P.; Tricarico, M.; Cottarelli, A.; Serafino, A.; Falchetti, R.; Ravagnan, G.; Turriziani, M.; Adamo, R.; Franzese, O.; et al. Resveratrol down-regulates the growth and telomerase activity of breast cancer cells in vitro. Int. J. Oncol. 2006, 28, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.-X.; Ding, J.-C.; Tang, Z.-M.; Li, J.-G.; Li, Y.-C.; Yan, Y.-H.; Sun, J.-C.; Zhang, C.-X. Effects of resveratrol on the proliferation, apoptosis and telomerase ability of human A431 epidermoid carcinoma cells. Oncol. Lett. 2016, 11, 3015–3018. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jove, R. The STATs of cancer—New molecular targets come of age. Nat. Rev. Cancer 2004, 4, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ihle, J.N. STATs: Signal transducers and activators of transcription. Cell 1996, 84, 331–334. [Google Scholar] [CrossRef]
- Blasius, R.; Reuter, S.; Henry, E.; Dicato, M.; Diederich, M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem. Pharmacol. 2006, 72, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Li, Y.Q.; Lv, Y.T.; Wang, J.M. Effect of curcumin on the proliferation, apoptosis, migration, and invasion of human melanoma A375 cells. Genet. Mol. Res. 2015, 14, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Zhang, X.; Hazarika, P.; Aggarwal, B.B.; Duvic, M. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: Potential role for STAT-3 and NF-kappaB signaling. J. Investig. Dermatol. 2010, 130, 2110–2119. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Hussain, A.R.; Manogaran, P.S.; Al-Hussein, K.; Platanias, L.C.; Gutierrez, M.I.; Bhatia, K.G. Curcumin suppresses growth and induces apoptosis in primary effusion lymphoma. Oncogene 2005, 24, 7022–7030. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, G.G.; Queisser, N.; Wolfson, M.L.; Fraga, C.G.; Adamo, A.M.; Oteiza, P.I. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin’s lymphoma cells. Int. J. Cancer 2008, 123, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.; Lee, H.; Jung, J.; Kong, M.; Lee, J.; Lee, J.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Resveratrol inhibits STAT-3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine 2016, 23, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Trung, L.Q.; Espinoza, J.L.; Takami, A.; Nakao, S. Resveratrol induces cell cycle arrest and apoptosis in malignant NK cells via JAK2/STAT-3 pathway inhibition. PLoS ONE 2013, 8, e55183. [Google Scholar]
- Wu, M.-L. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer. PLoS ONE 2014, 9, e89806. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.-X.; Li, H.; Wu, M.; Liu, X.-Y.; Zhong, M.; Chen, X.; Liu, J.; Zhang, Y. Inhibition of STAT3 signaling as critical molecular event in resveratrol-suppressed ovarian cancer cells. J. Ovarian Res. 2015, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, Y.; Sun, J.; Zhang, Y. Curcumin reduces the expression of Bcl-2 by upregulating miR-15a and miR-16 in MCF-7 cells. Med. Oncol. 2010, 27, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, Y.; Wu, C.; Ren, X.; Ti, X.; Shi, J.; Zhao, F.; Yin, H. Curcumin promotes apoptosis in human lung adenocarcinoma cells through miR-186 signaling pathway. Oncol. Rep. 2010, 24, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, T.; Ti, X.; Shi, J.; Wu, C.; Ren, X.; Yin, H. Curcumin promotes apoptosis in A549/DDP multidrug-resistant human lung adenocarcinoma cells through an miRNA signaling pathway. Biochem. Biophys. Res. Commun. 2010, 399, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jia, Z.; Li, A. Resveratrol repressed viability of U251 cells by miR-21 inhibiting of NF-κB pathway. Mol. Cell. Biochem. 2013, 382, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, H.; Xia, Q.; Li, P.; Kong, H.; Lei, P.; Wang, S.; Tu, Z. Resveratrol induces apoptosis of pancreatic cancers cells by inhibiting miR-21 regulation of BCL-2 expression. Clin. Transl. Oncol. 2013, 15, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ding, J.U.N.; Wu, Y. Resveratrol induces apoptosis of bladder cancer cells via miR-21 regulation of the Akt/Bcl-2 signaling pathway. Mol. Med. Rep. 2014, 9, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.; Baehrecke, E.; Blagosklonny, M.; El-Deiry, W.; Golstein, P.; Green, D.; et al. Classification of cell death. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell. Biol. 2012, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J. Pharmacol. Sci. 2013, 123, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chang, H.; Peng, X.; Bai, Q.; Yi, L.; Zhou, Y.; Zhu, J.; Mi, M. Resveratrol Inhibits Breast Cancer Stem-Like Cells and Induces Autophagy via Suppressing Wnt/b -Catenin Signaling Pathway. PLoS ONE 2014, 9, e102535. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yu, T.; Zhu, C.; Sun, H.; Qiu, Y.; Zhu, X.; Li, J. Resveratrol triggers protective autophagy through the Ceramide/Akt/mTOR pathway in melanoma B16 cells the ceramide/Akt/mTOR pathway in melanoma B16 cells. Nutr. Cancer 2014, 66, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, X.; Li, X.; Wang, C.; Zhang, X.; Liu, X.; Ding, X. Curcumin inhibits AP-2γ-induced apoptosis in the human malignant testicular germ cells in vitro. Acta Pharmacol. Sin. 2013, 34, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Ji, J.; Guo, Y. Biochemical and Biophysical Research Communications MST1 activation by curcumin mediates JNK activation, Foxo3a nuclear translocation and apoptosis in melanoma cells. Biochem. Biophys. Res. Commun. 2013, 441, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, H.; Huang, C.; Lin, J. Cycle arrest and apoptosis in MDA-MB-231/Her2 cells induced by curcumin. Eur. J. Pharmacol. 2012, 690, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Kuzuhara, T.; Echigo, N.; Fujii, A.; Suganuma, M.; Fujiki, H. Apoptosis of human lung cancer cells by curcumin mediated through up-regulation of “Growth arrest and DNA damage inducible genes 45 and 153”. Biol. Pharm. Bull. 2010, 33, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Milacic, V.; Banerjee, S.; Landis-piwowar, K.R.; Sarkar, F.H.; Majumdar, A.P.N.; Dou, Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008, 68, 7283–7292. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ke, C.; Cheng, H.; Huang, C.F.; Su, C. Curcumin-induced mitotic spindle defect and cell cycle arrest in human bladder cancer cells occurs partly through inhibition of aurora A. Mol. Pharmacol. 2011, 80, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, S.Y.; Kim, Y.; Park, J.O. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Langhans, S.A. Anaphase-promoting complex/cyclosome protein Cdc27 is a target for curcumin-induced cell cycle arrest and apoptosis. BMC Cancer 2012, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Krauthauser, C.; Maduskuie, V.; Fawcett, P.T.; Olson, J.M.; Rajasekaran, S.A. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer 2011, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.; Wu, C.; Won, S.; Shin, J.-W.; Liu, H.-S.; Su, C.-L. Kinase gene expression and subcellular protein expression pattern of protein kinase c isoforms in curcumin-treated human hepatocellular carcinoma hep 3B cells. Plant Foods Hum. Nutr. 2011, 66, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Meng, X.; Gao, X.; Tan, G. Curcumin decreases the expression of Pokemon by suppressing the binding activity of the Sp1 protein in human lung cancer cells. Mol. Biol. Rep. 2010, 37, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Singh, P.; Panda, D. Curcumin suppresses the dynamic instability of microtubules, activates the mitotic checkpoint and induces apoptosis in MCF-7 cells. FEBS J. 2010, 277, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Tang, Q.; Zhou, W.; Qiu, Y. Ras/ERK signaling pathway is involved in curcumin-induced cell cycle arrest and apoptosis in human gastric carcinoma AGS cells. J. Asian Nat. Prod. 2014, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Tian, W.; Ma, X. Curcumin induces apoptosis of HepG2 cells via inhibiting fatty acid synthase. Target. Oncol. 2014, 9, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Das, S.; Janarthan, M.; Ramachandran, H.K.; Chatterjee, M. Role of 5-lipoxygenase in resveratrol mediated suppression of 7,12-dimethylbenz (α) anthracene-induced mammary carcinogenesis in rats. Eur. J. Pharmacol. 2011, 668, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Crawford, D.R.; Lin, S.; Sebuyira, A.; Meng, R.; Westfall, E.; Tang, H.; Lin, S.; Yu, P.; Davis, P.J.; et al. Inducible COX-2-dependent apoptosis in human ovarian cancer cells. Carcinogenesis 2011, 32, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.X.; Zhan, ZX.; Hunag, Z.H.; Feng, M.; Xiong, J.P. Resveratrol treatment inhibits proliferation of and induces apoptosis in human colon cancer. Med. Sci. Monit. 2016, 22, 1101–1108. [Google Scholar]
- Chow, S.; Wang, J.; Chuang, S.; Chang, Y.; Chu, W.; Chen, W.; Chen, Y. Resveratrol-induced p53-independent apoptosis of human nasopharyngeal carcinoma cells is correlated with the downregulation of ΔNp63. Cancer Gene Ther. 2010, 17, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, F.; Lu, J.; Xia, Y.; He, L.; Chen, K.; Li, J.; Li, S.; Liu, T.; Zheng, Y.; et al. By reducing hexokinase 2, resveratrol induces apoptosis in HCC cells addicted to aerobic glycolysis and inhibits tumor growth in mice. Oncotarget 2015, 6, 13703–13717. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Samuel, S.K.; Levenson, A.S. Resveratrol enhances p53 acetylation and apoptosis in prostate cancer by inhibiting MTA1/NuRD complex. Int. J. Cancer 2010, 126, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Lee, Y.-J.; Ban, J.O.; Lee, Y.-J.; Lee, S.; Cho, M.; Nam, H.; Hong, J.T.; Shim, J. The flavonoid resveratrol suppresses growth of human malignant pleural mesothelioma cells through direct inhibition of specificity protein 1. Int. J. Mol. Med. 2012, 30, 21–27. [Google Scholar] [PubMed]
- Li, G.; He, S.; Chang, L.; Lu, H.; Zhang, H.; Zhang, H.; Chiu, J. GADD45α and annexin A1 are involved in the apoptosis of HL-60 induced by resveratrol. Phytomedicine 2011, 18, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.; Ranjan, S.; Das, D.; Siddharth, S. Resveratrol mediated cell death in cigarette smoke transformed breast epithelial cells is through induction of p21Waf1 / Cip1 and inhibition of long patch base excision repair pathway. Toxicol. Appl. Pharmacol. 2014, 275, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, S.; Troup, S.; Lu, X.; Callaghan, S.; Park, D.S.; Xing, Y.; Yang, X. Resveratrol induces apoptosis in breast cancer cells by E2F1-mediated up-regulation of ASPP1. Oncol. Rep. 2011, 25, 1713–1719. [Google Scholar] [PubMed]
- Kumar, B.; Iqbal, M.A.; Singh, R.K.; Bamezai, R.N.K. Biochimie resveratrol inhibits TIGAR to promote ROS induced apoptosis and autophagy. Biochimie 2015, 118, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Harris, N.H.; Johnson, A.D.; Lindvall, H.C.N.; Wang, G.; Ahmed, K. Protein kinase CK2 modulates apoptosis induced by resveratrol and epigallocatechin-3-gallate in prostate cancer cells. Mol. Cancer Ther. 2007, 6, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Galson, D.L.; Roodman, G.D.; Ouyang, H. Resveratrol triggers the pro-apoptotic endoplasmic reticulum stress response and represses pro-survival XBP1 signaling in human multiple myeloma cells. Exp. Hematol. 2011, 39, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Seeni, A.; Takahashi, S.; Takeshita, K.; Tang, M.; Sugiura, S.; Sato, S.; Shirai, T. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac. J. Cancer Prev. 2008, 9, 7–14. [Google Scholar] [PubMed]
- Lee, S.C.; Chan, J.; Clement, M.V.; Pervaiz, S. Functional proteomics of resveratrol-induced colon cancer cell apoptosis: Caspase-6-mediated cleavage of lamin A is a major signaling loop. Proteomics 2006, 6, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Lee, T.J.; Lee, S.H.; Seo, J.H.; Jeong, Y.J.; Park, J.W.; Kwon, T.K. Elevated gadd153/chop expression during resveratrol-induced apoptosis in human colon cancer cells. Biochem. Pharmacol. 2007, 73, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Wu, C.; Huang, S.; Wu, C.; Yo, Y.; Chen, Y.; Shiau, A.; Chou, C. Cathepsin L mediates resveratrol-induced autophagy and apoptotic cell death in cervical cancer cells. Autophagy 2009, 5, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Trincheri, N.F.; Nicotra, G.; Follo, C.; Castino, R.; Isidoro, C. Resveratrol induces cell death in colorectal cancer cells by a novel pathway involving lysosomal cathepsin D. Carcinogenesis 2007, 28, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, N.; Bahn, J.H.; Lee, S.H.; Eling, T.E.; Baek, S.J. Resveratrol-induced apoptosis is mediated by early growth response-1, Krüppel-like factor 4, and activating transcription factor 3. Cancer Prev. Res. 2011, 4, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.R.; Okuda, H.; Watabe, M.; Pai, S.K. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res. Treat. 2011, 130, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Ma, Z.; Dang, X.; Li, W.E.I.; Ma, Q. Effect of resveratrol on proliferation and apoptosis of human pancreatic cancer MIA PaCa-2 cells may involve inhibition of the Hedgehog signaling pathway. Mol. Med. Rep. 2014, 10, 2563–2567. [Google Scholar] [PubMed]
- Ryu, J.; Yoon, N.A.; Seong, H.; Jeong, J.Y.; Kang, S.; Park, N.; Choi, J.; Lee, D.H.; Roh, G.S.; Kim, H.J.; et al. Resveratrol induces glioma cell apoptosis through activation of tristetraprolin. Mol. Cells 2015, 38, 991–997. [Google Scholar] [PubMed]
- Tian, H.; Yu, Z. Resveratrol induces apoptosis of leukemia cell line K562 by modulation of sphingosine kinase-1 pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 2755–2762. [Google Scholar] [PubMed]
- Tomic, J.; Mccaw, L.; Li, Y.; Hough, M.R.; Ben-david, Y. Resveratrol has anti-leukemic activity associated with decreased O-GlcNAcylated proteins. Exp. Hematol. 2013, 41, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Vanamala, J.; Radhakrishnan, S.; Reddivari, L.; Bhat, V.B.; Ptitsyn, A.B. Resveratrol suppresses human colon cancer cell proliferation and induces apoptosis via targeting the pentose phosphate and the talin-FAK signaling pathways—A proteomic approach. Proteome Sci. 2011, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Faro, A.F.L.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr. Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef] [PubMed]
- D’Incalci, M.; Steward, W.P.; Gescher, A.J. Use of cancer chemopreventive phytochemicals as antineoplastic agents. Lancet Oncol. 2005, 6, 899–904. [Google Scholar] [CrossRef]
- López-Lázaro, M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008, 52, 103–127. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Dillon, S.P.; Dorri, Y.; D’Souza, A.; Scofield, R.H. Curcumin does not bind or intercalate into DNA and a note on the gray side of curcumin. Int. J. Cancer 2011, 128, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Maru, G.B. Understanding the molecular mechanisms of cancer prevention by dietary phytochemicals: From experimental models to clinical trials. World J. Biol. Chem. 2016, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Viswanathan, B.; Kesarwani, P.; Mehrotra, S. Dietary agents in cancer prevention: An immunological perspective. Photochem. Photobiol. 2012, 88, 1083–1098. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Singh, R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011, 1215, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Tokuda, H.; Satomi, Y.; Masuda, M.; Osaka, Y.; Yogosawa, S.; Wada, S.; Mou, X.Y.; Takayasu, J.; Murakoshi, M.; et al. Cancer prevention by antioxidants. BioFactors 2004, 22, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khar, A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med. Chem. 2006, 6, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.P.L.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Kumar, R.A.J.; Ahmad, N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms (Review). Int. J. Oncol. 2003, 23, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Stepanic, V.; Gasparovic, A.C.; Troselj, K.G.; Amic, D.; Zarkovic, N. Selected attributes of polyphenols in targeting oxidative stress in cancer. Curr. Top. Med. Chem. 2016, 15, 496–509. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as modulator of oxidative stress in cancer disease: New therapeutic strategies. Oxid. Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Adelzadeh, M.; Norouzi, Z.; Sarbolouki, M.N. Curcumin binding to DNA and RNA. DNA Cell Biol. 2009, 28, 201–208. [Google Scholar] [CrossRef] [PubMed]
- N’soukpoé-Kossi, C.N.; Bourassa, P.; Mandeville, J.S.; Bekale, L.; Tajmir-Riahi, H.A. Structural modeling for DNA binding to antioxidants resveratrol, genistein and curcumin. J. Photochem. Photobiol. B Biol. 2015, 151, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Baharuddin, P.; Satar, N.; Fakiruddin, K.S.; Zakaria, N.; Lim, M.N.; Yusoff, N.M.; Zakaria, Z.; Yahaya, B.H. Curcumin improves the efficacy of cisplatin by targeting cancer stem-like cells through p21 and cyclin D1-mediated tumour cell inhibition in non-small cell lung cancer cell lines. Oncol. Rep. 2016, 35, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Duarte, V.M.; Han, E.; Veena, M.S.; Salvado, A.; Jeffrey, D.; Liang, L.; Faull, K.F.; Srivatsan, E.S.; Wang, M.B. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKβ protein of the nuclear factor kB pathway. Mol. Cancer Ther. 2010, 9, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, G.; Wang, L.; Kang, J.; Wang, J. Curcumin p38-dependently enhances the anticancer activity of valproic acid in human leukemia cells. Eur. J. Pharm. Sci. 2010, 41, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.E.; Orlando, R.A. Curcumin reduces cytotoxicity of 5-Fluorouracil treatment in human breast cancer cells. J. Med. Food 2015, 18, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Vishnoi, K.; Mahata, S.; Tripathi, S.C.; Misra, S.P.; Misra, V.; Mehrotra, R.; Dwivedi, M.; Bharti, A.C. Berberine and curcumin target survivin and STAT-3 in gastric cancer cells and synergize actions of standard chemotherapeutic 5-fluorouracil. Nutr. Cancer 2015, 67, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kanwar, S.S.; Patel, B.B.; Nautiyal, J.; Sarkar, F.H.; Majumdar, A.P. Elimination of colon cancer stem-like cells by the combination of curcumin and FOLFOX. Transl. Oncol. 2009, 2, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-Z.; Du, J.-L.; Wang, Y.-L.; Li, J.; Wei, L.-X.; Guo, M.-Z. Synergistic effects of curcumin and bevacizumab on cell signaling pathways in hepatocellular carcinoma. Oncol. Lett. 2015, 9, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Shan, Q.; He, G.; Lin, J.; Gong, Y. Curcumin potentiates the anti-leukemia effects of imatinib by downregulation of the AKT/mTOR pathway and BCR/ABL gene expression in Ph+ acute lymphoblastic leukemia. Int. J. Biochem. Cell Biol. 2015, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Banik, N.L.; Ray, S.K. Synergistic anti-cancer mechanisms of curcumin and paclitaxel for growth inhibition of human brain tumor stem cells and LN18 and U138MG cells. Neurochem. Int. 2012, 61, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- De Ruiz Porras, V.; Bystrup, S.; Martínez-Cardús, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci. Rep. 2016, 6, 24675. [Google Scholar] [CrossRef] [PubMed]
- Zanotto-Filho, A.; Braganhol, E.; Klafke, K.; Figueiró, F.; Terra, S.R.; Paludo, F.J.; Morrone, M.; Bristot, I.J.; Battastini, A.M.; Forcelini, C.M.; et al. Autophagy inhibition improves the efficacy of curcumin/temozolomide combination therapy in glioblastomas. Cancer Lett. 2015, 358, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, Y.M.; Chang, G.C.; Yu, S.L.; Hsieh, W.Y.; Chen, J.J.W.; Chen, H.W.; Yang, P.C. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björklund, M.; Roos, J.; Gogvadze, V.; Shoshan, M. Resveratrol induces SIRT-1- and energy-stress-independent inhibition of tumor cell regrowth after low-dose platinum treatment. Cancer Chemother. Pharmacol. 2011, 68, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.M.; Al-Malki, H.S.; Al-Harthi, S.E.; El-Hanafy, A.A.; Elashmaoui, H.M.; Elshal, M.F. Modulatory role of resveratrol on cytotoxic activity of cisplatin, sensitization and modification of cisplatin resistance in colorectal cancer cells. Mol. Med. Rep. 2015, 12, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Kraehe, P.; Popper, B.; Goel, A.; Shakibaei, M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015, 98, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Koo, B.S.; Park, S.Y.; Kim, Y.M. Anti-angiogenic effects of resveratrol in combination with 5-fluorouracil on B16 murine melanoma cells. Mol. Med. Rep. 2015, 12, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Chavez, J.; Fonseca-Sanchez, M.A.; Arechaga-Ocampo, E.; Flores-Perez, A.; Palacios-Rodreguez, Y.; Domínguez-Góme, G.; Marchat, L.A.; Fuentes-Mera, L.; Mendoza-Hernandez, G.; Gariglio, P.; et al. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS ONE 2013, 8, e64378. [Google Scholar] [CrossRef] [PubMed]
- Kweon, S.H.; Song, J.H.; Kim, T.S. Resveratrol-mediated reversal of doxorubicin resistance in acute myeloid leukemia cells via downregulation of MRP1 expression. Biochem. Biophys. Res. Commun. 2010, 395, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Casanova, F.; Quarti, J.; Ferraz Da Costa, D.C.; Ramos, C.A.; Da Silva, J.L.; Fialho, E. Resveratrol chemosensitizes breast cancer cells to melphalan by cell cycle arrest. J. Cell. Biochem. 2012, 113, 2586–2596. [Google Scholar] [CrossRef] [PubMed]
- Filippi-Chiela, E.C.; Thomé, M.P.; Bueno e Silva, M.M.; Pelegrini, A.L.; Ledur, P.F.; Garicochea, B.; Zamin, L.L.; Lenz, G. Resveratrol abrogates the temozolomide-induced G2 arrest leading to mitotic catastrophe and reinforces the temozolomide-induced senescence in glioma cells. BMC Cancer 2013, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [PubMed]
- Rigolio, R.; Miloso, M.; Nicolini, G.; Villa, D.; Scuteri, A.; Simone, M.; Tredici, G. Resveratrol interference with the cell cycle protects human neuroblastoma SH-SY5Y cell from paclitaxel-induced apoptosis. Neurochem. Int. 2005, 46, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.P.; Miao, S.; Wu, Y.; Zhang, W.; Zhang, X.F.; Ma, H.Z.; Xin, H.L.; Feng, J.; Wen, A.D.; Li, Y. Resveratrol sensitizes tamoxifen in antiestrogen-resistant breast cancer cells with epithelial-mesenchymal transition features. Int. J. Mol. Sci. 2013, 14, 15655–15668. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Nigam, M.; Ranjan, V.; Zaidi, D.; Garg, V.K.; Sharma, S.; Chaturvedi, R.; Shankar, R.; Kumar, S.; Sharma, R.; et al. Resveratrol as an adjunct therapy in cyclophosphamide-treated MCF-7 cells and breast tumor explants. Cancer Sci. 2011, 102, 1059–1067. [Google Scholar] [CrossRef] [PubMed]



| Target | Effect | Cancer Type | Reference |
|---|---|---|---|
| GRP78 | downregulation | Colon | [117] |
| EphA2 | downregulation | Melanoma | [118] |
| SOCS1 & 3 | upregulation | Leukemia | [119] |
| Nrf2 | downregulation | Breast | [120] |
| miR-15a/16-1 | downregulation | Leukemia | [121] |
| DLEC1 | upregulation | Colon | [122] |
| Skp2 | downregulation | Glioma | [123] |
| Target | Effect | Cancer Type | Reference |
|---|---|---|---|
| PKC | downregulation | gastric | [124] |
| eEF1A2 | downregulation | ovarian | [125] |
| pro-IGFII | upregulation | breast | [126] |
| PTEN | upregulation | breast | [127] |
| MIC-1 | upregulation | pancreas | [128] |
| 6-PF1K | inhibition | breast | [129] |
| RNF20 | activation | breast | [130] |
| Nox5 | upregulation | lung | [131] |
| uH2B | downregulation | glioma | [132] |
| Target | Effect | Cancer Type | Reference |
|---|---|---|---|
| MTA-1/HDAC | downregulation | prostate | [257] |
| EGFR | downregulation | ovarian | [258] |
| MALAT-1 | downregulation | colorectal | [259] |
| TGF-β1/Smads | downregulation | colorectal | [260] |
| α5β1 integrins/hyaluronic acid | downregulation/upregulation | ovarian | [261] |
| tensin | upregulation | erythroleukemia | [262] |
| TGF-β1 | downregulation | lung | [263] |
| COX-2 | downregulation | colon adenocarcinoma | [264] |
| interleukin-18 | downregulation | hepatic melanoma | [265] |
| Target | Effect | Cancer Type | Reference |
|---|---|---|---|
| AP-2γ | inhibition | testicular | [368] |
| MST1 | activation | melanoma | [369] |
| Hexoquinase-2 | downregulation | colorectal | [370] |
| Skp2/Her2 | downregulation | breast | [371] |
| GADD45/153 | upregulation | lung | [372] |
| Proteasome | inhibition | colon | [373] |
| Aurora A | downregulation | bladder | [374] |
| AMPK | activation | colon | [375] |
| Cdc27/APC3 | inhibition | medulloblastoma | [376] |
| HDAC 4 | inhibition | medulloblastoma | [377] |
| PKC | downregulation | liver | [378] |
| Sp1 | inhibition | lung | [379] |
| Microtúbulo | inhibition | breast | [380] |
| Ras/ERK signaling | activation | gastric | [381] |
| Fatty acid synthase | inhibition | liver | [382] |
| Target | Effect | Cancer Type | Reference |
|---|---|---|---|
| 5-LOX | downregulation | mammary | [383] |
| COX 2 | upregulation | ovarian | [384,385] |
| ∆Np63 | downregulation | nasopharyngeal | [386] |
| Hexoquinase 2 | downregulation | hepatocellular | [387] |
| MTA1 | downregulation | prostate | [388] |
| Specificity protein 1 | inhibition | mesothelioma | [389] |
| GADD45α/Annexin A1 | upregulation | leukemia | [390] |
| p21 | upregulation | breast | [391] |
| ASPP1 | upregulation | breast | [392] |
| TIGAR | downregulation | Lung/breast | [393] |
| Casein kinase (CK2) | downregulation | prostate | [394] |
| IRE1α/XBP1 | upregulation | Multiple myeloma | [395] |
| Androgen receptor | downregulation | prostate | [396] |
| Caspase-6 | upregulation | colon | [397] |
| CHOP | upregulation | colon | [398] |
| Cathepsin L/B | activation | Cervical/colorectal | [399,400] |
| ATF3 | upregulation | colorectal | [401] |
| Fatty acid synthase | downregulation | breast | [402] |
| Hedgehog signaling | downregulation | pancreas | [403] |
| Tristetraprolin | activation | glioma | [404] |
| SphK1/S1P | downregulation | leukemia | [405] |
| Proteasome | activation | leukemia | [406] |
| Pentose phosphate and talin-FAK pathway | downregulation | colon | [407] |
| Polyphenol | Drug | Cancer Type | Reference |
|---|---|---|---|
| curcumin | cisplatin | lung | [426] |
| curcumin | cisplatin | head and neck | [427] |
| curcumin | valproic acid | leukemia | [428] |
| curcumin | gemcitabine | pancreatic | [429] |
| curcumin | 5-fluorouracil | breast | [430] |
| curcumin | 5-fluorouracil | gastric | [431] |
| curcumin | 5-fluorouracil + oxaliplatin | colon | [432] |
| curcumin | bevacizumab | liver | [433] |
| curcumin | imatinib | leukemia | [434] |
| curcumin | paclitaxel | brain | [435] |
| curcumin | oxaliplatin | colorectal | [436] |
| curcumin | temozolomide | glioblastoma | [437] |
| curcumin | gefitinib | lung | [438] |
| resveratrol | cisplatin | ovarian | [439] |
| resveratrol | cisplatin | colorectal | [440] |
| resveratrol | 5-fluorouracil | colorectal | [441] |
| resveratrol | 5-fluorouracil | melanoma | [442] |
| resveratrol | doxorubicin | breast | [443] |
| resveratrol | doxorubicin | leukemia | [444] |
| resveratrol | melphalan | breast | [445] |
| resveratrol | temozolomide | glioma | [446] |
| resveratrol | gemcitabine | pancreatic | [447] |
| resveratrol | paclitaxel | neuroblastoma | [448] |
| resveratrol | tamoxifen | breast | [449] |
| resveratrol | cyclophosphamide | breast | [450] |
| Cancer Treatment | |||
| Intervention | Study | Status | NCT Number |
| Curcumin and 5-fluoracil (5-FU) | Curcumin in combination with 5-FU for colon cancer | Recruiting | NCT02724202 (Phase 0) |
| Curcumin and capecitabine | Curcumin, capecitabine and radiation therapy followed by surgery for rectal cancer | Ongoing, but not recruiting | NCT00745134 (Phase II) |
| Curcumin | Trial of curcumin in advanced-pancreatic cancer | Completed | NCT00094445 (Phase II) |
| Curcumin | Phase II study of curcumin versus placebo for chemotherapy-treated breast cancer patients undergoing radiotherapy | Recruiting | NCT01740323 (Phase II) |
| Avastin and Curcumin | Avastin/folfiri in combination with curcumin in colorectal cancer patients with metastasis | Recruiting | NCT02439385 (Phase II) |
| Gemcitabine and curcumin | Gemcitabine With Curcumin for Pancreatic Cancer | Completed | NCT00192842 (Phase II) |
| Curcumin | Effect of Curcumin in Treatment of Squamous Cervical Intraepithelial Neoplasias (CINs) | Recruiting | NCT02554344 |
| Gemcitabine, curcumin and celecoxib | Phase III Trial of Gemcitabine, Curcumin and Celebrex in Patients with Metastatic Colon Cancer | Unknown | NCT00295035 |
| Curcumin and Docetaxel | Multicenter Study Comparing Taxotere Plus Curcumin Versus Taxotere Plus Placebo Combination in First-line Treatment of Prostate Cancer Metastatic Castration Resistant (CURTAXEL) (CURTAXEL) | Ongoing, but not recruiting | NCT02095717 (Phase II) |
| Curcumin and Docetaxel | Docetaxel With or Without a Phytochemical in Treating Patients with Breast Cancer | Recruiting | NCT00852332 (Phase II) |
| Gemcitabine, curcumin and celebrex | Phase III trial of gemcitabine, curcumin and celebrex in patients with advance or inoperable pancreatic cancer | Unknown | NCT00486460 (Phase III) |
| Curcumin and cholecalciferol | Curcumin and cholecalciferol in treating patients with previously untreated stage 0-II Chronic lymphocytic leukemia or small lymphocytic lymphoma | Recruiting | NCT0210042 (Phase II) |
| Curcumin and bioperine | Pilot study of curcumin (diferuloylmethane derivative) with or without bioperine in patients with multiple myeloma | Completed | NCT00113841 |
| Curcumin | Use of curcumin for treatment of intestinal adenomas in familial adenomatous polyposis (FAP) | Recruiting | NCT00927485 |
| Anthocyanins and curcumin | Randomized window of opportunity trial of anthocyanin extract and phospholipid curcumin in subjects with colorectal adenoma | Recruiting | NCT0194866 (Phase II) |
| Curcumin and Ashwagandha extract | Pilot study of curcumin formulation and Ashwagandha extract in advanced osteosarcoma | Unknown | NCT00689195 (Phase I/II) |
| Curcumin | Turmeric effect on reduction of serum prolactin and related hormonal change and adenoma size in prolactinoma patients | Unknown | NCT0134429 (Phase I) |
| Adverse Effects Management Induced by Chemotherapy | |||
| Intervention | Study | Status | NCT Number |
| Curcumin | Curcumin for the Prevention of Radiation-induced Dermatitis in Breast Cancer Patients | Completed | NCT01042938 (Phase II) |
| Curcumin | Radiosensitizing and Radioprotectve Effects of Curcumin in Prostate Cancer | Completed | NCT01917890 |
| Curcumin and FOLFOX | Combining Curcumin With FOLFOX Chemotherapy in Patients with Inoperable Colorectal Cancer (CUFOX) | Ongoing, but no Recruiting | NCT01490996 (Phase I/II) |
| Curcumin | Nanocurcumin for Prostate Cancer Patients Undergoing Radiotherapy (RT) | Recruiting | NCT02724618 (Phase II) |
| Curcumin and Tirosine kinase inhibitors | An Open-label Prospective Cohort Trial of Curcumin Plus Tyrosine Kinase Inhibitors (TKI) for EGFR -Mutant Advanced NSCLC (CURCUMIN) | Recruiting | NCT02321293 (Phase I) |
| Curcumin | Prophylactic Topical Agents in Reducing Radiation-Induced Dermatitis in Patients with Non-inflammatory Breast Cancer or Breast Cancer in Situ (Curcumin-II) | Ongoing, but no recruiting | NCT02556632 (Phase II) |
| Curcumin | Effect of curcumin addition to standard treatment on tumor-induced inflammation in endometrial carcinoma | Recruiting | NCT02017353 (Phase II) |
| Curcumin | Curcumin for prevention of oral mucositis in children using chemotherapy | Completed | NCT00475683 (Phase III) |
| Curcumin | Oral curcumin for radiation dermatitis in breast cancer patients | Completed | NCT01246973 (Phase II/III) |
| Chemoprevention | |||
| Intervention | Study | Status | NCT Number |
| Curcumin | Curcumin in Treating Patients with Familial Adenomatous Polyposis | Ongoing, but not recruiting | NCT00641147 (Phase II) |
| Curcumin | Curcumin in Preventing Gastric Cancer in Patients with Chronic Atrophic Gastritis or Gastric Intestinal Metaplasia | Not yet recruiting | NCT02782949 (Phase II) |
| Curcumin | Sulindac and plant compounds in preventing colon cancer | Completed | NCT00003365 |
| Curcumin | Curcumin for the chemoprevention of colorectal cancer | Completed | NCT00118989 (Phase II) |
| Curcumin and sulindac | The effects of curcuminoids on aberrant crypt foci in the human colon | Unknown | NCT00176618 |
| Curcumin | Randomized trial of adjuvant curcumin after prostatectomy | Recruiting | NCT02064673 |
| Cancer Treatment | |||
| Intervention | Study | Status | NCT Number |
| Resveratrol | Resveratrol for patients with colon cancer | Completed | NCT00256334 (Phase I) |
| Resveratrol | Resveratrol in treating patients with colorectal cancer that can be removed by surgery | Completed | NCT00433576 (Phase I) |
| Resveratrol | A biological study of resveratrol’s effects on notch-1 signaling in subjects with low grade gastrointestinal tumors | Ongoing, not recruiting | NCT01476592 |
| Resveratrol and others | Dietary intervention in follicular lymphoma (KLYMF) | Unknown | NCT00455416 (Phase II) |
| Adverse Effects Management Induced by Chemotherapy | |||
| Intervention | Study | Status | NCT Number |
| SRT501 (new formulation of resveratrol) | A clinical study to assess the safety, pharmacokinetics, and pharmacodynamics of SRT501 in subjects with colorectal cancer and hepatic metastases | Completed | NCT00920803 (Phase I) |
| Chemoprevention | |||
| Intervention | Study | Status | NCT Number |
| Resveratrol | UMCC 2003-064 Resveratrol in Preventing Cancer in Healthy Participants (IRB 2004-535) | Completed | NCT00098969 (Phase I) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan, A.R.; Silva, G.D.B.d.; Jornada, D.H.; Chiba, D.E.; Fernandes, G.F.d.S.; Man Chin, C.; Dos Santos, J.L. Unraveling the Anticancer Effect of Curcumin and Resveratrol. Nutrients 2016, 8, 628. https://doi.org/10.3390/nu8110628
Pavan AR, Silva GDBd, Jornada DH, Chiba DE, Fernandes GFdS, Man Chin C, Dos Santos JL. Unraveling the Anticancer Effect of Curcumin and Resveratrol. Nutrients. 2016; 8(11):628. https://doi.org/10.3390/nu8110628
Chicago/Turabian StylePavan, Aline Renata, Gabriel Dalio Bernardes da Silva, Daniela Hartmann Jornada, Diego Eidy Chiba, Guilherme Felipe dos Santos Fernandes, Chung Man Chin, and Jean Leandro Dos Santos. 2016. "Unraveling the Anticancer Effect of Curcumin and Resveratrol" Nutrients 8, no. 11: 628. https://doi.org/10.3390/nu8110628