Parasitology and One Health—Perspectives on Africa and Beyond
Abstract
:1. Introduction
1.1. Africa, Present and Future
1.2. COVID-19, Agriculture and Livestock Keeping
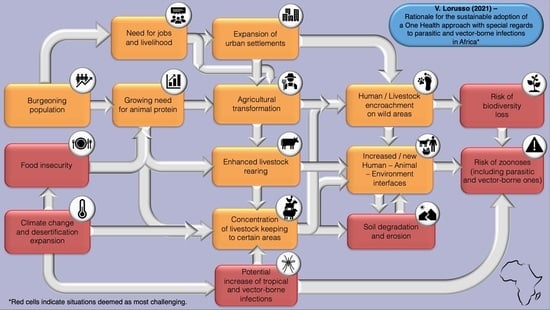
2. One Health and Parasitic and Arthropod-Borne Infections
3. One Health beyond Parasitic Zoonoses
4. One Health Approach for Research and Development of Parasiticides
5. Perspectives from Here
- i.
- The holistic perspective of the One Health approach here recommended encourages the involvement of parasitologists in issues pertaining not only to (i) mere parasitic conditions of humans and animals, but also to (ii) vector-borne infections caused by other pathogens (e.g., viruses and bacteria), entailing nevertheless parasitic arthropods in their epidemiology and therefore requiring entomological expertise in order to be tackled comprehensively and effectively. Moreover, the present analysis suggests that parasitologists should consider under the One Health approach not only (i) zoonoses, but also (ii) non-zoonotic veterinary parasitoses responsible for productivity losses in livestock, thus for food insecurity in their respective communities.With the role of agriculture being as fundamental to Africa’s ongoing and future socio-economic development, the analysis here provided underscores the indissolubility, when dealing with parasitic and vector-borne infections according to the One Health approach, of concepts such as (i) human health and (ii) animal health, as well as (iii) environment (e.g., plant and biodiversity’s) health. After all, the well-being of humans, animals and the environment is inextricably linked to agriculture and its several practices (e.g., agribusiness, agroecology, agroforestry and pastoralism) and to the management and use of wild resources, including flora, fauna and water. Accordingly, the durable insurance of the well-being of human beings and their livestock will necessarily need to encompass the preservation of habitats and biodiversity richness.
- ii.
- The global nature of this effort stems from the realisation that no public health issue has a merely local dimension, as it has been clearly shown by the COVID-19 pandemic. In the case of parasitic and vector-borne infections, there is no condition that can be considered as a null threat outside where it originates, especially if naïve areas of potential novel introduction harbour suitable habitats for the parasite or its intermediate host(s) or vector(s) to develop. For example, this is evidently the case for food-borne parasitoses (e.g., echinococcosis, toxoplasmosis and trematodiases) that could be carried to significant distances through trade of food produces [115] or for infections that can reach new areas through movement of infective vectors (e.g., mosquitoes) or intermediate hosts (e.g., snails) via human-made means of transports (e.g., airplanes or ships) [116,117,118] or by dispersion of vectors through migratory birds (e.g., Hyalomma ticks parasitising migratory birds and posing risks of new foci of CCHF) or other parasitised hosts [118,119,120] or through travel of infected hosts [118]. In the latter case, it should however be noted that a population of competent vectors would need to occur in the area of new introduction for a vector-borne infection to successfully establish itself [118].Undoubtedly, practicing the One Health approach globally requires great cooperation and coordination among institutions at national, regional, continental and intercontinental level, to ensure the constant exchange of information and the continuous advancement of surveillance and response systems. This should be done with the awareness that exchanging information among countries or regions can provide mutual benefits in terms of capacity building and thus preparedness and responsiveness in all geographies involved in such a dialogue. Accordingly, improving capacity in Europe on the detection and surveillance of “exotic” vector-borne infections that are currently endemic in some parts of Africa, such as RVF, can also provide enhanced training opportunities to African scientists and researchers, who, in turn, may further potentiate their monitoring systems by exchanging views and personnel with non-endemic third countries.
- iii.
- The interdisciplinary angle of this effort requires the consideration of parasitic and arthropod-borne infections under the lens of both life sciences and social sciences. Indeed, all types of control efforts can only be effective in a given area (from small to large, regardless of its size) if designed, implemented and evaluated by taking into account the social determinants of health, in which people are born, grow, live, work and age. These include factors like socio-economic status, education, neighbourhood and physical environment, employment, and social support networks, as well as access to health care of communities [121]. The involvement and “ownership” of local communities, that are the ultimate beneficiaries of interventions, whether treatments are addressed to humans or animals or the environment, should indeed be mandatory for all One Health (and beyond) interventions, dealing with all types of conditions, not only parasitic ones. Only a deep understanding of communities’ practices and customs can allow for the conception of potentially effective initiatives, which should be co-designed with recipient communities.For example, in the early 2010s, conversations with cattle keepers from northern Uganda (i.e., districts of Kaberamaido and Dokolo), reporting of being not rarely bitten by “colourful” ornate ticks (i.e., Amblyomma spp.) led to documenting for the first time in the country the occurrence of the zoonotic pathogen R. africae, causative agent of African Tick-Bite Fever (ATBF) [122], a condition often misdiagnosed with malaria- or flu-like syndromes [123]. Such finding highlighted the risk of exposure to ATBF of rural communities in northern Uganda, underpinning the importance of raising awareness on this rickettsiosis, particularly among persons handling cattle (e.g., herders, veterinarians and paraveterinarians) as well as among physicians practicing in these areas, and those who care for returning travellers [122]. It is thanks to farmers’ viewpoints that this investigation could be started, and such a public health risk could be unveiled.
- iv.
- The multisectoral nature of the approach here recommended entails the participation in One Health initiatives of all stakeholders potentially concerned, including civil society, academia, industry, institutions and their policy-makers. All parties’ contribution is essential for interventions to be successful. Academic parasitologists should therefore strive for engaging with civil society any time the investigations that they conduct may have possible repercussions on the latter. With data in hand, parasitologists as other scientists in the field of One Health, should engage in societal debate and render their research rationales, methodologies, findings and recommendations intelligible not only to the general public, but also to administrators. To some extent, the COVID-19 pandemic has shown that concepts such as antigenic or serological testing or even that of One Health itself, can become more widely accessible than they used to be beforehand, out of necessity. At the same time, academics and industry actors should proactively seek to collaborate with each other. The contribution of the private sector (e.g., pharmaceutical/biotech/vector control industries) is indeed essential in the fight against parasitic and vector-borne infections, as it allows to deliver “ready to use” solutions such as drugs, vaccines, insecticides and diagnostic tools. At the same time, serendipities happening in laboratories at universities and research institutes can lead to breakthrough discoveries that could be ultimately turned into “actual products”, responding to unmet needs on the ground, through win-win partnerships with the private sector.
- v.
- With multiple programmes being often conducted concurrently in neighbouring, if not overlapping, geographic areas, addressing either the same or different diseases, there is a need for harmonised actions. These would be possible through the establishment of a steady dialogue among key actors of projects’ cycles, including programmers and formulators (e.g., donors, local authorities and communities), implementers (e.g., funding grantees, principal investigators, programme coordinators, etc.) and monitoring and evaluation teams. Creating, whenever possible, synergies between incoming projects and previous and/or concomitant initiatives can allow to optimise results (i.e., outputs and outcomes) and minimise possible redundancies and “stakeholder fatigue”, for the sake of the common good. With global health gaining presumably increasingly more political attention in the wake of the COVID-19 pandemic [124,125,126], prioritising interventions based on burden of diseases (e.g., through Disability-Adjusted Life Years (DALY)) is undoubtedly an important instrument for agenda setting. In this view, the availability of reliable data, generated through robust methodologies and thorough analyses, is essential.
- vi.
- Finally, for it to be “ever topical” and effective, the One Health approach should also be forward-looking, and rely on institutional policies fostering (a) research and innovation, both at public and private level, and (b) continuing education and training in parasitology and entomology. Only through constant R&D efforts, entailing collaborations among academia, industry and PDPs, it can be hoped that more parasitic and arthropod-borne conditions of humans and animals or both, NTDs included, could be effectively controlled in the future. Fostering research and innovation as well as manufacturing capacity locally in Africa, not only could prove logistically practical and ultimately cost-effective, considering these efforts are addressed to endemic conditions of the continent, but can also provide the African burgeoning youth with major employment opportunities. This would also require tailored curricula to be put in place at local African universities. The know-how built by the Institut Pasteur de Dakar, only centre in Africa able to produce a yellow fever vaccine [127] and soon to produce vaccines against COVID-19 [128], as well as the institution of the University of Global Health Equity in Rwanda [129] and the One Health Research, Education and Outreach Centre in Africa (OHRECA) in Kenya [130] and the Africa One Health University Network (AFROHUN) [131] are just some encouraging examples in this respect, among other ongoing initiatives. Importantly, given the centrality of youth in education and the fundamental contribution of women scientists to Africa’s development [132], investing in research, innovation and training in parasitology and entomology can have an immensely empowering role and contribute to the overall attainment of the United Nations’ 2030 Agenda’s SDG #4 (“quality education”) and SDG #5 (“gender equality”).
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Author Statement
Glossary
- Zoonoses or zoonotic infections: Infections that are naturally transmitted from vertebrate animals to humans, and vice versa [64]. Zoonotic diseases are therefore conditions derived from such infections.
- Demographic dividend: Economic growth deriving from shifts in a population’s age structure, mainly when the share of the working age population (15–64-year-olds) is larger than the non-working age share of the population (<14-year-olds and >65-year-olds). This can occur when declining fertility leads to a bulge in the proportion of the population entering the labour force, in the presence of suitable jobs. If this young cohort is healthy, well-educated and empowered, and has a chance for decent work, it can accelerate sustainable development in the course of a generation [135].
- Parasitologists: Scientists devoted to the study of internal and/or external parasites (i.e., endoparasites (e.g., protozoa, nematodes, trematodes and cestodes) and ectoparasites (e.g., parasitic arthropods), respectively). The definition employed in this text is inclusive of that of entomologists (scientists devoted to the study of insects and other classes of arthropods).
- Endectocides: Antiparasitic products able to kill and control both internal (i.e., endoparasites) and external parasites (i.e., ectoparasites). Endoparasites usually controlled by endectocides include nematodes (e.g., gastrointestinal and/or bronchopulmonary nematodes), whereas target ectoparasites may vary according to the active compounds used, possibly including mange mites, one host-ticks (i.e., Boophilus spp.), fly larvae, etc. A typical example of endectocide is provided by products based on ivermectin and other compounds of the class of macrocyclic lactones (e.g., doramectin, moxidectin, milbemycin oxime, etc.).
- Repurposing (or repositioning): The process of finding new uses outside the scope of the original medical indication of a drug [136].
References
- Agence Française de Développement (AFD). AFD’s Atlas of Africa: Viewing the Continent from a New Angle. Published on 16 September 2020. Available online: https://www.afd.fr/en/actualites/afds-atlas-africa-viewing-continent-new-angle (accessed on 23 September 2021).
- Institut National d’études Démographiques (INED). WORLD–Estimations 2021. Available online: https://www.ined.fr/en/everything_about_population/data/all-countries/ (accessed on 27 September 2021).
- Institut National d’études Démographiques (INED). Projections by Continent. Update: November 2019. Available online: https://www.ined.fr/en/everything_about_population/data/world-projections/projections-by-continent/ (accessed on 27 September 2021).
- Food and Agriculture Organization (FAO). Africa Sustainable Livestock 2050; Animal Production and Health Report. No. 12; FAO: Rome, Italy, 2017; Available online: http://www.fao.org/3/ai7222e (accessed on 27 September 2021).
- Food and Agriculture Organization (FAO). The Future of Food and Agriculture–Alternative Pathways to 2050; Supplementary Material; FAO: Rome, Italy, 2018; p. 64. [Google Scholar]
- World Bank. Business and Livelihoods in African Livestock: Investments to Overcome Information Gaps; World Bank: Washington, DC, USA, 2014; Available online: https://openknowledge.worldbank.org/handle/10986/17801 (accessed on 27 September 2021).
- Rakotoarisoa, M.A.; Iafrate, M.; Paschali, M.; Food and Agriculture Organization (FAO). Why Has Africa Become a Net Food Importer? Explaining Africa Agricultural and Food Trade Deficits; Trade and Markets Division: Rome, Italy, 2011. [Google Scholar]
- African Development Bank (AfDB). Feed Africa. The High 5 for Transforming Africa. Available online: https://www.afdb.org/en/the-high-5/feed-africa (accessed on 27 September 2021).
- Food and Agriculture Organization (FAO). New UN Report Reveals that Hunger in Africa Continues to Rise. 13 February 2019. Available online: http://www.fao.org/news/story/en/item/1180443/icode/ (accessed on 27 September 2021).
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Akombi, B.J.; Agho, K.E.; Merom, D.; Renzaho, A.M.; Hall, J.J. Child malnutrition in sub-Saharan Africa: A meta-analysis of demographic and health surveys (2006–2016). PLoS ONE 2017, 12, e0177338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Regional Office for Africa. Obesity. Overview. Available online: https://www.afro.who.int/health-topics/obesity#:~:text=Once%20considered%20a%20high%2Dincome,to%2010.6%20million%20in%202014 (accessed on 27 September 2021).
- Africa Development Bank (AfDB). Feed Africa: Strategy for Agricultural Transformation in Africa 2016–2025. May 2016. Available online: https://www.afdb.org/fileadmin/uploads/afdb/Documents/Generic-Documents/Feed_Africa-_Strategy_for_Agricultural_Transformation_in_Africa_2016-2025.pdf (accessed on 24 September 2021).
- IFAD. The Field Report. Available online: https://www.ifad.org/thefieldreport/ (accessed on 28 September 2021).
- Jayaram, K.; Riese, J.; Sanghvi, S. Agriculture: Abundant Opportunities. McKinsey Quarterly, Summer 2010. Available online: https://www.mckinsey.com/featured-insights/middle-east-and-africa/africas-path-to-growth-sector-by-sector (accessed on 29 September 2021).
- African Union Development Agency (AUDA)-NEPAD. Comprehensive Africa Agriculture Development Programme (CAADP). Available online: https://www.nepad.org/cop/comprehensive-africa-agriculture-development-programme-caadp (accessed on 27 September 2021).
- New Alliance for Food Security and Nutrition. Available online: https://newalliance.travelvisabookings.com/about (accessed on 27 September 2021).
- Bacchi, U. Can Africa Deal with an Expected Boom in Demand for Meat? Thomson Reuters Foundation. 13 March 2017. Available online: https://www.reuters.com/article/us-africa-food-livestock-idUSKBN16K1V3 (accessed on 27 September 2021).
- Africa Group of Negotiators experts Support (AGNES). Desertification and Climate Change in Africa. Policy Brief No. 1. March 2020, pp. 1–8. Available online: https://agnes-africa.org/wp-content/uploads/2020/07/Policy-brief-1_Desertification-_Final_09032020.pdf (accessed on 27 September 2021).
- Steinfeld, H.; Gerber, P.; Wassenaar, T.; Castel, V.; Rosales, M.; De Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; FAO: Rome, Italy, 2006; p. 414. ISBN 978-92-5105571-7. Available online: http://www.fao.org/docrep/010/a0701e/a0701e00.HTM (accessed on 29 September 2021).
- African Development Bank (AfDB). Urbanization in Africa. 13 December 2012. Available online: https://blogs.afdb.org/fr/inclusive-growth/urbanization-africa-191 (accessed on 27 September 2021).
- Latino, L.R.; Pica-Ciamarra, U.; Wisser, D. Africa: The livestock revolution urbanizes. Glob. Food Sec. 2020, 26, 100399. [Google Scholar] [CrossRef] [PubMed]
- Neiderud, C.J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and disease emergence: Dynamics at the wildlife-livestock-human interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguimkeu, P.; Tadadjeu, S. Why is the number of COVID-19 cases lower than expected in Sub-Saharan Africa? A cross-sectional analysis of the role of demographic and geographic factors. World Dev. 2021, 138, 105251. [Google Scholar] [CrossRef]
- Kulohoma, B.W. COVID-19 risk factors: The curious case of Africa’s governance and preparedness. Sci. Afr. 2021, 13, e00948. [Google Scholar]
- Ezeh, A.; Silverman, M.; Stranges, S. The Conversation. The Impact of COVID-19 Has Been Lower in Africa. We Explore the Reasons. Contributor: Adams, J. 17 August 2021. Available online: https://theconversation.com/the-impact-of-covid-19-has-been-lower-in-africa-we-explore-the-reasons-164955 (accessed on 27 September 2021).
- Gondwe, G. Assessing the Impact of COVID-19 on Africa’s Economic Development. UNCTAD/ALDC/MISC/2020/3. July 2020, p. 21. Available online: https://unctad.org/system/files/official-document/aldcmisc2020d3_en.pdf (accessed on 27 September 2021).
- Barlow, P.; van Schalkwyk, M.C.; McKee, M.; Labonté, R.; Stuckler, D. COVID-19 and the collapse of global trade: Building an effective public health response. Lancet Planet. Health 2021, 5, e102–e107. [Google Scholar] [CrossRef]
- Ali Mohamed, E.M.; Alhaj Abdallah, S.M.; Ahmadi, A.; Lucero-Prisno, D.E. Food Security and COVID-19 in Africa: Implications and Recommendations. Am. J. Trop. Med. Hyg. 2021, 104, 1613–1615. [Google Scholar] [CrossRef]
- Oxford Business Group. Agriculture in Africa 2021: Focus Report. In Collaboration with OCP. April 2021. Available online: https://oxfordbusinessgroup.com/blog/bernardo-bruzzone/focus-reports/agriculture-africa-2021-focus-report (accessed on 26 September 2021).
- World Bank. GDP (Current US$)-Sub-Saharan Africa. Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?locations=ZG (accessed on 28 September 2021).
- Amankwah, A.; Gourlay, S. Food Security in the Face of COVID-19. Evidence from Africa. January 2021. Living Standards Measurement Study & World Bank Group. Available online: https://documents1.worldbank.org/curated/en/912661610964372485/pdf/Food-Security-in-the-Face-of-COVID-19-Evidence-from-Africa.pdf (accessed on 23 September 2021).
- OECD/FAO. Agriculture in Sub-Saharan Africa: Prospects and challenges for the next decade. In OECD-FAO Agricultural Outlook 2016–2025; OECD Publishing: Paris, France, 2016; Part I; Chapter 2. [Google Scholar]
- FAO; IFAD; WFP. The State of Food Insecurity in the World 2015. Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress, Food and Agriculture Organization Publications. 2015. Available online: https://www.fao.org/policy-support/tools-and-publications/resources-details/en/c/469455/ (accessed on 28 September 2021).
- World Bank. Rural Population (% of Total Population)-Sub-Saharan Africa. Available online: https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS?locations=ZG (accessed on 28 September 2021).
- Mercandalli, S.; Losch, B. Rural Migration in Sub−Saharan Africa: Patterns, Drivers and Relation to Structural Transformation; Belebema, M.N., Bélières, J.-F., Bourgeois, R., Dinbabo, M.F., Fréguin-Gresh, S., Mensah, C., Nshimbi, C.C., Eds.; FAO and CIRAD: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- NEPAD. African Agriculture, Transformation and Outlook; NEPAD: Johannesburg, South Africa, 2013; 72p. [Google Scholar]
- Heidhues, F.; Brüntrup, M. Subsistence agriculture in development: Its role in processes of structural change. In Subsistence Agriculture in Central and Eastern Europe: How to Break a Vicious Cycle? Abele, S., Frohberg, K., Eds.; Studies on the Agricultural and Food Sector in Central and Eastern Europe; Institute of Agricultural Development in Central and Eastern Europe (IAMO): Halle, Germany, 2003; Volume 22. [Google Scholar]
- Khalil, C.A.; Conforti, P.; Ergin, I.; Gennari, P. Food and Agriculture Organization (FAO). Defining Small-Scale Food Producers to Monitor Target 2.3 of the 2030 Agenda for Sustainable Development; Working Paper Series ESS/17-12; FAO Statistics Division: Rome, Italy, 2017. [Google Scholar]
- Murphy, S. Changing Perspectives: Small-Scale Farmers, Markets and Globalization (Revised Edition); IIED/Hivos: London/The Hague, UK, 2012. [Google Scholar]
- World Bank. Reaching the Rural Poor: A Renewed Strategy for Rural Development; World Bank: Washington, DC, USA, 2003; Available online: https://openknowledge.worldbank.org/handle/10986/14084 (accessed on 28 September 2021).
- Jayne, T.S.; Chamberlin, J.; Traub, L.; Sitko, N.; Muyanga, M.; Yeboah, F.K.; Anseeuw, W.; Chapoto, A.; Wineman, A.; Nkonde, C.; et al. Africa’s changing farm size distribution patterns: The rise of medium scale farms. Agric. Economics 2016, 47, 197–214. [Google Scholar] [CrossRef] [Green Version]
- Jayne, T.S.; Muyanga, M.; Wineman, A.; Ghebru, H.; Stevens, C.; Stickler, M.; Chapoto, A.; Anseeuw, W.; van der Westhuizen, D.; Nyange, D. Are medium-scale farms driving agricultural transformation in sub-Saharan Africa? Agric. Econ. 2019, 50, 75–95. [Google Scholar] [CrossRef] [Green Version]
- FARM Africa. Our Work with Livestock. Available online: https://www.farmafrica.org/agriculture/livestock (accessed on 28 September 2021).
- Otte, M.J.; Chilonda, P. Cattle and Small Ruminant Production Systems in Sub-Saharan Africa. A Systematic Review; Food and Agriculture Organization (FAO): Rome, italy, 2002; p. 98. [Google Scholar]
- De Leeuw, J.; Osano, P.; Said, M.; Ayantunde, A.; Dube, S.; Neely, C.; Vrieling, A.; Thornton, P.; Ericksen, P. The pastoral farming system. Balancing between tradition and transition. In Farming Systems and Food Security in Africa, 1st ed.; Dixon, J., Ed.; Routledge: London, UK, 2019; pp. 318–353. [Google Scholar]
- FAO. Pastoralism in Africa’s Drylands; FAO: Rome, Italy, 2018; p. 52. [Google Scholar]
- Avis, W. Rebuilding Pastoralist Livelihoods during and after Conflict; K4D Helpdesk Report 421; Institute of Development Studies: Brighton, UK, 2018. [Google Scholar]
- Grace, D.; Lindahl, J.; Wanyoike, F.; Bett, B.; Randolph, T.; Rich, K.M. Poor livestock keepers: Ecosystem-poverty-health interactions. Philos Trans R Soc Lond B Biol Sci. 2017, 372, 20160166. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.; Addison, J.; Bedelian, C.; Carabine, E.; Havlík, P.; Henderson, B.; Van De Steeg, J.; Thornton, P.K. Climate change and pastoralism: Impacts, consequences and adaptation. Rev. Sci. Tech. Off. Int. Epiz. 2016, 35, 417–433. [Google Scholar] [CrossRef]
- Roesel, K.; Grace, D. (Eds). Food Safety and Informal Markets: Animal Products in Sub-Saharan Africa; Routledge: London, UK, 2014. [Google Scholar]
- AUDA-NEPAD. Strengthening Rural Decent Jobs in Africa. 26 November 2020. Available online: https://www.nepad.org/news/strengthening-rural-decent-jobs-africa (accessed on 28 September 2021).
- World Health Organization (WHO). Food and Agriculture Organization of the United Nations (FAO) and World Organisation for Animal Health (OIE) Taking a Multisectoral, One Health Approach: A Tripartite Guide to Addressing Zoonotic Diseases in Countries. 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/EN_TripartiteZoonosesGuide_webversion.pdf (accessed on 25 September 2021).
- World Organisation for Animal Health (OIE). One Health. Available online: https://www.oie.int/en/what-we-do/global-initiatives/one-health/ (accessed on 25 September 2021).
- European Union. Global Health Summit. Rome Declaration. Available online: https://global-health-summit.europa.eu/rome-declaration_en (accessed on 28 September 2021).
- Njoroge, M.M.; Tirados, I.; Lindsay, S.W.; Vale, G.A.; Torr, S.J.; Fillinger, U. Exploring the potential of using cattle for malaria vector surveillance and control: A pilot study in western Kenya. Parasites Vectors. 2017, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.L.; Swain, S.; Lynch, P.A.; Sharma, S.K.; Haque, M.A.; Montgomery, J.; Thomas, M.B. Increasing the potential for malaria elimination by targeting zoophilic vectors. Sci. Rep. 2017, 7, 40551. [Google Scholar] [CrossRef] [Green Version]
- Khaligh, F.G.; Jafari, A.; Silivanova, E.; Levchenko, M.; Rahimi, B.; Gholizadeh, S. Endectocides as a complementary intervention in the malaria control program: A systematic review. Syst. Rev. 2021, 10, 30. [Google Scholar] [CrossRef]
- Hewitt, S.; Rowland, M. Control of zoophilic malaria vectors by applying pyrethroid insecticides to cattle. Trop. Med. Int. Health 1999, 4, 481–486. [Google Scholar] [CrossRef] [Green Version]
- WHO. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Khetarpal, N.; Khanna, I. Dengue Fever: Causes, Complications, and Vaccine Strategies. J. Immunol. Res. 2016, 2016, 6803098. [Google Scholar] [CrossRef] [Green Version]
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef]
- WHO. Control of Neglected Tropical Diseases. Neglected Zoonotic Diseases. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/neglected-zoonotic-diseases (accessed on 27 September 2021).
- Welburn, S.C.; Beange, I.; Ducrotoy, M.J.; Okello, A.L. The neglected zoonoses--the case for integrated control and advocacy. Clin. Microbiol. Infect. 2015, 21, 433–443. [Google Scholar] [CrossRef] [Green Version]
- United Nations. General Assembly. Seventieth Session. Resolution Adopted by the General Assembly on 25 September 2015. Available online: https://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed on 29 September 2021).
- Uniting to Combat Neglected Tropical Diseases. Ten Achievements of the London Declaration on Neglected Tropical Diseases Posted: 30 January 2020. Available online: https://unitingtocombatntds.org/news/ten-achievements-of-the-london-declaration-on-neglected-tropical-diseases/ (accessed on 29 September 2021).
- World Health Organization (WHO). Neglected Tropical Diseases: 2020 Preventive Chemotherapy Treatment Coverage Declines due to COVID-19 Disruptions. 24 September 2021 Departmental News, Geneva, Switzerland. Available online: https://www.who.int/news/item/24-09-2021-neglected-tropical-diseases-2020-preventive-chemotherapy-treatment-coverage-declines-due-to-covid-19-disruptions (accessed on 28 September 2021).
- Laing, G.; Vigilato, M.A.N.; Cleaveland, S.; Thumbi, S.M.; Blumberg, L.; Salahuddin, N.; Abdela-Ridder, B.; Harrison, W. One Health for neglected tropical diseases. Trans R. Soc. Trop. Med. Hyg. 2021, 115, 182–184, Erratum in: Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 940. [Google Scholar] [CrossRef]
- Fillâtre, P.; Revest, M.; Tattevin, P. Crimean-Congo hemorrhagic fever: An update. Med. Mal. Infect. 2019, 49, 574–585, Erratum in: Med. Mal. Infect. 2020, 50, 95–96. [Google Scholar] [CrossRef]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley fever: Biology and epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Elbir, H.; Raoult, D.; Drancourt, M. Relapsing fever borreliae in Africa. Am. J. Trop. Med. Hyg. 2013, 89, 288–292. [Google Scholar] [CrossRef] [Green Version]
- Cazorla, C.; Socolovschi, C.; Jensenius, M.; Parola, P. Tick-borne diseases: Tick-borne spotted fever rickettsioses in Africa. Infect. Dis. Clin. N. Am. 2008, 22, 531–544. [Google Scholar] [CrossRef]
- Parola, P. Rickettsia felis: From a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin. Microbiol. Infect. 2011, 17, 996–1000. [Google Scholar] [CrossRef] [Green Version]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Colombo, M.; Traversa, D. Canine and Feline Parasitology: Analogies, Differences, and Relevance for Human Health. Clin. Microbiol. Rev. 2021, 34, e0026620. [Google Scholar]
- AFP/Fox News. Uganda Confirms at Least One Case of Crimean-Congo Fever. 16 August 2013. Available online: https://www.foxnews.com/world/uganda-confirms-at-least-one-case-of-crimean-congo-fever (accessed on 25 September 2021).
- WHO Africa. How Previous Ebola Virus Disease Outbreaks Helped Uganda Respond to COVID-19 Outbreak. 28 August 2021. Available online: https://www.afro.who.int/news/how-previous-ebola-virus-disease-outbreaks-helped-uganda-respond-covid-19-outbreak (accessed on 28 September 2021).
- Schmidt-Sane, M.M.; Nielsen, J.O.; Chikombero, M.; Lubowa, D.; Lwanga, M.; Gamusi, J.; Kabanda, R.; Kaawa-Mafigiri, D. Challenges to Ebola preparedness during an ongoing outbreak: An analysis of borderland livelihoods and trust in Uganda. PLoS ONE 2020, 15, e0230683. [Google Scholar] [CrossRef]
- Mirembe, B.B.; Musewa, A.; Kadobera, D.; Kisaakye, E.; Birungi, D.; Eurien, D.; Nyakarahuka, L.; Balinandi, S.; Tumusiime, A.; Kyondo, J.; et al. Sporadic outbreaks of crimean-congo haemorrhagic fever in Uganda, July 2018-January 2019. PLoS Negl. Trop. Dis. 2021, 15, e0009213. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Africa. Government of Uganda Confirms Outbreak of Crimean-Congo Hemorrhagic and Rift Valley Fevers. 24 January 2018. Available online: https://www.afro.who.int/news/government-uganda-confirms-outbreak-crimean-congo-hemorrhagic-and-rift-valley-fevers (accessed on 25 September 2021).
- Kamani, J.; Massetti, L.; Olubade, T.; Balami, J.A.; Samdi, K.M.; Traub, R.J.; Colella, V.; González-Miguel, J. Canine gastrointestinal parasites as a potential source of zoonotic infections in Nigeria: A nationwide survey. Prev. Vet. Med. 2021, 192, 105385. [Google Scholar] [CrossRef]
- Warembourg, C.; Wera, E.; Odoch, T.; Bulu, P.M.; Berger-González, M.; Alvarez, D.; Abakar, M.F.; Maximiano Sousa, F.; Cunha Silva, L.; Alobo, G.; et al. Comparative Study of Free-Roaming Domestic Dog Management and Roaming Behavior Across Four Countries: Chad, Guatemala, Indonesia, and Uganda. Front. Vet. Sci. 2021, 8, 617900. [Google Scholar] [CrossRef] [PubMed]
- Van Blerk, H. African Lions: Who are Africa’s Rising Middle Class? IPSOS Views #15. February 2018. Available online: https://www.ipsos.com/en-ug/african-lions-who-are-africas-rising-middle-class (accessed on 27 October 2021).
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; de la Rue, M. Ecology and Life Cycle Patterns of Echinococcus Species. Adv. Parasitol. 2017, 95, 213–314. [Google Scholar] [PubMed]
- Catalano, S.; Sène, M.; Diouf, N.D.; Fall, C.B.; Borlase, A.; Léger, E.; Bâ, K.; Webster, J.P. Rodents as Natural Hosts of Zoonotic Schistosoma Species and Hybrids: An Epidemiological and Evolutionary Perspective from West Africa. J. Infect. Dis. 2018, 218, 429–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grace, D.; Songe, M.; Knight-Jones, T. Impact of neglected diseases on animal productivity and public health in Africa. In Proceedings of the 21st conference of the World Organisation for Animal Health (OIE) Regional Commission for Africa, Rabat, Morocco, 16–20 February 2015. [Google Scholar]
- Crump, A.; Ōmura, S. Ivermectin, ‘Wonder drug’ from Japan: The human use perspective. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Mectizan® Donation Program. Local Power–Global Change. History of the Program. Available online: https://mectizan.org/what/history/# (accessed on 26 September 2021).
- The Ivermectin Roadmappers; Billingsley, P.; Binka, F.; Chaccour, C.; Foy, B.; Gold, S.; Gonzalez-Silva, M.; Jacobson, J.; Jagoe, G.; Jones, C.; et al. A Roadmap for the Development of Ivermectin as a Complementary Malaria Vector Control Tool. Am. J. Trop. Med. Hyg. 2020, 102, 3–24. [Google Scholar]
- Selzer, P.M.; Epe, C. Antiparasitics in Animal Health: Quo Vadis? Trends Parasitol. 2021, 37, 77–89. [Google Scholar] [CrossRef]
- Gower, C.M.; Vince, L.; Webster, J.P. Should we be treating animal schistosomiasis in Africa? The need for a One Health economic evaluation of schistosomiasis control in people and their livestock. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 244–247. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L.; Basso, A.; Guidi, A. Schistosomiasis control: Praziquantel forever? Mol. Biochem. Parasitol. 2014, 195, 23–29. [Google Scholar] [CrossRef]
- Awadzi, K.; Boakye, D.A.; Edwards, G.; Opoku, N.O.; Attah, S.K.; Osei-Atweneboana, M.Y.; Lazdins-Helds, J.K.; Ardrey, A.E.; Addy, E.T.; Quartey, B.T.; et al. An investigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann. Trop. Med. Parasitol. 2004, 98, 231–249. [Google Scholar] [CrossRef]
- Lustigman, S.; McCarter, J.P. Ivermectin resistance in Onchocerca volvulus: Toward a genetic basis. PLoS Negl. Trop. Dis. 2007, 1, e76. [Google Scholar] [CrossRef] [Green Version]
- Vale, N.; Gouveia, M.J.; Rinaldi, G.; Brindley, P.J.; Gärtner, F.; Correia da Costa, J.M. Praziquantel for Schistosomiasis: Single-Drug Metabolism Revisited, Mode of Action, and Resistance. Antimicrob. Agents Chemother. 2017, 61, e02582-16. [Google Scholar] [CrossRef] [Green Version]
- Drugs for Neglected Diseases Initiative (DNDi). 2021. Available online: https://dndi.org/ (accessed on 28 September 2021).
- Medicines for Malaria Venture (MMV). 2021. Available online: https://www.mmv.org/ (accessed on 28 September 2021).
- GHIT Fund. 2021. Available online: https://www.ghitfund.org/ (accessed on 28 September 2021).
- FIND Diagnosis for All. 2021. Available online: https://www.finddx.org/ (accessed on 28 September 2021).
- PATH. 2021. Available online: https://www.path.org/about/ (accessed on 28 September 2021).
- Innovative Vector Control Consortium. 2021. Available online: https://www.ivcc.com/ (accessed on 29 September 2021).
- World Health Organisation (WHO) Africa. Neglected Tropical Diseases (NTD). The Burden of Neglected Tropical Diseases in the AFRO Region. Available online: https://www.afro.who.int/node/8924 (accessed on 26 September 2021).
- Worrall, E.; Basu, S.; Hanson, K. Is malaria a disease of poverty? A review of the literature. Trop. Med. Int. Health 2005, 10, 1047–1059. [Google Scholar] [CrossRef]
- Bill & Melinda Gates Foundation. Private and Public Partners Unite to Combat 10 Neglected Tropical Diseases by 2020. Available online: https://www.gatesfoundation.org/ideas/media-center/press-releases/2012/01/private-and-public-partners-unite-to-combat-10-neglected-tropical-diseases-by-2020 (accessed on 28 September 2021).
- Drugs for Neglected Diseases Initiative (DNDi). Diseases. Available online: https://dndi.org/diseases/ (accessed on 26 September 2021).
- Drugs for Neglected Diseases Initiative (DNDi). Filaria: River Blindness. Developing a Rapid Cure for Millions at Risk of Blindness. Available online: https://dndi.org/diseases/filaria-river-blindness/ (accessed on 26 September 2021).
- Drugs for Neglected Diseases Initiative (DNDi). Filaria: River Blindness. Available online: https://dndi.org/research-development/portfolio/emodepside/ (accessed on 29 September 2021).
- Global Alliance for Livestock Veterinary Medicines (GALVmed). Our Approach. Available online: https://www.galvmed.org/about-us/our-approach/ (accessed on 28 September 2021).
- Global Alliance for Livestock Veterinary Medicines (GALVmed). Human & Animal Health. Available online: https://www.galvmed.org/livestock-and-diseases/human-and-animal-health/ (accessed on 28 September 2021).
- Kabululu, M.L.; Ngowi, H.A.; Mlangwa, J.E.D.; Mkupasi, E.M.; Braae, U.C.; Colston, A.; Cordel, C.; Poole, E.J.; Stuke, K.; Johansen, M.V. TSOL18 vaccine and oxfendazole for control of Taenia solium cysticercosis in pigs: A field trial in endemic areas of Tanzania. PLoS Negl. Trop. Dis. 2020, 14, e0008785. [Google Scholar] [CrossRef]
- Global Alliance for Livestock Veterinary Medicines (GALVmed). Swine Programmes. Available online: https://www.galvmed.org/work/product-development/swine-programmes/ (accessed on 28 September 2021).
- Rossati, A. Global Warming and Its Health Impact. Int. J. Occup. Environ. Med. 2017, 8, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef]
- Buisson, Y.; Marié, J.L.; Davoust, B. Ces maladies infectieuses importées par les aliments [These infectious diseases imported with food]. Bull. Soc. Pathol Exot. 2008, 101, 343–347. [Google Scholar] [CrossRef]
- Gratz, N.G.; Steffen, R.; Cocksedge, W. Why aircraft disinsection? Bull. World Health Organ. 2000, 78, 995–1004. [Google Scholar]
- Tatem, A.J.; Hay, S.I.; Rogers, D.J. Global traffic and disease vector dispersal. Proc. Natl. Acad. Sci. USA 2006, 103, 6242–6247. [Google Scholar] [CrossRef] [Green Version]
- De La Rocque, S.; Balenghien, T.; Halos, L.; Dietze, K.; Claes, F.; Ferrari, G.; Guberti, V.; Slingenbergh, J. A review of trends in the distribution of vector-borne diseases: Is international trade contributing to their spread? Rev. Sci. Tech. 2011, 30, 119–130. [Google Scholar] [CrossRef]
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick Borne Dis. 2012, 3, 95–99. [Google Scholar] [CrossRef]
- Toma, L.; Mancuso, E.; d’Alessio, S.G.; Menegon, M.; Spina, F.; Pascucci, I.; Monaco, F.; Goffredo, M.; Di Luca, M. Tick species from Africa by migratory birds: A 3-year study in Italy. Exp Appl. Acarol. 2021, 83, 147–164. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Social Determinants of Health. Overview. Available online: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed on 29 September 2021).
- Lorusso, V.; Gruszka, K.A.; Majekodunmi, A.; Igweh, A.; Welburn, S.C.; Picozzi, K. Rickettsia africae in Amblyomma variegatum ticks, Uganda and Nigeria. Emerg. Infect. Dis. 2013, 19, 1705–1707. [Google Scholar] [CrossRef]
- Katswara, T.; Mukaratirwa, S. Knowledge, attitudes and practices on African tick bite fever of rural livestock communities living in a livestock-wildlife interface area in the Eastern Cape Province of South Africa. BMC Infect. Dis. 2021, 21, 497. [Google Scholar] [CrossRef]
- Gavas, M.; Pleeck, S. Global Trends in 2021: How COVID-19 Is Transforming International Development. CGD Notes. March 2021, pp. 1–16. Available online: https://www.cgdev.org/publication/global-trends-2021-how-covid-transforming-international-development (accessed on 29 September 2021).
- Reid, M.; Abdool-Karim, Q.; Geng, E.; Goosby, E. How will COVID-19 Transform Global Health Post-Pandemic? Defining Research and Investment Opportunities and Priorities. PLoS Med. 2021, 18, e1003564. [Google Scholar] [CrossRef]
- Wells, N. COVID-19 Focusses Investors on Global Health Priorities. Swiss Info. Opinion. International Geneva. 31 May 2021. Available online: https://www.swissinfo.ch/eng/covid-19-focusses-investors-on-global-health-priorities/46649132 (accessed on 30 September 2021).
- Relief Web. Fight against Yellow Fever: Institut Pasteur Foundation in Dakar to Set Up New Vaccine Production Unit. 4 February 2016. Available online: https://reliefweb.int/report/world/fight-against-yellow-fever-institut-pasteur-foundation-dakar-set-new-vaccine-production (accessed on 29 September 2021).
- European Commission. Republic of Senegal and Team Europe Agree to Build a Manufacturing Plant to Produce Vaccines Against COVID-19 and other Endemic Diseases. Press Release. 9 July 2021. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_3562 (accessed on 29 September 2021).
- University of Global Health Equity (UGHE). Available online: https://ughe.org/ (accessed on 29 September 2021).
- ILRI. One Health Centre in Africa. Available online: https://www.ilri.org/research/facilities/one-health-centre (accessed on 29 September 2021).
- Africa One Health University Network (AFROHUN). Available online: https://afrohun.org/ (accessed on 29 September 2021).
- World Health Organisation (WHO) TDR. African Women in Science. Available online: https://www.who.int/tdr/research/gender/Women_overview_piece.pdf?ua=1 (accessed on 30 September 2021).
- Horton, R.; Lo, S. Planetary health: A new science for exceptional action. Lancet 2015, 386, 1921–1922, Erratum in 2015, 386, 1944. [Google Scholar] [CrossRef] [Green Version]
- The Lancet. Zoonoses: Beyond the human-animal-environment interface. Lancet 2020, 396, 1. [Google Scholar] [CrossRef]
- UNFPA. The Demographic Dividend Atlas for Africa. Tracking the Potential for a Demographic Dividend. September 2017, p. 151. Available online: https://www.unfpa.org/sites/default/files/resource-pdf/UNFPA_African_Atlas_KW_RS_SZ.pdf (accessed on 28 September 2021).
- Langedijk, J.; Mantel-Teeuwisse, A.K.; Slijkerman, D.S.; Schutjens, M.H. Drug repositioning and repurposing: Terminology and definitions in literature. Drug Discov. Today 2015, 20, 1027–1034. [Google Scholar] [CrossRef]

| Neglected Tropical Diseases (NTDs) | Causative Agent(s) | Parasitic Aetiology | Arthropod-Borne | Zoonotic |
|---|---|---|---|---|
| Buruli ulcer | Mycobacterium ulcerans | |||
| Dengue and chikungunya | dengue virus (DENV) chikungunya virus (CHIKV) | ✓ | ✓ | |
| Echinococcosis (hydatidosis) | Echinococcus granulosus complex | ✓ | ✓ | |
| Food-borne trematodiases | Fasciola spp.; Paragonimus spp. | ✓ | ✓ | |
| Guinea worm disease (Dracunculiasis) | Dracunculus medinensis | ✓ | ✓ | |
| Human African trypanosomiasis (sleeping sickness) | Trypanosoma brucei | ✓ | ✓ | ✓ |
| Leishmaniasis | Leishmania infantum; Leishmania donovani; Leishmania major; Leishmania aethiopica; Leishmania tropica | ✓ | ✓ | ✓ |
| Leprosy (Hansen disease) | Mycobacterium leprae | |||
| Lymphatic Filariasis | Wuchereria bancrofti | ✓ | ✓ | |
| Mycetoma | Madurella spp.; Streptomyces spp.; Actinomadura spp.; Exophiala spp. | |||
| Onchocerciasis (River blindness) | Onchocerca volvulus | ✓ | ✓ | |
| Rabies | Rabies virus (RV) | ✓ | ||
| Scabies | Sarcoptes scabiei var. hominis | ✓ | ||
| Schistosomiasis (Bilharziasis) | Schistosoma haematobium; Schistosoma mansoni and hybrid forms | ✓ | ✓ | |
| Snakebite envenoming | Several toxins in snakes’ venoms | |||
| Soil-Transmitted Helminthiases | Ascaris lumbricoides and Strongyloides stercoralis (roundworms); Trichuris trichiura (whipworm); Necator americanus and Ancylostoma duodenale (hookworms) | ✓ | ✓ (S. stercoralis) | |
| Taeniasis/ cysticercosis | Taenia solium/ Cysticercus cellulosae | ✓ | ✓ | |
| Blinding trachoma | Chlamydia trachomatis | |||
| Endemic treponematoses (yaws) | Treponema spp. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, V. Parasitology and One Health—Perspectives on Africa and Beyond. Pathogens 2021, 10, 1437. https://doi.org/10.3390/pathogens10111437
Lorusso V. Parasitology and One Health—Perspectives on Africa and Beyond. Pathogens. 2021; 10(11):1437. https://doi.org/10.3390/pathogens10111437
Chicago/Turabian StyleLorusso, Vincenzo. 2021. "Parasitology and One Health—Perspectives on Africa and Beyond" Pathogens 10, no. 11: 1437. https://doi.org/10.3390/pathogens10111437