pH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers
Abstract
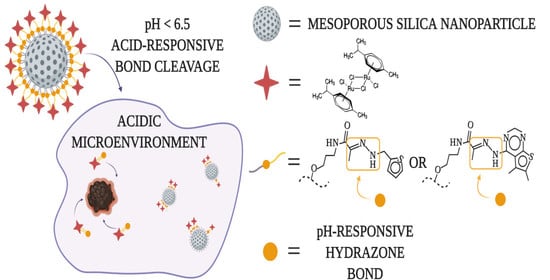
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of MSN and Functionalization with Ligands
2.4. Reaction MSN Materials with Dichloro(p-Cymene)ruthenium(II) Dimer
2.5. Drug Release Investigation
2.6. Determination of Cell Viability
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rapoport, B.L. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front. Pharmacol. 2017, 8, 19. [Google Scholar] [CrossRef]
- Monsuez, J.-J.; Charniot, J.-C.; Vignat, N.; Artigou, J.-Y. Cardiac side-effects of cancer chemotherapy. Int. J. Cardiol. 2010, 144, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Baudino, T.A. Send Orders for Reprints to [email protected] Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Drug Discov. Technol. 2015, 12, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Zak, J.K. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greish, K.; Iyer, A.K.; Fang, J.; Kawasuji, M.; Maeda, H. Enhanced permeability and retention (EPR) effect and tumor-selective delivery of anticancer drugs. Deliv. Protein Pept. Drugs Cancer 2006, 10, 14. [Google Scholar]
- Knežević, N.Ž.; Durand, J.-O. Targeted Treatment of Cancer with Nanotherapeutics Based on Mesoporous Silica Nanoparticles. Chempluschem 2015, 80, 26–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Barui, S.; Cauda, V. Multimodal decorations of mesoporous silica nanoparticles for improved cancer therapy. Pharmaceutics 2020, 12, 527. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Cordeiro, R.A.; Faneca, H. Silica-Based Gene Delivery Systems: From Design to Therapeutic Applications. Pharmaceutics 2020, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Chan, R.; Ji, Y.; Lu, J.; Liao, Y.-P.; Okene, M.; Lin, J.; Lin, P.; Chang, C.H.; et al. Improved Efficacy and Reduced Toxicity Using a Custom-Designed Irinotecan-Delivering Silicasome for Orthotopic Colon Cancer. ACS Nano 2019, 13, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona Composition Can Affect the Mechanisms Cells Use to Internalize Nanoparticles. ACS Nano 2019, 13, 11107–11121. [Google Scholar] [CrossRef]
- Li, H.; Yan, W.; Suo, X.; Peng, H.; Yang, X.; Li, Z.; Zhang, J.; Liu, D. Nucleus-targeted nano delivery system eradicates cancer stem cells by combined thermotherapy and hypoxia-activated chemotherapy. Biomaterials 2019, 200, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Lozano, D.; Matsumoto, K.; Komatsu, A.; Manzano, M.; Tamanoi, F.; Vallet-Regí, M. Designing Mesoporous Silica Nanoparticles to Overcome Biological Barriers by Incorporating Targeting and Endosomal Escape. ACS Appl. Mater. Interfaces 2021, 13, 9656–9666. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Kaluđerović, G.N. Silicon-based nanotheranostics. Nanoscale 2017, 9, 12821–12829. [Google Scholar] [CrossRef]
- Böhme, I.; Bosserhoff, A.K. Acidic tumor microenvironment in human melanoma. Pigment Cell Melanoma Res. 2016, 29, 508–523. [Google Scholar] [CrossRef]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.E.; Ardenkjær-Larsen, J.H.; In‘t Zandt, R.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef]
- Yang, K.; Luo, H.; Zeng, M.; Jiang, Y.; Li, J.; Fu, X. Intracellular pH-Triggered, Targeted Drug Delivery to Cancer Cells by Multifunctional Envelope-Type Mesoporous Silica Nanocontainers. ACS Appl. Mater. Interfaces 2015, 7, 17399–17407. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Yang, L.-Y.; Ouyang, X.-K.; Huang, F. Folic Acid and PEI Modified Mesoporous Silica for Targeted Delivery of Curcumin. Pharmaceutics 2019, 11, 430. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Peng, S.; Lin, W.; Wang, J.; Zhang, L. Multistage pH-responsive mesoporous silica nanohybrids with charge reversal and intracellular release for efficient anticancer drug delivery. J. Colloid Interface Sci. 2019, 555, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, Q.; Shen, X.; Sun, Q.; Mu, C.; Gu, H.; Cai, K. A pH-responsive nanocontainer based on hydrazone-bearing hollow silica nanoparticles for targeted tumor therapy. J. Mater. Chem. B 2016, 4, 4594–4604. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yao, Q.; Qian, K.; Tian, L.; Cheng, Z.; Yang, D.; Wang, Y. Synthesis, antiproliferative activity and mechanism of gallium(III)-thiosemicarbazone complexes as potential anti-breast cancer agents. Eur. J. Med. Chem. 2018, 154, 91–100. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Virag, P.; Cenariu, M.; Prashar, S.; Fajardo, M.; Fischer-Fodor, E.; Gómez-Ruiz, S. Anti-cancer Applications of Titanocene-Functionalised Nanostructured Systems: An Insight into Cell Death Mechanisms. Chem. A Eur. J. 2014, 20, 10811–10828. [Google Scholar] [CrossRef]
- Ghalandari, B.; Divsalar, A.; Saboury, A.A.; Parivar, K. The new insight into oral drug delivery system based on metal drugs in colon cancer therapy through β-lactoglobulin/oxali-palladium nanocapsules. J. Photochem. Photobiol. B Biol. 2014, 140, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Tabrizi, L.; Chiniforoshan, H. Ruthenium(II) p-cymene complexes of naphthoquinone derivatives as antitumor agents: A structure−activity relationship study. J. Organomet. Chem. 2016, 822, 211–220. [Google Scholar] [CrossRef]
- Orhan, E.; Garci, A.; Riedel, T.; Soudani, M.; Dyson, P.J.; Therrien, B. Cytotoxic double arene ruthenium metalla-cycles that overcome cisplatin resistance. J. Organomet. Chem. 2016, 803, 39–44. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Robalo, M.P.; Tomaz, A.I.; Carvalho, A.; Fernandes, A.R.; Marques, F.; Folgueira, M.; Yáñez, J.; Vázquez-García, D.; López Torres, M.; et al. RuII(p-cymene) Compounds as Effective and Selective Anticancer Candidates with No Toxicity In Vivo. Inorg. Chem. 2018, 57, 13150–13166. [Google Scholar] [CrossRef]
- Murray, B.S.; Babak, M.V.; Hartinger, C.G.; Dyson, P.J. The development of RAPTA compounds for the treatment of tumors. Coord. Chem. Rev. 2016, 306, 86–114. [Google Scholar] [CrossRef]
- Ludwig, G.; Kaluđerović, G.N.; Rüffer, T.; Bette, M.; Korb, M.; Block, M.; Paschke, R.; Lang, H.; Steinborn, D. Cationic arene ruthenium(II) complexes with chelating P-functionalized alkyl phenyl sulfide and sulfoxide ligands as potent anticancer agents. Dalt. Trans. 2013, 42, 3771–3774. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, G.N.; Krajnović, T.; Momcilovic, M.; Stosic-Grujicic, S.; Mijatović, S.; Maksimović-Ivanić, D.; Hey-Hawkins, E. Ruthenium(II) p-cymene complex bearing 2,2′-dipyridylamine targets caspase 3 deficient MCF-7 breast cancer cells without disruption of antitumor immune response. J. Inorg. Biochem. 2015, 153, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, G.; Mojić, M.; Bulatović, M.; Mijatović, S.; Maksimović-Ivanić, D.; Steinborn, D.; Kaluđerović, G.N. Biological Potential of Halfsandwich Ruthenium(II) and Iridium(III) Complexes. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2016, 16, 1455–1460. [Google Scholar] [CrossRef]
- Carter, R.; Westhorpe, A.; Romero, M.J.; Habtemariam, A.; Gallevo, C.R.; Bark, Y.; Menezes, N.; Sadler, P.J.; Sharma, R.A. Radiosensitisation of human colorectal cancer cells by ruthenium(II) arene anticancer complexes. Sci. Rep. 2016, 6, 20596. [Google Scholar] [CrossRef] [Green Version]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured materials functionalized with metal complexes: In search of alternatives for administering anticancer metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Edeler, D.; Arlt, S.; Petković, V.; Ludwig, G.; Drača, D.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Delivery of [Ru(η6-p-cymene)Cl2{Ph2P(CH2)3SPh-κP}] using unfunctionalized and mercapto functionalized SBA-15 mesoporous silica: Preparation, characterization and in vitro study. J. Inorg. Biochem. 2018, 180, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž.; Stojanovic, V.; Chaix, A.; Bouffard, E.; El Cheikh, K.; Morère, A.; Maynadier, M.; Lemercier, G.; Garcia, M.; Gary-Bobo, M.; et al. Ruthenium(II) multifunctionalized porous silicon nanoparticles for two-photon near-infrared light responsive imaging and photodynamic cancer therapy. J. Mater. Chem. B 2016, 4, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous silica nanoparticles functionalised with a photoactive ruthenium(II) complex: Exploring the formulation of a metal-based photodynamic therapy photosensitiser. Dalton Trans. 2019, 48, 5940–5951. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.-Y. Functionalized mesoporous silica nanoparticle-based visible light responsive controlled release delivery system. Chem. Commun. 2011, 47, 2817–2819. [Google Scholar] [CrossRef] [PubMed]
- Frasconi, M.; Liu, Z.; Lei, J.; Wu, Y.; Strekalova, E.; Malin, D.; Ambrogio, M.W.; Chen, X.; Botros, Y.Y.; Cryns, V.L. Photoexpulsion of surface-grafted ruthenium complexes and subsequent release of cytotoxic cargos to cancer cells from mesoporous silica nanoparticles. J. Am. Chem. Soc. 2013, 135, 11603–11613. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Carmona, M.; Ho, Q.P.; Morand, J.; García, A.; Ortega, E.; Erthal, L.C.S.; Ruiz-Hernandez, E.; Santana, M.D.; Ruiz, J.; Vallet-Regí, M.; et al. Amino-Functionalized Mesoporous Silica Nanoparticle-Encapsulated Octahedral Organoruthenium Complex as an Efficient Platform for Combatting Cancer. Inorg. Chem. 2020, 59, 10275–10284. [Google Scholar] [CrossRef]
- Ma, B.; He, L.; You, Y.; Mo, J.; Chen, T. Controlled synthesis and size effects of multifunctional mesoporous silica nanosystem for precise cancer therapy. Drug Deliv. 2018, 25, 293–306. [Google Scholar] [CrossRef]
- Lv, G.; Qiu, L.; Liu, G.; Wang, W.; Li, K.; Zhao, X.; Lin, J. pH sensitive chitosan-mesoporous silica nanoparticles for targeted delivery of a ruthenium complex with enhanced anticancer effects. Dalton Trans. 2016, 45, 18147–18155. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Zhu, H.; Pang, G.; Zheng, W.; Wong, Y.-S.; Chen, T. Cancer-Targeted Monodisperse Mesoporous Silica Nanoparticles as Carrier of Ruthenium Polypyridyl Complexes to Enhance Theranostic Effects. Adv. Funct. Mater. 2014, 24, 2754–2763. [Google Scholar] [CrossRef]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science & Business Media: New York, NY, USA, 2012; Volume 16, ISBN 1402023030. [Google Scholar]
- Drača, D.; Mijatović, S.; Krajnović, T.; Pristov, J.B.; Đukić, T.; Kaluđerović, G.N.; Wessjohann, L.A.; Maksimović-Ivanić, D. The synthetic tubulysin derivative, tubugi-1, improves the innate immune response by macrophage polarization in addition to its direct cytotoxic effects in a murine melanoma model. Exp. Cell Res. 2019, 380, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Knežević, N.Ž. Visible light responsive anticancer treatment with an amsacrine-loaded mesoporous silica-based nanodevice. RSC Adv. 2013, 3, 19388–19392. [Google Scholar] [CrossRef]
- Vaz-Ramos, J.; Cordeiro, R.; Castro, M.M.C.A.; Geraldes, C.F.G.C.; Costa, B.F.O.; Faneca, H.; Durães, L. Supercritically dried superparamagnetic mesoporous silica nanoparticles for cancer theranostics. Mater. Sci. Eng. C 2020, 115, 111124. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, S.; Piludu, M.; Casula, M.F.; Vallet-Regì, M.; Monduzzi, M.; Salis, A. Interactions between bovine serum albumin and mesoporous silica nanoparticles functionalized with biopolymers. Chem. Eng. J. 2018, 340, 42–50. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.Y. Light- and pH-responsive release of doxorubicin from a mesoporous silica-based nanocarrier. Chem. A Eur. J. 2011, 17, 3338–3342. [Google Scholar] [CrossRef] [PubMed]
- Bíró, L.; Farkas, E.; Buglyó, P. Hydrolytic behaviour and chloride ion binding capability of [Ru(η6-p-cym)(H2O)3]2+: A solution equilibrium study. Dalton Trans. 2012, 41, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Drača, D.; Edeler, D.; Saoud, M.; Dojčinović, B.; Dunđerović, D.; Đmura, G.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Antitumor potential of cisplatin loaded into SBA-15 mesoporous silica nanoparticles against B16F1 melanoma cells: In vitro and in vivo studies. J. Inorg. Biochem. 2021, 217, 111383. [Google Scholar] [CrossRef]
- Dömötör, O.; Kiss, M.A.; Gál, G.T.; May, N.V.; Spengler, G.; Nové, M.; Gašparović, A.Č.; Frank, É.; Enyedy, É.A. Solution equilibrium, structural and cytotoxicity studies on Ru (η6-p-cymene) and copper complexes of pyrazolyl thiosemicarbazones. J. Inorg. Biochem. 2020, 202, 110883. [Google Scholar] [CrossRef] [PubMed]





| Material | BET Surface Area (m2/g) | Total Volume of Mesopores (cm3/g) | Average Pore Diameter (nm) | BJH Pore Diameter (nm) |
|---|---|---|---|---|
| MSN | 1046 | 0.6 | 3.4 | 2.7 |
| MSN-PA | 753 | 0.6 | 2.8 | 2.2 |
| MSN-H1 | 703 | 0.2 | 2.5 | 2.2 |
| MSN-H2 | 696 | 0.2 | 2.5 | 2.1 |
| MSN-H1[Ru] | 298 | 0.1 | 2.2 | <2 |
| MSN-H2[Ru] | 238 | 0.2 | 2.5 | <2 |
| Material | pH 5.0 | pH 6.0 | pH 7.4 |
|---|---|---|---|
| MSN[Ru] | 1.50 ± 0.01 | 0.95 ± 0.02 | 0.64 ± 0.01 |
| MSN-H1[Ru] | 26.28 ± 0.13 | 26.13 ± 0.08 | 19.47 ± 0.17 |
| MSN-H2[Ru] | 19.91 ± 0.11 | 19.60 ± 0.06 | 16.66 ± 0.03 |
| Material | pH 7.2 | pH 5.0 | ||
|---|---|---|---|---|
| MTT Assay | CV Assay | MTT Assay | CV Assay | |
| MSN-H1[Ru] | >100 | >100 | 4.00 ± 0.86 | 0.63 ± 0.15 |
| MSN-H2[Ru] | >100 | >100 | 2.49 ± 1.02 | 0.63 ± 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, M.; Morgan, I.; Ilić, N.; Saoud, M.; Pergal, M.V.; Kaluđerović, G.N.; Knežević, N.Ž. pH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers. Pharmaceutics 2021, 13, 460. https://doi.org/10.3390/pharmaceutics13040460
Mladenović M, Morgan I, Ilić N, Saoud M, Pergal MV, Kaluđerović GN, Knežević NŽ. pH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers. Pharmaceutics. 2021; 13(4):460. https://doi.org/10.3390/pharmaceutics13040460
Chicago/Turabian StyleMladenović, Minja, Ibrahim Morgan, Nebojša Ilić, Mohamad Saoud, Marija V. Pergal, Goran N. Kaluđerović, and Nikola Ž. Knežević. 2021. "pH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers" Pharmaceutics 13, no. 4: 460. https://doi.org/10.3390/pharmaceutics13040460