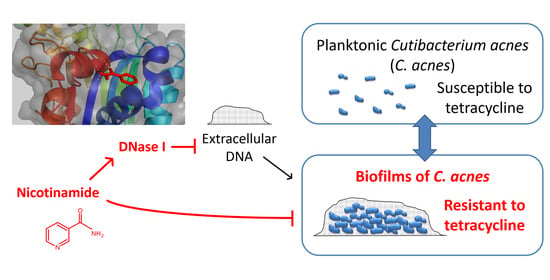
Activation of Deoxyribonuclease I by Nicotinamide as a New Strategy to Attenuate Tetracycline-Resistant Biofilms of Cutibacterium acnes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Establishment of an In Vitro Culture System for Biofilms of C. acnes
3.2. NAM Potentiates the Efficacy of the Suboptimal Dosing of TCN against C. acnes
3.3. Nicotinamide Alone Suppresses Biofilms of C. acnes In Vitro
3.4. Nicotinamide Activates DNase I and Enhances the Antibiofilm Effect of the Enzyme against C. acnes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global Skin Disease Morbidity and Mortality: An Update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Prim. 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Pathak, R.; Mary, P.B.; Jha, D.; Sardana, K.; Gautam, H.K. New insights into acne pathogenesis: Exploring the role of acne-associated microbial populations. Dermatol. Sin. 2016, 34, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Coenye, T.; Peeters, E.; Nelis, H.J. Biofilm formation by Propionibacterium acnes is associated with increased resistance to antimicrobial agents and increased production of putative virulence factors. Res. Microbiol. 2007, 158, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.J.; Choi, D.K.; Sohn, K.C.; Seo, M.S.; Lee, H.E.; Lee, Y.; Seo, Y.J.; Lee, Y.H.; Shi, G.; Zouboulis, C.C.; et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J. Invest. Dermatol. 2014, 134, 2747–2756. [Google Scholar] [CrossRef] [Green Version]
- Mayslich, C.; Grange, P.A.; Dupin, N. Cutibacterium acnes as an Opportunistic Pathogen: An Update of Its Virulence-Associated Factors. Microorganisms 2021, 9, 303. [Google Scholar] [CrossRef]
- Bienenfeld, A.; Nagler, A.R.; Orlow, S.J. Oral Antibacterial Therapy for Acne Vulgaris: An Evidence-Based Review. Am. J. Clin. Dermatol. 2017. [Google Scholar] [CrossRef]
- Walsh, T.R.; Efthimiou, J.; Dreno, B. Systematic review of antibiotic resistance in acne: An increasing topical and oral threat. Lancet Infect. Dis. 2016, 16, e23–e33. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, P.; Barath, Z.; Gajdacs, M. It’s Not Easy Being Green: A Narrative Review on the Microbiology, Virulence and Therapeutic Prospects of Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2021, 10, 42. [Google Scholar] [CrossRef]
- Senobar Tahaei, S.A.; Stajer, A.; Barrak, I.; Ostorhazi, E.; Szabo, D.; Gajdacs, M. Correlation Between Biofilm-Formation and the Antibiotic Resistant Phenotype in Staphylococcus aureus Isolates: A Laboratory-Based Study in Hungary and a Review of the Literature. Infect. Drug Resist. 2021, 14, 1155–1168. [Google Scholar] [CrossRef]
- Hoiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.O.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69 (Suppl. 1), i37–i40. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Jahns, A.C.; Lundskog, B.; Ganceviciene, R.; Palmer, R.H.; Golovleva, I.; Zouboulis, C.C.; McDowell, A.; Patrick, S.; Alexeyev, O.A. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: A case-control study. Br. J. Dermatol. 2012, 167, 50–58. [Google Scholar] [CrossRef]
- Burkhart, C.G.; Burkhart, C.N. Expanding the microcomedone theory and acne therapeutics: Propionibacterium acnes biofilm produces biological glue that holds corneocytes together to form plug. J. Am. Acad. Dermatol. 2007, 57, 722–724. [Google Scholar] [CrossRef] [PubMed]
- Aubin, G.G.; Portillo, M.E.; Trampuz, A.; Corvec, S. Propionibacterium acnes, an emerging pathogen: From acne to implant-infections, from phylotype to resistance. Med. Mal. Infect. 2014, 44, 241–250. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Ratjen, F. Effect of dornase alfa on inflammation and lung function: Potential role in the early treatment of cystic fibrosis. J. Cyst. Fibros. 2012, 11, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Otte, N.; Borelli, C.; Korting, H.C. Nicotinamide—Biologic actions of an emerging cosmetic ingredient. Int. J. Cosmet. Sci. 2005, 27, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Shalita, A.R.; Smith, J.G.; Parish, L.C.; Sofman, M.S.; Chalker, D.K. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int. J. Dermatol. 1995, 34, 434–437. [Google Scholar] [CrossRef]
- Khodaeiani, E.; Fouladi, R.F.; Amirnia, M.; Saeidi, M.; Karimi, E.R. Topical 4% nicotinamide vs. 1% clindamycin in moderate inflammatory acne vulgaris. Int. J. Dermatol. 2013, 52, 999–1004. [Google Scholar] [CrossRef]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Ryu, S.; Park, Y.; Kim, B.; Cho, S.M.; Lee, J.; Lee, H.H.; Gurley, C.; Song, K.; Johnson, A.; Armstrong, C.A.; et al. Inhibitory and anti-inflammatory effects of the Helicobacter pylori-derived antimicrobial peptide HPA3NT3 against Propionibacterium acnes in the skin. Br. J. Dermatol. 2014, 171, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Parsiegla, G.; Noguere, C.; Santell, L.; Lazarus, R.A.; Bourne, Y. The structure of human DNase I bound to magnesium and phosphate ions points to a catalytic mechanism common to members of the DNase I-like superfamily. Biochemistry 2012, 51, 10250–10258. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunitz, M. Crystalline desoxyribonuclease; digestion of thymus nucleic acid; the kinetics of the reaction. J. Gen. Physiol. 1950, 33, 363–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunitz, M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J. Gen. Physiol. 1950, 33, 349–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, H.R.; Raghava, G.P. Identification of NAD interacting residues in proteins. BMC Bioinform. 2010, 11, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giangreco, I.; Packer, M.J. Pharmacophore binding motifs for nicotinamide adenine dinucleotide analogues across multiple protein families: A detailed contact-based analysis of the interaction between proteins and NAD(P) cofactors. J. Med. Chem. 2013, 56, 6175–6189. [Google Scholar] [CrossRef]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Tetz, G.V.; Artemenko, N.K.; Tetz, V.V. Effect of DNase and antibiotics on biofilm characteristics. Antimicrob. Agents Chemother. 2009, 53, 1204–1209. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.; Liao, Z.; Tan, F.; Zhu, Z.; Jiang, Y.; Cao, Y. Effect of Nicotinamide Against Candida albicans. Front. Microbiol. 2019, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Doroshenko, N.; Tseng, B.S.; Howlin, R.P.; Deacon, J.; Wharton, J.A.; Thurner, P.J.; Gilmore, B.F.; Parsek, M.R.; Stoodley, P. Extracellular DNA impedes the transport of vancomycin in Staphylococcus epidermidis biofilms preexposed to subinhibitory concentrations of vancomycin. Antimicrob. Agents Chemother. 2014, 58, 7273–7282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baelo, A.; Levato, R.; Julian, E.; Crespo, A.; Astola, J.; Gavalda, J.; Engel, E.; Mateos-Timoneda, M.A.; Torrents, E. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control. Release Off. J. Control. Release Soc. 2015, 209, 150–158. [Google Scholar] [CrossRef]
- Novotny, L.A.; Jurcisek, J.A.; Goodman, S.D.; Bakaletz, L.O. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 2016, 10, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Hoffler, U.; Gehse, M.; Gloor, M.; Pulverer, G. Enzyme production of propionibacteria from patients with acne vulgaris and healthy persons. Acta Dermatovenereol. 1985, 65, 428–432. [Google Scholar]
- Hoeffler, U. Enzymatic and hemolytic properties of Propionibacterium acnes and related bacteria. J. Clin. Microbiol. 1977, 6, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.P.; Rani, R. Effect of nicotinamide on 12-O-tetradecanoyl-phorbol-13-acetate exposed mouse skin endonuclease activity and DNA synthesis. Biomed. Environ. Sci. BES 2000, 13, 122–130. [Google Scholar]
- Fujihara, J.; Yasuda, T.; Kunito, T.; Fujii, Y.; Takatsuka, H.; Moritani, T.; Takeshita, H. Two N-linked glycosylation sites (Asn18 and Asn106) are both required for full enzymatic activity, thermal stability, and resistance to proteolysis in mammalian deoxyribonuclease I. Biosci. Biotechnol. Biochem. 2008, 72, 3197–3205. [Google Scholar] [CrossRef] [Green Version]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Brackman, G.; Forier, K.; Al Quntar, A.A.; de Canck, E.; Enk, C.D.; Srebnik, M.; Braeckmans, K.; Coenye, T. Thiazolidinedione derivatives as novel agents against Propionibacterium acnes biofilms. J. Appl. Microbiol. 2014, 116, 492–501. [Google Scholar] [CrossRef]
- Simion, F.A.; Rhein, L.D.; Grove, G.L.; Wojtkowski, J.M.; Cagan, R.H.; Scala, D.D. Sequential order of skin responses to surfactants during a soap chamber test. Contact Dermat. 1991, 25, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Sulzberger, M.; Folster, H.; Sattler, M.; Rippke, F.; Gronniger, E. Inhibition of Propionibacterium acnes associated biofilm formation by Decanediol. J. Dermatol. Sci. 2016, 83, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Brackman, G.; Rigole, P.; de Witte, E.; Honraet, K.; Rossel, B.; Nelis, H.J. Eradication of Propionibacterium acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomed. Int. J. Phytother. Phytopharmacol. 2012, 19, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Feuillolay, C.; Pecastaings, S.; Le Gac, C.; Fiorini-Puybaret, C.; Luc, J.; Joulia, P.; Roques, C. A Myrtus communis extract enriched in myrtucummulones and ursolic acid reduces resistance of Propionibacterium acnes biofilms to antibiotics used in acne vulgaris. Phytomed. Int. J. Phytother. Phytopharmacol. 2016, 23, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Wunnoo, S.; Saising, J.; Voravuthikunchai, S.P. Rhodomyrtone inhibits lipase production, biofilm formation, and disorganizes established biofilm in Propionibacterium acnes. Anaerobe 2017, 43, 61–68. [Google Scholar] [CrossRef]
- Revathy, J.; Karthika, S.; Sentila, R.; Michael, A. In vitro evaluation of the efficacy of chicken egg yolk antibodies (IgY) generated against Propionibacterium acnes. Int. J. Cosmet. Sci. 2014, 36, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gajdacs, M.; Spengler, G. The Role of Drug Repurposing in the Development of Novel Antimicrobial Drugs: Non-Antibiotic Pharmacological Agents as Quorum Sensing-Inhibitors. Antibiotics 2019, 8, 270. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne is an inflammatory disease and alterations of sebum composition initiate acne lesions. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C. Linking diet to acne metabolomics, inflammation, and comedogenesis: An update. Clin. Cosmet. Investig. Dermatol. 2015, 8, 371–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, D.W.; Winters, G.C.; Brownsey, R.W.; Kulpa, J.E.; Gilliland, K.L.; Thiboutot, D.M.; Hofland, H.E. Inhibition of Sebum Production with the Acetyl Coenzyme A Carboxylase Inhibitor Olumacostat Glasaretil. J. Invest. Dermatol. 2017, 137, 1415–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, Y.-H.; Liu, D.; Chen, Y.-C.; Liao, M.-H.; Lee, W.-R.; Shen, S.-C. Activation of Deoxyribonuclease I by Nicotinamide as a New Strategy to Attenuate Tetracycline-Resistant Biofilms of Cutibacterium acnes. Pharmaceutics 2021, 13, 819. https://doi.org/10.3390/pharmaceutics13060819
Shih Y-H, Liu D, Chen Y-C, Liao M-H, Lee W-R, Shen S-C. Activation of Deoxyribonuclease I by Nicotinamide as a New Strategy to Attenuate Tetracycline-Resistant Biofilms of Cutibacterium acnes. Pharmaceutics. 2021; 13(6):819. https://doi.org/10.3390/pharmaceutics13060819
Chicago/Turabian StyleShih, Yi-Hsien, Donald Liu, Yen-Chou Chen, Ming-Hsuan Liao, Woan-Ruoh Lee, and Shing-Chuan Shen. 2021. "Activation of Deoxyribonuclease I by Nicotinamide as a New Strategy to Attenuate Tetracycline-Resistant Biofilms of Cutibacterium acnes" Pharmaceutics 13, no. 6: 819. https://doi.org/10.3390/pharmaceutics13060819