Natural Polymer Chitosan as Super Disintegrant in Fast Orally Disintegrating Meloxicam Tablets: Formulation and Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
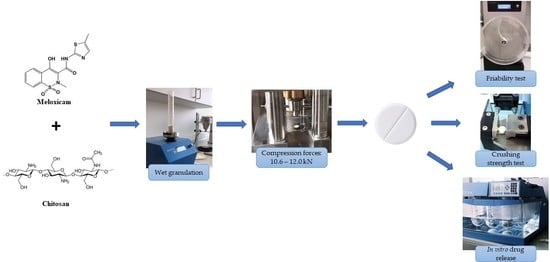
2.2. Formulation of Fast Disintegrating Tablets of Meloxicam
2.3. Physicochemical Characterization of Granules
2.3.1. Determination of Densities
2.3.2. Tablet Evaluation
2.3.3. Compact Density
2.3.4. Crushing Strength
2.3.5. Friability
2.3.6. Disintegration Time
2.4. HPLC Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Characterization of Tablets
3.2. Post Formulation Studies
3.3. Stability Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashish, P.; Harsoliya, M.S.; Pathan, J.K.; Shruti, S. A review-formulation of mouth dissloving tablet. Int. J. Pharm. Clin. Sci. 2011, 1, 1–8. [Google Scholar]
- Siddiqui, N.; Garg, G.; Sharma, P.K. Fast dissolving tablets: Preparation, characterization and evaluation: An overview. Intern. J. Pharm. Sci. Rev. Res. 2010, 4, 87–96. [Google Scholar]
- Jyoti, V.; Prajapati, S.K.; Irchhiaya, R. An overview on superdisintegrants: A Review. Eur. J. Pharm. Med. Res. 2017, 4, 252–260. [Google Scholar]
- Slavkova, M.; Breitkreutz, J. Orodispersible drug formulations for children and elderly. Eur. J. Pharm. Sci. 2015, 75, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Nautiyal, U.; Mali, R.R. A review on fast dissolving tablet. Int. J. Recent Adv. Sci. Technol. 2015, 2, 20–28. [Google Scholar] [CrossRef]
- Bala, R.; Khanna, S.; Pawar, P. Polymers in fast disintegrating tablets—A review. Asian J. Pharm. Clin. Res. 2012, 5, 8–14. [Google Scholar]
- Amaliyar, P.R.; Patel, H.; Chaudhary, S.A.; Shah, H.; Patel, A.; Suva, M.A. A brief review on natural and synthetic superdisintegrants. Invent J. 2014, 3, 1–6. [Google Scholar]
- Sharma, V.; Arora, V.; Ray, C. Use of natural superdisintegrsant in mouth dissolving tablet—An emerging trend. Int. Bull. Drug Res. 2010, 1, 46–54. [Google Scholar]
- Alam, M.T.; Parvez, N.; Sharma, P.K. FDA-Approved Natural Polymers for Fast Dissolving Tablets. J. Pharm. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F.M. A chitosan-based liposome formulation enhances the in vitro wound healing efficacy of substance P neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [Green Version]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the encapsulation of natural products: The case of chitosan biopolymer as a matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Bruscato, F.N.; Danti, A.G. Pharmaceutical Tablets Containing Chitin and Chitosan as a Disintegrant. U.S. Patent US4086335A, 25 April 1978. [Google Scholar]
- Emmanuel, O.O.; Musiliu, O.A.; Ekaetel, I.A. Evaluation of callinectes chitosan as a superdisintegrant in metronidazole tablet. Int. J. Pharm. Pharm. Sci. 2017, 9, 111–118. [Google Scholar]
- Sigma Aldrich Product Specification. Available online: Https://Www.sigmaaldrich.com/Catalog/Product/Aldrich/448877?Lang=En&Region=LT (accessed on 14 May 2021).
- Brezovska, M.; Jampilek, J.; Opatrilova, R. A Review of HPLC Methods Used for Determining the Presence of Meloxicam. Curr. Pharm. Anal. 2013, 9, 69–76. [Google Scholar]
- Khemariya, P.; Gajbhiye, R.; Vaidya, D.; Jadon, S.; Mishra, S.; Shukla, A.; Bhargava, M.; Singhai, K.; Goswami, S. Preparation and evaluation of mouth dissolving tablets of meloxicam. Int. J. Drug Deliv. 2010, 2, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Jafar, M.; Ali, S. Development and evaluation of Meloxicam solid dispersion loaded buccal patches. JAPS 2011, 1, 77–82. [Google Scholar]
- Jaafar, I.S.; Sabar, M.H.; Mahmood, S.Z. Formulation and In-vitro Evaluation of Fast Dissolving Tablets of Meloxicam Solid Dispersion. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 202–207. [Google Scholar]
- Markl, D.; Zeitler, J.A. A review of disintegration mechanisms and measurement techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, T.; Ghosh, A.; Prasad, D. A review on new generation orodispersible tablets and its future prospective. Int. J. Pharm. Pharm. Sci. 2011, 3, 1–7. [Google Scholar]
- Kouchak, M.; Avadi1, M.; Abbaspour, M.; Jahangiri, A.; Boldaji, S.K. Effect of different molecular weights of chitosan on preparation and characterization of insulin loaded nanoparticles by ion gelation method. Int. J. Drug Dev. Res. 2012, 4, 271–277. [Google Scholar]
- Mohanachandran, P.S.; Sindhumol, P.G.; Kiran, T.S. Superdisintegrants: An Overview. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 105–109. [Google Scholar]
- Fukami, J.; Yonemochi, E.; Yoshihashi, Y.; Terada, K. Evaluation of rapidly disintegrating tablets containing glycine and carboxymethylcellulose. Int. J. Pharm. 2006, 310, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Fukami, J.; Ozawa, A.; Yoshihashi, Y.; Yonemochi, E. Development of Fast Disintegrating Compressed Tablets Using Amino Acid as Disintegration Accelerator: Evaluation of Wetting and Disintegration of Tablet on the Basis of Surface Free Energy. Chem. Pharm. Bull. 2005, 53, 1536–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monica, E.; Rollando, R.; Sitepu, R.; Nisah, D.R.K.; Irwati, L.N.; Listio, S.D.L. Formulation of Fast Disintegrating Tablet Paracetamol Employing Selected Super-disintegrant. Int. J. Res. Pharm. Sci. 2020, 11, 4323–4333. [Google Scholar] [CrossRef]
- Goel, H.; Vora, N.; Tiwar, A.K.; Rana, V. Understanding the mechanism for paradoxical effect of ionized and unionized chitosan: Orodispersible tablets of Ondansetron Hydrochloride. Pharm. Dev. Technol. 2009, 14, 476–484. [Google Scholar] [CrossRef]
- EDQM. European Pharmacopoeia Online, 8th ed.; Council of Europe: Strasbourg, France, 2016. [Google Scholar]
- Ali, H.; Zafar, F.; Khan, S.; Yasmeen, R.; Bushra, R.; Baloch, S. Design and optimization of fast dispersible formulations of multi strength meloxicam tablets using response surface methodology. FARMACIA 2019, 67, 4. [Google Scholar] [CrossRef]
- Jafar, M.; Mhg, D.; Shareef, A. Enhancement of dissolution and antiinfammatory effect of meloxicam using solid dispersions. J. Pharma 2010, 2, 22–27. [Google Scholar]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- ICH QIA (R2). Stability Testing Guidelines: Stability Testing of New Drug Substances and Products. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products (accessed on 14 May 2021).
- Aucamp, M.E. Assessment of the Tableting Properties of Chitosan through Wet Granulation and Direct Compression Formulations. Master’s Thesis, Northwest University, Potchefstroom, South Africa, 2004. [Google Scholar]
- Nagar, P.; Singh, K.; Chauhan, I.; Verma, M.; Yasir, M. Orally disintegrating tablets: Formulation, preparation, techniques and evaluation. J. Appl. Pharm. Sci. 2011, 1, 35–45. [Google Scholar]
- Kumar, M.V.; Pooja, S.; Rajat, K.; Saraogi, G.K.; Singhai, A.K. Orally disinegrating tablets: A review. Int. Res. J. Pharm. 2011, 2, 16–22. [Google Scholar]
- Nagar, M.; Yadav, A.V. Cinnarizine orodispersible tablets: A Chitosan based fast mouth dissolving technology. Int. J. PharmTech Res. 2009, 1, 1079–1091. [Google Scholar]

| No. | Ingredients (mg) | Formulations | |||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | ||
| 1. | Meloxicam | 7.5 | 7.5 | 7.5 | 7.5 |
| 2. | Chitosan | - | 14 | - | - |
| Intragranular | - | 7 | - | - | |
| Extragranular | - | 7 | - | - | |
| 3. | Croscarmellose sodium | - | - | 14 | - |
| Intragranular | - | - | 7 | - | |
| Extragranular | - | - | 7 | - | |
| 4. | Sodium starch glycolate | - | - | - | 14 |
| Intragranular | - | - | - | 7 | |
| Extragranular | - | - | - | 7 | |
| 5. | Microcrystalline cellulose | 27.5 | 27.5 | 27.5 | 27.5 |
| 6. | Magnesium stearate | 2 | 2 | 2 | 2 |
| 7. | Sorbitol | 134 | 120 | 120 | 120 |
| Intragranular | 122.5 | 115.5 | 115.5 | 115.5 | |
| Extragranular | 11.5 | 4.5 | 4.5 | 4.5 | |
| 8. | Mannitol | 29 | 29 | 29 | 29 |
| Total weight: | 200 | 200 | 200 | 200 | |
| Properties | Formulations | |||
|---|---|---|---|---|
| F1 | F2 | F3 | F4 | |
| Tapped density (g/cm3) | 0.708 ± 0.037 | 0.571 ± 0.043 | 0.75 ± 0.074 | 0.833 ± 0.065 |
| Bulk density (g/cm3) | 0.567 ± 0.033 | 0.513 ± 0.029 | 0.601 ± 0.039 | 0.667 ± 0.025 |
| Carr‘s index (%) | 19.31 ± 0.89 | 10.26 ± 0.76 | 16.05 ± 1.02 | 15.78 ± 0.94 |
| Hausner’s ratio | 1.221 ± 0.367 | 1.104 ± 0.413 | 1.149 ± 0.281 | 1.178 ± 0.547 |
| Compression Force, kN | Average Weight (mg) (n = 20) | Drug Content (%) (n = 10) | ||
|---|---|---|---|---|
| F1 | F2 | F1 | F2 | |
| 10.6 | 0.201 ± 0.006 | 0.198 ± 0.007 | 95.5 ± 0.33 | 99.3 ± 0.74 |
| 10.8 | 0.202 ± 0.007 | 0.200 ± 0.007 | 97.1 ± 0.18 | 98.7 ± 0.31 |
| 11.0 | 0.199 ± 0.004 | 0.200 ± 0.006 | 99.7 ± 0.55 | 101.4 ± 0.77 |
| 11.2 | 0.202 ± 0.007 | 0.202 ± 0.005 | 101.3 ± 0.17 | 99.7 ± 0.46 |
| 11.4 | 0.201 ± 0.008 | 0.201 ± 0.007 | 94.4 ± 0.74 | 97.6 ± 0.71 |
| 11.6 | 0.199 ± 0.009 | 0.202 ± 0.006 | 103.7 ± 0.35 | 99.1 ± 0.36 |
| 11.8 | 0.201 ± 0.007 | 0.200 ± 0.008 | 98.9 ± 0.73 | 98.7 ± 0.39 |
| 12.0 | 0.197 ± 0.012 | 0.199 ± 0.015 | 102.6 ± 0.69 | 97.1 ± 0.46 |
| F3 | F4 | F3 | F4 | |
| 15.2 | 0.201 ± 0.0017 | 0.200 ± 0.0024 | 99.2 ± 0.47 | 98.1 ± 0.42 |
| 15.4 | 0.200 ± 0.0014 | 0.201 ± 0.0016 | 96.5 ± 0.15 | 102.0 ± 0.88 |
| 15.6 | 0.200 ± 0.0018 | 0.201 ± 0.0018 | 98.8 ± 0.68 | 98.6 ± 0.62 |
| 15.8 | 0.200 ± 0.0018 | 0.201 ± 0.0018 | 98.3 ± 0.71 | 99.7 ± 0.31 |
| 16.0 | 0.200 ± 0.0016 | 0.201 ± 0.0009 | 101.6 ± 0.39 | 97.5 ± 0.12 |
| 16.2 | 0.201 ± 0.0015 | 0.201 ± 0.0018 | 97.9 ± 0.77 | 99.1 ± 0.42 |
| 16.4 | 0.200 ± 0.0017 | 0.200 ± 0.0014 | 99.4 ± 0.74 | 101.5 ± 0.81 |
| Compression Force, kN | Compact Density (g/cm3) | Crushing Strength (kg/cm2) | Friability (%) ±SD (n = 20) | |||
|---|---|---|---|---|---|---|
| F1 | F2 | F1 | F2 | F1 | F2 | |
| 10.6 | 0.93 ± 0.01 | 0.89 ± 0.01 | 3.20 ± 0.14 * | 0.85 ± 0.84 | 1.70 ± 0.03 * | 2.00 ± 0.05 |
| 10.8 | 0.94 ± 0.01 | 0.92 ± 0.01 | 4.91 ± 0.22 * | 3.00 ± 4.41 | 0.48 ± 0.05 * | 0.94 ± 0.03 |
| 11.0 | 0.96 ± 0.01 | 0.94 ± 0.00 | 6.00 ± 0.16 * | 3.63 ± 3.14 | 0.47 ± 0.05 | 0.50 ± 0.05 |
| 11.2 | 0.99 ± 0.00 | 0.96 ± 0.00 | 7.35 ± 0.15 * | 4.17 ± 5.68 | 0.46 ± 0.03 | 0.48 ± 0.05 |
| 11.4 | 1.00 ± 0.01 | 0.97 ± 0.01 | 9.38 ± 0.21 * | 4.33 ± 6.77 | 0.45 ± 0.04 | 0.47 ± 0.03 |
| 11.6 | 1.01 ± 0.00 | 1.00 ± 0.01 | 10.90 ± 0.16 * | 7.61 ± 3.14 | 0.43 ± 0.07 | 0.44 ± 0.01 |
| 11.8 | 1.02 ± 0.01 | 1.01 ± 0.01 | 12.31 ± 0.14 * | 8.43± 5.10 | 0.42 ± 0.05 | 0.43 ± 0.03 |
| 12.0 | 1.04 ± 0.01 | 1.03 ± 0.01 | 13.00 ± 0.15 * | 10.66 ± 6.11 | 0.41 ± 0.05 | 0.42 ± 0.05 |
| F3 | F4 | F3 | F4 | F3 | F4 | |
| 15.2 | 1.24 ± 0.01 | 1.20 ± 0.02 | 0.9 ± 1.93 + | - | 6.79 ± 1.17 + | 49.8 ± 3.7 |
| 15.4 | 1.45 ± 0.02 | 1.44 ± 0.02 | 1.25 ± 1.85 + | 1.20 ± 1.6 | 6.06 ± 0.67 | 3.49 ± 0.18 |
| 15.6 | 1.50 ± 0.02 | 1.47 ± 0.01 | 1.60 ± 2.02 + | 1.52 ± 1.26 | 1.69 ± 0.08 + | 3.29 ± 0.17 |
| 15.8 | 1.55 ± 0.02 | 1.53 ± 0.01 | 2.50 ± 2.25 + | 2.04 ± 1.92 | 0.87 ± 0.03 | 1.22 ± 0.067 |
| 16.0 | 1.61 ± 0.01 | 1.58 ± 0.01 | 2.90 ± 0.76 + | 2.59 ± 1.83 | 0.67 ± 0.041 | 0.98 ± 0.066 |
| 16.2 | 1.62 ± 0.01 | 1.60 ± 0.02 | 3.48 ± 2.02 + | 3.03 ± 2.5 | 0.52 ± 0.12 | 0.78 ± 0.05 |
| 16.4 | 1.64 ± 0.02 | 1.63 ± 0.02 | 3.87 ± 1.22 + | 3.67 ± 2.06 | 0.32 ± 0.07 | 0.48 ± 0.037 |
| Compression Force, kN | Wetting Time (s) | Water Absorption, % | Disintegration Time (s) | |||
|---|---|---|---|---|---|---|
| F1 | F2 | F1 | F2 | F1 | F2 | |
| 10.6 | 789.31 ± 4.1 | 11.65 ± 0.87 | 7.68 ± 0.98 | 40.94 ± 1.41 | 80.86 ± 0.58 * | 18.65 ± 1.2 |
| 10.8 | 971.98 ± 1.6 * | 16.95 ± 0.88 | 7.53 ± 0.74 | 36.9 ± 0.98 | 119.34 ± 0.19 * | 40.01 ± 1.12 |
| 11.0 | 1057.3 ± 3.6 * | 23.38 ± 2.13 | 6.85 ± 0.66 | 32.81 ± 1.13 | 125.72 ± 1.88 * | 59.65 ±5.75 |
| 11.2 | 1574 ± 3.2 * | 68.13 ± 2.1 | 6.43 ± 0.48 | 22.43 ± 1.15 | 206.97 ± 0.45 * | 106.59 ± 2.61 |
| 11.4 | 1698.35 ± 3.7 * | 92.65 ± 1.2 | 6.28 ± 0.78 | 17.37 ± 0.74 | 219.44 ± 2.39 * | 140.04 ±7.69 |
| 11.6 | 1985.78 ± 3.1 * | 144.64 ± 0.71 | 6.01 ± 0.33 | 12.66 ± 0.56 | 260.1 ± 4.95 * | 235.37 ± 2.24 |
| 11.8 | 2338.4 ± 2.5 * | 214.8 ± 2.16 | 5.67 ± 0.81 | 10.76 ± 0.58 | 279.18 ± 2.8 * | 296.56 ±3.61 |
| 12.0 | 2541.8 ± 3.1 * | 256.55 ± 1.88 | 5.14 ± 0.45 | 10.74 ± 0.74 | 394.32 ± 5.36 * | 353.34 ± 5.14 |
| F3 | F4 | F3 | F4 | F3 | F4 | |
| 15.2 | 11.68 ± 0.81 + | 6.54 ± 0.63 | 40.76 ± 1.45 + | 55.35 ± 1.53 | 12.82 ± 0.83 + | 16.68 ± 0.618 |
| 15.4 | 16.18 ± 0.79 | 12.57 ± 1.47 | 29.43 ± 1.35 | 40.5 ± 1.47 | 17.86 ± 0.66 | 18.05 ± 0.96 |
| 15.6 | 18.9 ± 1.11 | 15.75 ± 0.76 | 31.65 ± 1.28 | 34.84 ± 1.11 | 18.96 ± 1.65 | 22.69 ± 1.16 |
| 15.8 | 27.37 ± 2.09 | 18.71 ± 1.45 | 24.36 ± 1.43 | 35.96 ± 0.86 | 21.28 ± 0.83 | 27.16 ± 1.41 |
| 16.00 | 29.56 ± 1.71 | 20.71 ± 1.26 | 23.33 ± 1.52 | 34.15 ± 1.62 | 22.16 ± 1.91 | 35.73 ± 0.83 |
| 16.2 | 51.64 ± 1.56 + | 21.25 ± 2.15 | 17.4 ± 1.45 + | 30.99 ± 1.81 | 41.74 ± 0.8 + | 54.49 ± 1.24 |
| 16.4 | 58.78 ± 0.73 + | 42.58 ± 3.29 | 9.81 ±1.62 + | 22.94 ± 1.69 | 45.15 ± 2.53 + | 60.82 ± 1.52 |
| Formulations | Evaluation Parameters | Duration in Months | ||
|---|---|---|---|---|
| 0 | 3 | 6 | ||
| F2-10.8 kN | Physical changes | No changes | No changes | No changes |
| Hardness (kg/cm2) | 3.00 ± 4.41 | 2.97 ± 2.93 | 2.97 ± 2.75 | |
| Disintegration time (s) | 40.01 ± 1.12 | 39.78 ± 0.93 | 39.69 ± 0.75 | |
| Drug content (%) | 98.70 ± 0.31 | 98.30 ± 0.28 | 98.40 ± 0.30 | |
| Dissolution (%) | 98.68 ± 0.63 | 98.45 ± 0.53 | 98.60 ± 0.68 | |
| F2-11.0 kN | Physical changes | No changes | No changes | No changes |
| Hardness (kg/cm2) | 3.63 ± 3.14 | 3.62 ± 2.71 | 3.62 ± 3.03 | |
| Disintegration time (s) | 59.65 ± 5.75 | 59.62 ± 6.71 | 59.54 ± 6.03 | |
| Drug content (%) | 101.40 ± 0.77 | 101.00 ± 0.65 | 101.2 ± 0.67 | |
| Dissolution (%) | 90.54 ± 1.71 | 90.37 ± 1.68 | 90.40 ± 1.65 | |
| F2-11.2 kN | Physical changes | No changes | No changes | No changes |
| Hardness (kg/cm2) | 4.17 ± 5.68 | 4.15 ± 1.99 | 4.14 ± 1.26 | |
| Disintegration time (s) | 106.59 ± 2.61 | 106.53 ± 1.79 | 106.46 ± 1.16 | |
| Drug content (%) | 99.70 ± 0.46 | 99.50 ± 0.35 | 99.30 ± 0.42 | |
| Dissolution (%) | 80.84 ± 1.67 | 80.72 ± 1.58 | 80.69 ± 1.72 | |
| F3-16.2 kN | Physical changes | No changes | No changes | No changes |
| Hardness (kg/cm2) | 3.48 ± 2.02 | 3.47 ± 2.90 | 3.42 ± 2.75 | |
| Disintegration time (s) | 41.74 ± 0.80 | 40.76 ± 0.90 | 39.91 ± 0.65 | |
| Drug content (%) | 97.90 ± 0.74 | 97.30 ± 0.88 | 98.0 ± 0.79 | |
| Dissolution (%) | 84.97 ± 2.41 | 84.17 ± 2.73 | 83.95 ± 2.54 | |
| F3-16.4 kN | Physical changes | No changes | No changes | No changes |
| Hardness (kg/cm2) | 3.87 ± 1.22 | 3.79 ± 1.71 | 3.82 ± 1.53 | |
| Disintegration time (s) | 45.15 ± 5.65 | 46.52 ± 6.70 | 46.74 ±5.03 | |
| Drug content (%) | 99.40 ± 0.42 | 98.60 ± 0.98 | 98.2 ± 0.77 | |
| Dissolution (%) | 86.51 ± 1.74 | 85.81 ± 1.96 | 85.04 ± 2.05 | |
| F4-16.0 kN | Physical changes | No changes | No changes | No changes |
| Hardness (kg/cm2) | 2.59 ± 1.83 | 2.63 ± 1.90 | 2.60 ± 1.75 | |
| Disintegration time (s) | 35.73 ± 0.83 | 36.70 ± 0.93 | 36.59 ± 0.75 | |
| Drug content (%) | 97.50 ± 0.39 | 98.20 ± 0.55 | 98.01 ± 0.27 | |
| Dissolution (%) | 71.97 ± 0.79 | 71.31 ± 1.46 | 70.07 ± 1.82 | |
| F4-16.2 kN | Physical changes | No changes | No changes | No changes |
| Hardness (kg/cm2) | 3.03 ± 2.5 | 3.22 ± 2.70 | 3.16 ± 2.13 | |
| Disintegration time (s) | 54.49 ± 1.24 | 54.92 ± 1.71 | 55.24 ±1.03 | |
| Drug content (%) | 99.1 ± 0.81 | 99.80 ± 0.56 | 98.6 ± 0.73 | |
| Dissolution (%) | 72.55 ± 1.15 | 71.81 ± 1.71 | 71.22 ± 2.66 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Draksiene, G.; Venclovaite, B.; Pudziuvelyte, L.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. Natural Polymer Chitosan as Super Disintegrant in Fast Orally Disintegrating Meloxicam Tablets: Formulation and Evaluation. Pharmaceutics 2021, 13, 879. https://doi.org/10.3390/pharmaceutics13060879
Draksiene G, Venclovaite B, Pudziuvelyte L, Ivanauskas L, Marksa M, Bernatoniene J. Natural Polymer Chitosan as Super Disintegrant in Fast Orally Disintegrating Meloxicam Tablets: Formulation and Evaluation. Pharmaceutics. 2021; 13(6):879. https://doi.org/10.3390/pharmaceutics13060879
Chicago/Turabian StyleDraksiene, Gailute, Brigita Venclovaite, Lauryna Pudziuvelyte, Liudas Ivanauskas, Mindaugas Marksa, and Jurga Bernatoniene. 2021. "Natural Polymer Chitosan as Super Disintegrant in Fast Orally Disintegrating Meloxicam Tablets: Formulation and Evaluation" Pharmaceutics 13, no. 6: 879. https://doi.org/10.3390/pharmaceutics13060879