Isohydricity of Two Different Citrus Species under Deficit Irrigation and Reclaimed Water Conditions
Abstract
:1. Introduction
2. Results
2.1. Effects of the Irrigation Strategies on Water Relations, Hydraulic Conductance, and ABA
2.2. Relationship between Stomata and Environmental and Plant Physiological Factors
3. Discussion
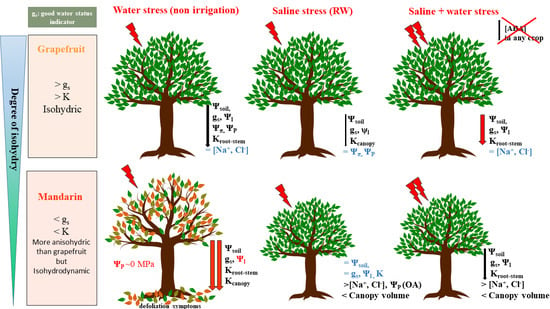
3.1. Water Relations of the Grapefruit and Mandarin Crops under Saline and Water Stresses
3.2. Relationship between Water Relations and Hydraulic Conductance: Near-Isohidric or Anisohydric Behavior
4. Materials and Methods
4.1. Experimental Conditions and Plant Materials
4.2. Water Sources and Irrigation Treatments
4.3. Measurements
4.3.1. Plant Water Status
4.3.2. Leaf Chemical Analysis
4.4. Statistical Design and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamshidi, S.; Zand-Parsa, S.; Niyogi, D. Assessing crop water stress index of citrus using in-situ measurements, landsat, and sentinel-2 data. Int. J. Remote Sens. 2021, 42, 1893–1916. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Alarcón, J.J.; Nortes, P.A.; Bayona, J.M.; Maestre-Valero, J.; Nicolás, E. Mid-long term effects of saline reclaimed water irrigation and regulated deficit irrigation on fruit quality of citrus. J. Sci. Food Agric. 2020, 100, 1350–1357. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, Y. Research on reclaimed water from the past to the future: A review. Environ. Develop. Sustain. 2021. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Nortes, P.A.; Alarcón, J.J.; Nicolás, E. Determination of 15N stable isotope natural abundances for assessing the use of saline reclaimed water in grapefruit. Environ. Eng. Manag. J. 2014, 13, 2525–2530. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Nortes, P.A.; Pedrero, F.; Mounzer, M.; Alarcón, J.J.; Bayona, J.M.; Nicolás, E. Assessment of the viability of using saline reclaimed water in grapefruit in medium to long term. Span. J. Agric. Res. 2014, 12, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Romero-Trigueros, C.; Nortes, P.; Alarcón, J.J.; Hunink, J.E.; Parra, M.; Contreras, S.; Droogers, P.; Nicolás, E. Effects of saline reclaimed waters and deficit irrigation on Citrus physiology assessed by UAV remote sensing. Agric. Water Manag. 2017, 183, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Nicolás, E.; Romero-Trigueros, C.; Nortes, P.A.; Pedrero, F.; Bayona, J.M.; Maestre-Valero, J.F.; Alarcón, J.J. Chapter 7: Long-term physiological and agronomic responses of citrus irrigated with saline reclaimed water. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Academic Press: Cambridge, MA, USA, 2018; pp. 131–147. ISBN 978-0-12-813164-0. [Google Scholar] [CrossRef]
- Grattan, S. Evaluation of the Impact of Boron on Citrus Orchards in Riverside Country; Riverside County Water Task Force: Riverside, CA, USA, 2013; 78p. [Google Scholar]
- García-Sánchez, F.; Syvertsen, J.P.; Gimeno, V.; Botia, P.; Pérez-Pérez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant 2007, 130, 532–542. [Google Scholar] [CrossRef]
- Pantin, F.; Monnet, F.; Jannaud, D.; Costa, J.M.; Renaud, J.; Muller, B.; Simonneau, T.; Genty, B. The dual effect of abscisic acid on stomata. New Phytol. 2013, 97, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, C.; Buckley, T.N.; Cernusaket, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [Green Version]
- Pou, A.; Medrano, H.; Magdalena, T. Anisohydric behaviour in grapevines results in better performance under moderate water stress and recovery than isohydric behaviour. Plant Soil 2012, 359, 335–349. [Google Scholar] [CrossRef]
- Dal Santo, S.; Palliotti, A.; Zenoni, S.; Tornielli, G.B.; Fasoli, M.; Paci, P.; Tombesi, S.; Frioni, T.; Silvestroni, O.; Bellincontro, A.; et al. Distinct transcriptome responses to water limitation in isohydric and anisohydric grapevine cultivars. BMC Genom. 2016, 17, 815. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, H.; Mathias, F.; Schuldt, B. A whole-plant perspective of isohydry: Stem-level support for leaf-level plant water regulation. Tree Physiol. 2021, 41, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Domínguez, C.M.; Brodribb, T.J. Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol. 2020, 225, 126–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.J.; Bi, M.H.; Nie, Z.F.; Jiang, H.; Liu, X.D.; Fang, X.W.; Brodribb, T.J. Evolution of stomatal closure to optimize water-use efficiency in response to dehydration in ferns and seed plants. New Phytol. 2021, 230, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, U.; Degu, A.; Fait, A.; Rachmilevitch, S. Near isohydric grapevine cultivar displays higher photosynthetic efficiency and photorespiration rates under drought stress as compared with near anisohydric grapevine cultivar. Physiol. Plant. 2013, 147, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, F.C.; Smith, D.D.; Woodruff, D.R.; Marias, D.E.; MacCulloh, K.A.; Howard, A.R.; Magedman, A.L. Stomatal kinetics and photosynthetic gas exchange along a continuum of isohydric to anisohydric regulation of plant water status. Plant Cell Environ. 2017, 40, 1618–1628. [Google Scholar] [CrossRef]
- Mira-García, A.B. Evaluation of Physiological Indicators of Water Stress in Verna Lemon Tree. Master’s Thesis, Orihuela Higher Polytechnic School, Miguel Hernández University of Elche, Elche, Spain, 2016. [Google Scholar]
- Banuls, J.; Serna, M.D.; Legaz, F.; Talon, M.; Primo-Millo, E. Growth and gas exchange parameters of Citrus plants stressed with different salts. J. Plant Physiol. 1997, 150, 194–199. [Google Scholar] [CrossRef]
- Vivaldi, A.G.; Lopriore, G.; Romero-Trigueros, C.; Pedrero, F.; Camposeo, S.; Álvarez, S. Physiological responses of almond trees under regulated deficit irrigation using saline and desalinated reclaimed water. Agric. Water Manag. 2021. accepted. [Google Scholar]
- McAdam, S.A.M.; Brodribb, T.J. Linking turgor with ABA biosynthesis: Implications for stomatal responses to vapour pressure deficit across land plants. Plant Physiol. 2016, 171, 00380. [Google Scholar] [CrossRef] [Green Version]
- Dodd, I.C. Abscisic acid and stomatal closure: A hydraulic conductance conundrum? New Phytol. 2013, 197, 6–8. [Google Scholar] [CrossRef]
- Shang, Y.; Yang, D.; Ha, Y.; Lee, J.Y.; Kim, J.Y.; Oh, M.; Nam, K.H. Open stomata 1 exhibits dual serine/threonine and tyrosine kinase activity in regulating abscisic acid signaling. J. Exp. Bot. 2021, 72, 5494–5507. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.A.; Hoffmann, T.; Teplova, I.; Grill, E.; Muller, A. Generation of active pools of abscisic acid revealed by in vivo Imaging of water-stressed Arabidopsis. Plant Physiol. 2005, 137, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Israelsson, M.; Siegel, R.S.; Young, J.; Hashimoto, M.; Iba, K.; Schroeder, J.I. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr. Opin. Plant Biol. 2006, 9, 654–663. [Google Scholar] [CrossRef]
- Reynolds-Henne, C.E.; Langenegger, A.; Mani, J.; Schenk, N.; Zumsteg, A.; Feller, U. Interactions between temperature, drought and stomatal opening in legumes. Environ. Exp. Bot. 2010, 68, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Brodribb, T.J.; Holbrook, N.M. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant Cell Environ. 2004, 27, 820–827. [Google Scholar] [CrossRef]
- Christmann, A.A.; Weiler, E.W.; Steudle, E.; Grill, E. Hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef]
- Saliendra, N.Z.; Sperry, J.S.; Comstock, J.P. Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula-occidentalis. Planta 1995, 196, 357–366. [Google Scholar] [CrossRef]
- Mrad, A.; Sevanto, S.; Domec, J.-C.; Liu, Y.; Nakad, M.; Katul, G. A Dynamic Optimality Principle for Water Use Strategies Explains Isohydric to Anisohydric Plant Responses to Drought. Front. For. Glob. Change 2019, 2, 49. [Google Scholar] [CrossRef] [Green Version]
- Sperry, J.S.; Hacke, U.G.; Oren, R.; Comstock, J.P. Water deficit and hydraulic limits to leaf water supply. Plant Cell Environ. 2002, 25, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Dominguez, C.M.; Buckley, T.N.; Egea, G.; de Cires, A.; Hernandez-Santana, V.; Martorell, S.; Diaz-Espejo, A. Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ. 2016, 39, 2014–2026. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Tejero, I.; Jimenez-Bocanegra, J.A.; Martinez, G.; Romero, R.; Duran-Zuazo, V.H.; Muriel-Fernandez, J. Positive impact of regulated deficit irrigation on yield and fruit quality in a commercial citrus orchard [Citrus sinensis (L.) Osbeck, cv. salustiano]. Agric. Water Manag. 2010, 97, 614–622. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Bayona, J.M.; Nortes, P.; Alarcón, J.J.; Nicolás, E. Determination of crop water stress index by thermometry in grapefruit trees irrigated with saline reclaimed water combined with deficit irrigation. Remote Sens. 2019, 11, 757. [Google Scholar] [CrossRef] [Green Version]
- Sampaio, A.H.R.; Silva, R.O.; Brito, R.B.F.; Soares, W.D.; Gesteira, A.D.; Souza, L.D.; Coelho, M.A. Sweet orange acclimatisation to water stress: A rootstock dependency. Sci. Hortic. 2021, 276, 109727. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Intrigliolo, D.S.; Primo-Millo, E.; Forner-Giner, M.A. Relationships between xylem anatomy, root hydraulic conductivity, leaf/root ratio and transpiration rates in citrus trees on different rootstocks. Physiol. Plant. 2010, 139, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, V.; Syvertsen, J.P.; Simon, I.; Martinez, V.; Camara-Zapata, J.M.; Nieves, M.; Garcia-Sanchez, F. Interstock of ‘Valencia’ Orange Affects the Flooding Tolerance in ‘Verna’ Lemon Trees. Hortscience 2012, 47, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.L.; Flexas, J. From one side to two sides: The effects of stomatal distribution on photosynthesis. New Phytol. 2020, 228, 1754–1766. [Google Scholar] [CrossRef]
- Rodríguez-Dominguez, C.M.; Hernández-Santana, V.; Buckley, T.N.; Fernández, J.E.; Díaz-Espejo, A. Sensitivity of olive leaf turgor to air vapour pressure deficit correlates with diurnal maximum stomatal conductance. Agric. For. Meteorol. 2019, 272–273, 156–165. [Google Scholar] [CrossRef]
- Pedrero, F.; Maestre-Valero, J.F.; Mounzer, O.; Nortes, P.A.; Alcobendas, R.; Romero-Trigueros, C.; Bayona, J.M.; Alarcón, J.J.; Nicolás, E. Response of young ‘Star Ruby’ grapefruit trees to regulated deficit irrigation with saline reclaimed wáter. Agric. Water Manag. 2015, 158, 51–60. [Google Scholar] [CrossRef]
- Nicolas, E.; Alarcón, J.J.; Mounzer, O.; Pedrero, F.; Nortes, P.A.; Alcobendas, R.; Romero-Trigueros, C.; Bayona, J.M.; Maestre-Valero, J.F. Long-term physiological and agronomic responses of mandarin trees to irrigation with saline reclaimed water. Agric. Water Manag. 2016, 166, 1–8. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Graham, J.H. Hydraulic conductivity of roots, mineral nutrition, and leaf gas exchange of Citrus rootstocks. J. Am. Soc. Hortic. Sci. 1985, 110, 865–869. [Google Scholar]
- Forner-Giner, M.A.; Rodriguez-Gamir, J.; Primo-Millo, E. Hydraulic and Chemical Responses of Citrus Seedlings to Drought and Osmotic Stress. J. Plant Growth Regul. 2011, 30, 353–366. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Rodríguez-Gamir, J.; Martínez-Cuenca, M.R.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.A. Relationship between hydraulic conductance and citrus dwarfing by the Flying Dragon rootstock (Poncirus trifoliata L. Raft var. Monstrosa). Trees 2013, 27, 629–638. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Santana, V.; Rodríguez-Domínguez, C.; Fernández, J.E.; Espejo-Díaz, A. Role of leaf hydraulic conductance in the regulation of stomatal conductance in almond and olive in response to water stress. Tree Physiol. 2016, 36, 725–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Froux, F.; Ducrey, M.; Dreyer, E.; Huc, R. Vulnerability to embolism differs in roots and shoots and among three Mediterranean conifers: Consequences for stomatal regulation of water loss? Trees 2005, 19, 137–144. [Google Scholar] [CrossRef]
- Grassi, G.; Ripullone, F.; Borghetti, M.; Raddi, S.; Magnani, F. Contribution of diffusional and non-diffusional limitations to midday depression of photosynthesis in Arbutus unedo L. Trees 2009, 23, 1149–1161. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Meinzer, F.C.; Qi, J.H.; Goldstein, G.; Cao, K.F. Midday stomatal conductance is more related to stem rather than leaf water status in subtropical deciduous and evergreen broadleaf trees. Plant Cell Environ. 2013, 36, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Brodribb, T.J.; Jordan, G.J. Internal coordination between hydraulics and stomatal control in leaves. Plant Cell Environ. 2008, 31, 1557–1564. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbo, M.; Diaz-Espejo, A.; Galmes, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Schultz, H.R. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell Environ. 2003, 26, 1393–1405. [Google Scholar] [CrossRef]
- Mounzer, O.; Pedrero, F.; Nortes, P.A.; Bayona, J.M.; Nicolás, E.; Alarcón, J.J. Transient soil salinity under the combined effect of reclaimed water and regulated deficit drip irrigation of Mandarin trees. Agric. Water Manage. 2013, 120, 23–29. [Google Scholar] [CrossRef]
- Corso, D.; Delzon, S.; Lamarque, L.J.; Cochard, H.; Torres-Ruiz, J.M.; King, A.; Brodribb, T. Neither xylem collapse, cavitation, or changing leaf conductance drive stomatal closure in wheat. Plant Cell Environ. 2020, 43, 854–865. [Google Scholar] [CrossRef]
- Girona, J.; Mata, M.; del Campo, J.; Arbonés, A.; Bartra, E.; Marsal, J. The use of midday leaf water potential for scheduling deficit irrigation in vineyards. Irrig. Sci. 2006, 24, 115–127. [Google Scholar] [CrossRef]
- Mirás-Ávalos, J.M.; Pérez-Sarmiento, F.; Alcobendas, R.; Alarcón, J.J.; Mounzer, O.; Nicolás, E. Using midday stem water potential for scheduling deficit irrigation in mid–late maturing peach trees under Mediterranean conditions. Irrig. Sci. 2016, 34, 161–173. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M. Evolution of the Stomatal Regulation of Plant Water Content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.M.; Garcia-Olmos, B.; Andujar, S.; Rodríguez-Morán, M.; Moreno, M.; Porras, I. Effects of calcium on growth and nutritional state of citrus seedlings under NaCl stress. In Proceedings of the 28th International Horticultural Congress on Science and Horticulture for People (IHC)/International Symposium on Climwater—Horticultural Use of Water in a Changing Climate, Lisbon, Portugal, 22–27 August 2010; Volume 922, pp. 55–60. [Google Scholar]
- Chapman, H.D.; Reuther, W.; Batchelor, L.D.; Weber, H.J. The mineral nutrition of citrus. In The Citrus Industry; Reuther, W., Ed.; University of California: Berkeley, CA, USA, 1968; Volume 2, pp. 127–289. [Google Scholar]
- Levy, Y.; Shamhevet, J. Ranking the tolerance of citrus rootstocks by juice analysis. Sci. Hortic. 1990, 45, 89–98. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Parra, M.; Bayona, J.M.; Nortes, P.; Alarcón, J.J.; Nicolás, E. Effect of deficit irrigation and reclaimed water on yield and quality of grapefruits at harvest and postharvest. LWT Food Sci. Technol. 2017, 85, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Yahmed, B.J.; Novillo, P.; Garcia-Lor, A.; Salvador, A.; Ben Mimoun, M.; Luro, F.; Talon, M.; Ollitrault, P.; Morillon, R. Salt tolerance traits revealed in mandarins (Citrus reticulata Blanco) are mainly related to root-to-shoot Cl− translocation limitation and leaf detoxification processes. Sci. Hortic. 2015, 191, 90–100. [Google Scholar] [CrossRef]
- Patakas, A.; Noitsakis, B. Cell wall elasticity as a mechanism to maintain favourable water relations during leaf ontogeny in grapevines. Am. J. Enol. Vitic. 1997, 48, 352–356. [Google Scholar]
- Ocheltree, T.W.; Nippert, J.B.; Prasad, P.V.V. Stomatal sensitivity of C3 and C4 grasses. Plant Cell Environ. 2014, 37, 132–139. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.G.; Syvertsen, J.P.; Botia, P.; Garcia-Sanchez, F. Leaf water relations and net gas exchange responses of salinized Carrizo citrange seedlings during drought stress and recovery. Ann. Bot. 2007, 100, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Pedrero, F.; Maestre-Valero, J.F.; Mounzer, O.; Alarcón, J.J.; Nicolás, E. Physiological and agronomic mandarin trees performance under saline reclaimed water combined with regulated deficit irrigation. Agric. Water Manag. 2014, 146, 228–237. [Google Scholar] [CrossRef]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/Anisohydry: A Plant-Environment Interaction Rather Than a Simple Hydraulic Trait. Trends Plant Sci. 2018, 23(2), 112–120. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Sperry, J.S.; Katul, G.G.; Pataki, D.E.; Ewers, B.E.; Phillips, N.; Schafer, K.V.R. Survey and synthesis of intra-and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Vogt, U.K. Hydraulic vulnerability, vessel refilling, and seasonal courses of stem water potential of Sorbus aucuparia L. and Sambucus nigra L. J. Exp. Bot. 2001, 52, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Franks, P.J.; Drake, P.L.; Froend, R.H. Anisohydric but isohydrodynamic: Seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ. 2007, 30, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Novick, K.A.; Konings, A.G.; Gentine, P. Beyond soil water potential: An expanded view on isohydricity including land–atmosphere interactions and phenology. Plant Cell Environ. 2019, 42, 1802–1815. [Google Scholar] [CrossRef]
- Cunningham, S.C. Photosynthetic responses to vapour pressure deficit in temperate and tropical evergreen rainforest trees of Australia. Oecologia 2005, 142, 521–528. [Google Scholar] [CrossRef]
- Creese, C.; Oberbauer, S.; Rundel, P.; Sack, L. Are fern stomatal responses to different stimuli coordinated? Testing responses to light, vapor pressure deficit, and CO2 for diverse species grown under contrasting irradiances. New Phytol. 2014, 204, 92–104. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, P.; Shen, W.; Niu, J.; Zhu, L.; Ni, G. Biophysical limits to responses of water flux to vapor pressure deficit in seven tree species with contrasting land use regimes. Agric. For. Meteorol. 2015, 200, 258–269. [Google Scholar] [CrossRef]
- Shirke, P.A.; Pathre, U.V. Influence of leaf-to-air vapour pressure deficit on the biochemistry and physiology of photosynthesis in Prosopis juliflora. J. Exp. Bot. 2004, 55, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Damour, G.; Simonneau, T.; Cochard, H.; Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 2010, 33, 1419–1438. [Google Scholar] [CrossRef] [PubMed]
- Novick, K.A.; Ficklin, D.L.; Stoy, P.C.; Williams, C.A.; Bohrer, G.; Oishi, A.C.; Papuga, S.A.; Blanken, P.D.; Noormets, A.; Sulman, B.N.; et al. The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Change 2016, 6, 1023–1087. [Google Scholar] [CrossRef] [Green Version]
- Mott, K.A.; Peak, D. Stomatal responses to humidity and temperature in darkness. Plant Cell Environ. 2010, 33, 1084–1090. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Gamir, J.; Xue, J.M.; Clearwater, M.J.; Meason, D.F.; Clinton, P.W.; Domec, J.C. Aquaporin regulation in roots controls plant hydraulic conductance, stomatal conductance, and leaf water potential in Pinus radiata under water stress. Plant Cell Environ. 2019, 42, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Zekri, M.; Parsons, L.R. Growth and root hydraulic conductivity of several citrus rootstocks under salt and polyethylene glycol stresses. Physiol. Plant 1989, 77, 99–106. [Google Scholar] [CrossRef]
- Rodríguez-Gamir, J.; Ancillo, G.; Legaz, F.; Primo-Millo, E.; Forner-Giner, M.A. Influence of salinity on PIP gene expression in citrus roots and its relationship with root hydraulic conductance, transpiration and chloride exclusion from leaves. Environ. Exp. Bot. 2012, 78, 163–166. [Google Scholar] [CrossRef]
- Dodd, I.C. Root-to-shoot signalling: Assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant Soil 2005, 274, 251–270. [Google Scholar] [CrossRef]
- Liang, J.S.; Zhang, J.H.; Wong, M.H. How do roots control xylem sap ABA concentration in response to soil drying? Plant Cell Physiol. 1997, 38, 10–16. [Google Scholar] [CrossRef]
- Castro, P.; Puertolas, J.; Dodd, I.C. Stem girdling uncouples soybean stomatal conductance from leaf water potential by enhancing leaf xylem ABA concentration. Environ. Exp. Bot. 2019, 159, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.N. Optimal carbon partitioning helps reconcile the apparent divergence between optimal and observed canopy profiles of photosynthetic capacity. New Phytol. 2021, 230, 2246–2260. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration Guidelines for Computing Crop Water Requirements; FAO Irrigation and Drainage Paper no 56; FAO: Rome, Italy, 1998; pp. 15–27. [Google Scholar]
- Bastida, F.; Torres, I.F.; Abadía, J.; Romero-Trigueros, C.; Alarcón, J.J.; García, C.; Nicolás, E. Comparing the impacts of drip irrigation by freshwater and reclaimed waste-water on the soil microbial community of two citrus species. Agric. Water Manag. 2018, 203, 53–62. [Google Scholar] [CrossRef]
- Maas, E.V. Salinity and citriculture. Tree Physiol. 1993, 12, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Turner, N.C. Measurements of plant water status by pressure chamber technique. Irrig. Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- McCutchan, H.; Shackel, K.A. Stem-water potential as a sensitive indicator of water-stress in prune trees (Prunus-domestica L cv French). J. Am. Soc. Hort. Sci. 1992, 117, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Gucci, R.; Xiloyannis, C.; Flore, J.A. Gas-exchange parameters, water relations and carbohydrate paririoning in leaves of field-grown prunus-domestica following fruit removal. Physiol. Plant. 1991, 83, 497–505. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol. 2003, 132, 2166–2173. [Google Scholar] [CrossRef] [Green Version]
- Quarrie, S.A.; Whitford, P.N.; Appleford, N.E.J.; Wang, T.L.; Cook, S.K.; Henson, I.E.; Loveys, B.R. A monoclonal-antibody to(s)—Abscisic Acid—its characterization and use in a radioimmunoassay for measuring abscisic-acid in crude extracts of cereal and lupin leaves. Planta 1988, 173, 330–339. [Google Scholar] [CrossRef]







| Crop | Treatment | Ψ100s (MPa) | OA (MPa) | Cl− (%) | Na+ (%) |
|---|---|---|---|---|---|
| Grapefruit | TW-fI | −1.59 ± 0.13 a | - | 0.44 ± 0.02 a | 0.050 ± 0.005 a |
| TW-nI | −1.45 ± 0.11 a | −0.14 | 0.32 ± 0.11 a | 0.036 ± 0.004 a | |
| RW-fI | −1.39 ± 0.21 a | −0.20 | 0.57 ± 0.02 a | 0.066 ± 0.026 a | |
| RW-nI | −1.86 ± 0.01 b | 0.27 | 0.40 ± 0.28 a | 0.074 ± 0.038 a | |
| Mandarin | TW-fI | −1.73 ± 0.11 a | 0.18 ± 0.02 a | 0.046 ± 0.010 a | |
| TW-nI | −1.98 ± 0.08 b | 0.25 | 0.15 ± 0.07 a | 0.055 ± 0.020 a | |
| RW-fI | −1.69 ± 0.10 a | 0.04 | 0.64 ± 0.08 b | 0.070 ± 0.009 a | |
| RW-nI | −1.88 ± 0.03 ab | 0.15 | 0.55 ± 0.05 b | 0.073 ± 0.006 a |
| Crop | Treatment | Kroot–stem (mol·MPa−1·m−2·s−1) | Kcanopy (mol·MPa−1·m−2·s−1) |
|---|---|---|---|
| Grapefruit | TW-fI | 4.51 ± 0.28 b | 8.93 ± 1.81 b |
| TW-nI | 2.89 ± 0.29 a | 8.47 ± 2.26 b | |
| RW-fI | 4.54 ± 0.22 b | 4.26 ± 0.93 a | |
| RW-nI | 2.23 ± 0.20 a | 9.65 ± 3.20 b | |
| ANOVA | Qw | ns | ns |
| Aw | * | * | |
| Qw * Aw | ns | * | |
| Mandarin | TW-fI | 4.87 ± 0.81 b | 2.47 ± 0.81 b |
| TW-nI | 0.69 ± 0.11 a | 1.51 ± 0.23 a | |
| RW-fI | 5.30 ± 0.38 b | 2.48 ± 0.43 b | |
| RW-nI | 0.86 ± 0.18 a | 2.41 ± 1.26 b | |
| ANOVA | Qw | ns | ns |
| Aw | *** | ** | |
| Qw * Aw | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Trigueros, C.; Gambín, J.M.B.; Nortes Tortosa, P.A.; Cabañero, J.J.A.; Nicolás Nicolás, E. Isohydricity of Two Different Citrus Species under Deficit Irrigation and Reclaimed Water Conditions. Plants 2021, 10, 2121. https://doi.org/10.3390/plants10102121
Romero-Trigueros C, Gambín JMB, Nortes Tortosa PA, Cabañero JJA, Nicolás Nicolás E. Isohydricity of Two Different Citrus Species under Deficit Irrigation and Reclaimed Water Conditions. Plants. 2021; 10(10):2121. https://doi.org/10.3390/plants10102121
Chicago/Turabian StyleRomero-Trigueros, Cristina, Jose María Bayona Gambín, Pedro Antonio Nortes Tortosa, Juan José Alarcón Cabañero, and Emilio Nicolás Nicolás. 2021. "Isohydricity of Two Different Citrus Species under Deficit Irrigation and Reclaimed Water Conditions" Plants 10, no. 10: 2121. https://doi.org/10.3390/plants10102121