Thermal Stability of Allyl-Functional Phthalonitriles-Containing Benzoxazine/Bismaleimide Copolymers and Their Improved Mechanical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Preparation of DABA-Ph/BMI Prepolymer
2.3. Preparation of DABA-Ph/BMI Copolymers
2.4. Fabrication of DABA-Ph/BMI/GF Composite Laminates
2.5. Characterizations
3. Results and Discussion
3.1. Designing of DABA-Ph/BMI Pre-Polymers and Their Curing Behaviors
3.2. Processing Properties of DABA-Ph/BMI Pre-Polymers
3.3. The Structure and Possible Polymerization Processes of DABA-Ph/BMI Pre-Polymers
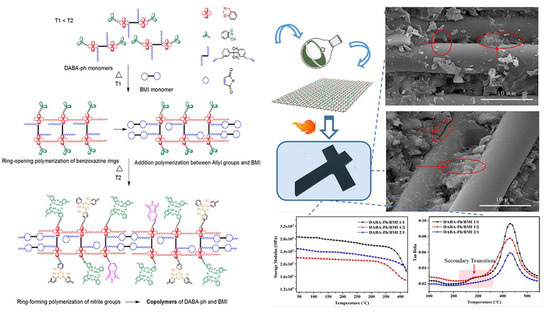
3.4. Mechanical Properties of the Composite Laminates and Their Interface Property
3.5. Dynamic Thermal Mechanical Properties of the DABA-Ph/BMI/GF Composite Laminates
3.6. Thermal Stability of the DABA-Ph/BMI/GF Composite Laminates
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bulgakov, B.A.; Sulimov, A.V.; Babkin, A.V.; Afanasiev, D.V.; Solopchenko, A.V.; Afanaseva, E.S.; Kepman, A.V.; Avdeev, V.V. Flame-retardant carbon fiber reinforced phthalonitrile composite for high-temperature applications obtained by resin transfer molding. Mendeleev Commun. 2017, 27, 257–259. [Google Scholar] [CrossRef]
- Bulgakov, B.A.; Sulimov, A.V.; Babkin, A.V.; Kepman, A.V.; Malakho, A.P.; Avdeev, V.V. Dual-curing thermosetting monomer containing both propargyl ether and phthalonitrile groups. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Derradji, M.; Ramdani, N.; Zhang, T.; Wang, J.; Gong, L.-D.; Xu, X.-D.; Lin, Z.-W.; Henniche, A.; Rahoma, H.K.S.; Liu, W.-B. Thermal and mechanical properties enhancements obtained by reinforcing a bisphenol-a based phthalonitrile resin with silane surface-modified alumina nanoparticles. Polym. Compos. 2017, 38, 1549–1558. [Google Scholar] [CrossRef]
- Xu, M.; Liu, M.; Dong, S.; Liu, X. Design of low temperature self-cured phthalonitrile-based polymers for advanced glass fiber composite laminates. J. Mater. Sci. 2013, 48, 8108–8116. [Google Scholar] [CrossRef]
- Ding, Y.; Lu, B.; Wang, P.; Wang, G.; Ji, J. PLA-PBAT-PLA tri-block copolymers: Effective compatibilizers for promotion of the mechanical and rheological properties of PLA/PBAT blends. Polym. Degrad. Stab. 2018, 147, 41–48. [Google Scholar] [CrossRef]
- Augustine, D.; Mathew, D.; Nair, C.P.R. Phthalonitrile resin bearing cyanate ester groups: Synthesis and characterization. RSC Adv. 2015, 5, 91254–91261. [Google Scholar] [CrossRef]
- Augustine, D.; Mathew, D.; Nair, C.P.R. Mechanistic and kinetic aspects of the curing of phthalonitrile monomers in the presence of propargyl groups. Polymer 2015, 60, 308–317. [Google Scholar] [CrossRef]
- Guo, H.; Zou, Y.; Chen, Z.; Zhang, J.; Zhan, Y.; Yang, J.; Liu, X. Effects of self-promoted curing behaviors on properties of phthalonitrile/epoxy copolymer. High Perform. Polym. 2012, 24, 571–579. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, M.; Chen, J.; Zeng, S.; Huang, Y.; Feng, Z.; Xu, Q.; Yan, C.; Gu, Y. Synthesis, polymerization kinetics, and high-frequency dielectric properties of novel main-chain benzoxazine copolymers. React. Funct. Polym. 2018, 122, 158–166. [Google Scholar] [CrossRef]
- Liu, C.; Sun, M.; Zhang, B.; Zhang, X.; Li, J.; Wang, L.; Xue, G.; Zhao, M.; Song, C.; Li, Q. Preparation and properties of acetylene-terminated benzoxazine/epoxy copolymers. React. Funct. Polym. 2017, 120, 98–103. [Google Scholar] [CrossRef]
- Zabihi, O.; Khodabandeh, A.; Mostafavi, S.M. Preparation, optimization and thermal characterization of a novel conductive thermoset nanocomposite containing polythiophene nanoparticles using dynamic thermal analysis. Polym. Degrad. Stab. 2012, 97, 3–13. [Google Scholar] [CrossRef]
- Deveci, S.; Antony, N.; Eryigit, B. Effect of carbon black distribution on the properties of polyethylene pipes—Part 1: Degradation of post yield mechanical properties and fracture surface analyses. Polym. Degrad. Stab. 2018, 148, 75–85. [Google Scholar] [CrossRef]
- Huang, W.; He, W.; Long, L.; Yan, W.; He, M.; Qin, S.; Yu, J. Highly efficient flame-retardant glass-fiber-reinforced polyamide 6T system based on a novel DOPO-based derivative: Flame retardancy, thermal decomposition, and pyrolysis behavior. Polym. Degrad. Stab. 2018, 148, 26–41. [Google Scholar] [CrossRef]
- Xiong, X.; Zhou, L.; Ren, R.; Ma, X.; Chen, P. Thermal, mechanical properties and shape memory performance of a novel phthalide-containing epoxy resins. Polymer 2018, 140, 326–333. [Google Scholar] [CrossRef]
- Harsha, A.P.; Tewari, U.S.; Venkatraman, B. Solid particle erosion behaviour of various polyaryletherketone composites. Wear 2003, 254, 693–712. [Google Scholar] [CrossRef]
- Sastri, S.B.; Keller, T.M. Phthalonitrile cure reaction with aromatic diamines. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1885–1890. [Google Scholar] [CrossRef]
- Keller, T.M.; Price, T.R. Amine-Cured Bisphenol-Linked Phthalonitrile Resins. J. Macromol. Sci. Part A Chem. 2006, 18, 931–937. [Google Scholar] [CrossRef]
- Cao, G.P.; Chen, W.J.; Liu, X.B. Synthesis and thermal properties of the thermosetting resin based on cyano functionalized benzoxazine. Polym. Degrad. Stab. 2008, 93, 739–744. [Google Scholar] [CrossRef]
- Xu, M.; Luo, Y.; Lei, Y.; Liu, X. Phthalonitrile-based resin for advanced composite materials: Curing behavior studies. Polym. Test. 2016, 55, 38–43. [Google Scholar] [CrossRef]
- Xu, M.; Ren, D.; Chen, L.; Li, K.; Liu, X. Understanding of the polymerization mechanism of the phthalonitrile-based resins containing benzoxazine and their thermal stability. Polymer 2018, 143, 28–29. [Google Scholar] [CrossRef]
- Xu, M.Z.; Jia, K.; Liu, X.B. Effect of bisphenol-A on the structures and properties of phthalonitrile-based resin containing benzoxazine. Express Polym. Lett. 2015, 9, 567–581. [Google Scholar] [CrossRef]
- Xu, M.; Jia, K.; Liu, X. Self-cured phthalonitrile resin via multistage polymerization mediated by allyl and benzoxazine functional groups. High Perform. Polym. 2016, 28, 1161–1171. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Chen, P.; Xia, L.; Xiong, X. Novel Bismaleimide Resins Modified by Allyl Compound Containing Liquid Crystalline Structure. Adv. Polym. Technol. 2018, 37, 281–289. [Google Scholar] [CrossRef]
- Ge, M.; Miao, J.-T.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Building and origin of bio-based bismaleimide resins with good processability, high thermal, and mechanical properties. J. Appl. Polym. Sci. 2018, 135, 45947. [Google Scholar] [CrossRef]
- Radue, M.S.; Varshney, V.; Baur, J.W.; Roy, A.K.; Odegard, G.M. Molecular Modeling of Cross-Linked Polymers with Complex Cure Pathways: A Case Study of Bismaleimide Resins. Macromolecules 2018, 51, 1830–1840. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Wang, X.; Lu, S.; Wang, B. The role of maleic anhydride functionalized graphene oxide in improving the interfacial properties of carbon fibre/bismaleimide composites. Polym. Int. 2018, 67, 276–282. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Xu, Q.; Fu, F.; Zhang, Y.; Endo, T.; Liu, X. Significant Improvement on Polybenzoxazine Toughness Achieved by Amine/Benzoxazine Copolymerization-Induced Phase Separation. Macromol. Chem. Phys. 2018, 219, 1700517. [Google Scholar] [CrossRef]
- Xu, M.; Yang, X.; Zhao, R.; Liu, X. Copolymerizing Behavior and Processability of Benzoxazine/Epoxy Systems and Their Applications for Glass Fiber Composite Laminates. J. Appl. Polym. Sci. 2013, 128, 1176–1184. [Google Scholar] [CrossRef]
- Chua, J.; Tu, Q. A Molecular Dynamics Study of Crosslinked Phthalonitrile Polymers: The Effect of Crosslink Density on Thermomechanical and Dielectric Properties. Polymers 2018, 10, 64. [Google Scholar] [CrossRef]
- Augustine, D.; Mathew, D.; Nair, C.P.R. End-functionalized thermoplastic-toughened phthalonitrile composites: Influence on cure reaction and mechanical and thermal properties. Polym. Int. 2015, 64, 146–153. [Google Scholar] [CrossRef]
- Laskoski, M.; Schear, M.B.; Neal, A.; Dominguez, D.D.; Ricks-Laskoski, H.L.; Hervey, J.; Keller, T.M. Improved synthesis and properties of aryl ether-based oligomeric phthalonitrile resins and polymers. Polymer 2015, 67, 185–191. [Google Scholar] [CrossRef]
- Hidalgo, J.; Jimenez-Morales, A.; Torralba, J.M. Thermal stability and degradation kinetics of feedstocks for powder injection moulding—A new way to determine optimal solid loading? Polym. Degrad. Stab. 2013, 98, 1188–1195. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Wei, P.; Jiang, P.; Zhao, X.; Yu, H. Synthesis of a novel hybrid synergistic flame retardant and its application in PP/IFR. Polym. Degrad. Stab. 2011, 96, 1134–1140. [Google Scholar] [CrossRef]












| Sample | Ti (°C) | Tp (°C) | Tp′ (°C) | Tp″ (°C) | ΔH1 (J/g) | ΔH2 (J/g) |
|---|---|---|---|---|---|---|
| DABA-Ph | 227.75 | 272.8 | - | 295.6 | 44.8 | 75.6 |
| DABA-Ph/BMI-2-1 | 197.41 | 238.2 | 263.3 | 287.1 | 90.6 | 71.2 |
| DABA-Ph/BMI-1-1 | 199.35 | 239.4 | - | 290.3 | 134.6 | 56.6 |
| DABA-Ph/BMI-1-2 | 198.55 | 239.7 | - | 304.7 | 181.1 | 33.1 |
| Sample | Initial Viscosity (Pa·s) | Gel-Time (min) | Gelling Viscosity (Pa·s) |
|---|---|---|---|
| DABA-Ph | 2.471 | 22.7 | 417.2 |
| DABA-Ph/BMI-2-1 | 0.447 | 61.4 | 63.26 |
| DABA-Ph/BMI-1-1 | 0.175 | 52.1 | 62.11 |
| DABA-Ph/BMI-1-2 | 0.113 | 18.3 | 146.1 |
| Sample | Initial Storage Modulus at 50 °C (MPa) | Tg (°C) |
|---|---|---|
| DABA-Ph # | 28,975 | 382 |
| DABA-Ph/BMI-2-1 | 24,781 | 430.1 |
| DABA-Ph/BMI-1-1 | 28,556 | 430.9 |
| DABA-Ph/BMI-1-2 | 21,944 | 430.5 |
| Sample | Ti (°C) | T5% (°C) | Tm (°C) | Char Yield at 600 °C (%) |
|---|---|---|---|---|
| DABA-Ph | 450.7 | 497.3 | 502.2 | 86.4 |
| DABA-Ph/BMI-2-1 | 452.8 | 484.2 | 496.5 | 84.5 |
| DABA-Ph/BMI-1-1 | 443.3 | 466.4 | 462.7 | 81.3 |
| DABA-Ph/BMI-1-2 | 439.5 | 459.6 | 454.1 | 80.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Lei, Y.; Ren, D.; Chen, L.; Li, K.; Liu, X. Thermal Stability of Allyl-Functional Phthalonitriles-Containing Benzoxazine/Bismaleimide Copolymers and Their Improved Mechanical Properties. Polymers 2018, 10, 596. https://doi.org/10.3390/polym10060596
Xu M, Lei Y, Ren D, Chen L, Li K, Liu X. Thermal Stability of Allyl-Functional Phthalonitriles-Containing Benzoxazine/Bismaleimide Copolymers and Their Improved Mechanical Properties. Polymers. 2018; 10(6):596. https://doi.org/10.3390/polym10060596
Chicago/Turabian StyleXu, Mingzhen, Yangxue Lei, Dengxun Ren, Lin Chen, Kui Li, and Xiaobo Liu. 2018. "Thermal Stability of Allyl-Functional Phthalonitriles-Containing Benzoxazine/Bismaleimide Copolymers and Their Improved Mechanical Properties" Polymers 10, no. 6: 596. https://doi.org/10.3390/polym10060596