Development of Biodegradable Cosmetic Patch Using a Polylactic Acid/Phycocyanin–Alginate Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PLA/Phycocyanin–Alginate Composites
2.3. Characterizations
2.3.1. Antioxidant Assay
2.3.2. Cell Cytotoxicity Analysis
- Cell culture
- Phycocyanin treatment
- MTT assay
2.3.3. Materials Characterizations
3. Results and Discussion
3.1. Preliminary Test (Cell Cytotoxicity Test and Antioxidant Activity)
3.1.1. Cell Cytotoxicity Test
3.1.2. Antioxidant Activity
3.2. Optimization of Preparation Conditions
3.2.1. Optimization of Phycocyanin/Alginate Ratio
3.2.2. Optimization of Stirring Time
3.2.3. Optimization of Mixing Temperature
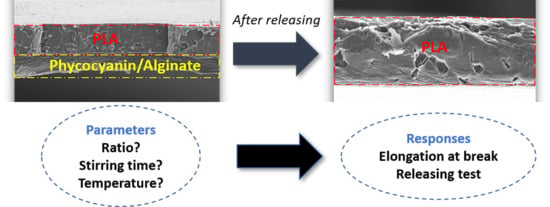
3.3. Morphological Properties of PLA/Phycocyanin–Alginate Composite (Scanning Electron Microscopy (SEM))
3.4. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR–FTIR) of PLA/Phycocyanin–Alginate Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sofokleous, P.; Stride, E.; Edirisinghe, M. Preparation, characterization, and release of amoxicillin from electrospun fibrous wound dressing patches. Pharm. Res. 2013, 30, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Elsner, J.J.; Zilberman, M. Antibiotic-eluting bioresorbable composite fibers for wound healing applications: Microstructure, drug delivery and mechanical properties. Acta Biomater. 2009, 5, 2872–2883. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Kermani, Z.; Faghihi, G.; Asilian, A.; Hamishehkar, H.; Jamshidi, A. Preparation and evaluation of cosmetic patches containing lactic and glycolic acids. Indian J. Dermatol. Venereol. Leprol. 2006, 72, 432. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Agarwal, S.; Chander, R.; Singh, A.; Yadav, P. Patch testing in patients with suspected cosmetic dermatitis: A retrospective study. J. Cosmet. Dermatol. 2018, 17, 95–100. [Google Scholar] [CrossRef]
- Ahmad, Z.; Stride, E.; Edirisinghe, M. Novel preparation of transdermal drug-delivery patches and functional wound healing materials. J. Drug Target. 2009, 17, 724–729. [Google Scholar] [CrossRef]
- Byeon, S.Y.; Cho, M.K.; Shim, K.H.; Kim, H.J.; Song, H.G.; Shin, H.S. Development of a spirulina extract/alginate-imbedded pcl nanofibrous cosmetic patch. J. Microbiol. Biotechnol. 2017, 27, 1657–1663. [Google Scholar] [CrossRef] [Green Version]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef]
- Vatankhah, E. Rosmarinic acid-loaded electrospun nanofibers: In vitro release kinetic study and bioactivity assessment. Eng. Life Sci. 2018, 18, 732–742. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Park, J.; Chu, G.S.; Kim, K.S.; Sung, J.H.; Kim, B. Transdermal delivery of cosmetic ingredients using dissolving polymer microneedle arrays. Biotechnol. Bioprocess Eng. 2015, 20, 543–549. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Ali, F.B.; Awale, R.J.; Fakhruldin, H.; Anuar, H. Plasticizing poly(lactic acid) using epoxidized palm oil for environmental friendly packaging material. Malays. J. Anal. Sci. 2016, 20, 1153–1158. [Google Scholar] [CrossRef]
- Adli, S.A.; Ali, F.; Azmi, A.S. Preparation and Characterization of PLA/Algae Nanocomposite for Packaging Industry. J. Adv. Res. Mater. Sci. 2018, 1, 19–25. [Google Scholar]
- Jung, S.M.; Kim, D.S.; Ju, J.H.; Shin, H.S. Assessment of Spirulina-PCL nanofiber for the regeneration of dermal fibroblast layers. Vitr. Cell Dev. Biol. Anim. 2013, 49, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Suksaeree, J.; Karnsopa, P.; Wannaphruek, N.; Prasomkij, J.; Panrat, K.; Pichayakorn, W. Transdermal Delivery of Nicotine Using Pectin Isolated from Durian Fruit-Hulls-Based Polymer Blends as a Matrix Layer. J. Polym. Environ. 2018, 26, 3216–3225. [Google Scholar] [CrossRef]
- Kim, C.; Shim, J.; Han, S.; Chang, I. The skin-permeation-enhancing effect of phosphatidylcholine: Caffeine as a model active ingredient. J. Cosmet. Sci. 2002, 53, 363–374. [Google Scholar]
- Chu, W.L.; Lim, Y.W.; Radhakrishnan, A.K.; Lim, P.E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement. Altern. Med. 2010, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Omura, S.; Maruyama, M. Regulation of growth factors-associated cell migration by C-phycocyanin scaffold in dermal wound healing. Clin. Exp. Pharmacol. Physiol. 2012, 39, 13–19. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicryl-hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Asthana, G.S.; Sharma, S.; Asthana, A. Formulation and evaluation of alginate-based mucoadhesive buccal patch for delivery of antimigraine drug. Asian J. Pharm. Clin. Res. 2018, 11, 185–191. [Google Scholar] [CrossRef]
- Grasmeijer, F.; Hagedoorn, P.; Frijlink, H.W.; de Boer, A.H. Drug Content Effects on the Dispersion Performance of Adhesive Mixtures for Inhalation. PLoS ONE 2013, 8, 1–18. [Google Scholar] [CrossRef]
- De Morais, M.G.; da Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from Microalgae: Properties, Extraction and Purification, with Some Recent Applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]










| Phycocyanin Concentration (µg/mL) | DPPH Scavenging Activity (% Inhibition) |
|---|---|
| 19.53 | 39.26 |
| 39.06 | 46.16 |
| 78.13 | 48.98 |
| 156.25 | 52.17 |
| 312.50 | 59.46 |
| 625.00 | 67.90 |
| Phycocyanin/Alginate Ratio | Percentage Release (w/w%) |
|---|---|
| 50/50 | 30.52 |
| 40/60 | 30.18 |
| 30/70 | 34.93 |
| 20/80 | 36.45 |
| 10/90 | 45.56 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adli, S.A.; Ali, F.; Azmi, A.S.; Anuar, H.; Nasir, N.A.M.; Hasham, R.; Idris, M.K.H. Development of Biodegradable Cosmetic Patch Using a Polylactic Acid/Phycocyanin–Alginate Composite. Polymers 2020, 12, 1669. https://doi.org/10.3390/polym12081669
Adli SA, Ali F, Azmi AS, Anuar H, Nasir NAM, Hasham R, Idris MKH. Development of Biodegradable Cosmetic Patch Using a Polylactic Acid/Phycocyanin–Alginate Composite. Polymers. 2020; 12(8):1669. https://doi.org/10.3390/polym12081669
Chicago/Turabian StyleAdli, Sarah Amalina, Fathilah Ali, Azlin Suhaida Azmi, Hazleen Anuar, Nur Aimi Mohd Nasir, Rosnani Hasham, and Mohamad Khairul Hafiz Idris. 2020. "Development of Biodegradable Cosmetic Patch Using a Polylactic Acid/Phycocyanin–Alginate Composite" Polymers 12, no. 8: 1669. https://doi.org/10.3390/polym12081669