Protein-Based Films and Coatings for Food Industry Applications
Abstract
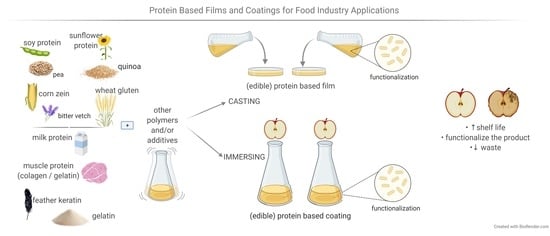
:1. Introduction
2. Materials Used for Protein-Based Packaging and Edible Packaging
3. Proteins Used for Food Films or Coatings
3.1. Animal Protein-Based Packages
3.2. Vegetable Protein-Based Packages
4. Protein-Based Films and Coating Functionalization
5. Antioxidant, Antimicrobial/Antifungal Activity of Protein-Based Films
6. Physicochemical Properties of Protein-Based Packages
6.1. Mechanical Properties
6.2. Thickness
6.3. Water Vapor Permeability (WVP)
6.4. Moisture Content
6.5. Sensory Properties
7. Safety Issues
8. Economic Perspectives
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kaewprachu, P.; Rawdkuen, S. Application of active edible film as food packaging for food preservation and extending shelf life. In Microbes in Food and Health; Springer: Berlin/Heidelberg, Germany, 2016; pp. 185–205. [Google Scholar]
- Brandenburg, A.; Weller, C.; Testin, R. Edible films and coatings from soy protein. J. Food Sci. 1993, 58, 1086–1089. [Google Scholar] [CrossRef]
- Erginkaya, Z.; Kalkan, S.; Ünal, E. Use of antimicrobial edible films and coatings as packaging materials for food safety. In Food Processing: Strategies for Quality Assessment; Springer: Berlin/Heidelberg, Germany, 2014; pp. 261–295. [Google Scholar]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef]
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Nychas, G.E.; Tassou, C.; Chorianopoulos, N. Use of Fourier transform infrared spectroscopy for monitoring the shelf life of ham slices packed with probiotic supplemented edible films after treatment with high pressure processing. Food Res. Int. 2018, 106, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Pop, O.L.; Brandau, T.; Vodnar, D.C.; Socaciu, C. Study of bifidobacterium lactic 300b survival during encapsulation, coating and freeze drying process and the release in alkaline media. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. Agric. 2012, 69, 372–379. [Google Scholar]
- Qian, Y.F.; Zheng, L.J.; Song, R.Y.; Du, B. Electrospinning of Pullulan Nanofibers for Food Package Materials. Adv. Mater. Res. 2013, 821–822, 1321–1325. [Google Scholar] [CrossRef]
- Von Schantz, L.; Schagerlöf, H.; Nordberg Karlsson, E.; Ohlin, M. Characterization of the substitution pattern of cellulose derivatives using carbohydrate-binding modules. BMC Biotechnol. 2014, 14, 113. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, E.M.C.; Lourenço, R.V.; Bittante, A.M.Q.B.; Moraes, I.C.F.; do Amaral Sobral, P.J. Gelatin-based films reinforced with montmorillonite and activated with nanoemulsion of ginger essential oil for food packaging applications. Food Packag. Shelf Life 2016, 10, 87–96. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Active edible films based on semi-refined κ-carrageenan: Antioxidant and color properties and application in chicken breast packaging. Food Packag. Shelf Life 2020, 24, 100476. [Google Scholar] [CrossRef]
- Pop, O.L.; Pop, C.R.; Dufrechou, M.; Vodnar, D.C.; Socaci, S.A.; Dulf, F.V.; Minervini, F.; Suharoschi, R. Edible Films and Coatings Functionalization by Probiotic Incorporation: A Review. Polymers 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamróz, E.; Kopel, P.; Tkaczewska, J.; Dordevic, D.; Jancikova, S.; Kulawik, P.; Milosavljevic, V.; Dolezelikova, K.; Smerkova, K.; Svec, P. Nanocomposite Furcellaran Films—The Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation. Polymers 2019, 11, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Huang, J.; Xing, H.; Gao, Q.; Li, Y.; Li, X. Edible coating packaging and its preservation effect to cherry tomatoes. In Proceedings of the China Academic Conference on Printing & Packaging and Media Technology, Xi’an, China, 25–27 November 2016; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Cruz-Diaz, K.; Cobos, Á.; Fernández-Valle, M.E.; Díaz, O.; Cambero, M.I. Characterization of edible films from whey proteins treated with heat, ultrasounds and/or transglutaminase. Application in cheese slices packaging. Food Packag. Shelf Life 2019, 22, 100397. [Google Scholar] [CrossRef]
- Adilah, Z.A.M.; Jamilah, B.; Hanani, Z.A.N. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Maria Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Ghoshal, G. Recent Trends in Active, Smart, and Intelligent Packaging for Food Products. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 10; pp. 343–374. [Google Scholar]
- Hanani, Z.N.; Roos, Y.; Kerry, J. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Parimi, N.S.; Singh, M.; Kastner, J.R.; Das, K.C.; Forsberg, L.S.; Azadi, P. Optimization of Protein Extraction from Spirulina platensis to Generate a Potential Co-Product and a Biofuel Feedstock with Reduced Nitrogen Content. Front. Energy Res. 2015, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal. Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Liang, L.; Wang, C.; Li, S.; Chu, X.; Sun, K. Nutritional compositions of Indian Moringa oleifera seed and antioxidant activity of its polypeptides. Food Sci. Nutr. 2019, 7, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasco, L.; Acuti, G.; Bani, P.; Dalle Zotte, A.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Gençdağ, E.; Görgüç, A.; Yılmaz, F.M. Recent advances in the recovery techniques of plant-based proteins from agro-industrial by-products. Food Rev. Int. 2020, 1–22. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef]
- Pardo-Ibáñez, P.; Lopez-Rubio, A.; Martínez-Sanz, M.; Cabedo, L.; Lagaron, J.M. Keratin–polyhydroxyalkanoate melt-compounded composites with improved barrier properties of interest in food packaging applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Moreira, M.d.R.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial effectiveness of bioactive packaging materials from edible chitosan and casein polymers: Assessment on carrot, cheese, and salami. J. Food Sci. 2011, 76, M54–M63. [Google Scholar] [CrossRef]
- Ramos, M.; Valdes, A.; Beltran, A.; Garrigós, M.C. Gelatin-based films and coatings for food packaging applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Sun, J.; Gao, W. Site-selective protein modification with polymers for advanced biomedical applications. Biomaterials 2018, 178, 413–434. [Google Scholar] [CrossRef]
- Fabra, M.; Jiménez, A.; Atarés, L.; Talens, P.; Chiralt, A. Effect of fatty acids and beeswax addition on properties of sodium caseinate dispersions and films. Biomacromolecules 2009, 10, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Sobral, P.J.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res. Int. 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H.; Wu, J. Milk protein-based edible film mechanical strength changes due to ultrasound process. J. Food Sci. 1996, 61, 824–828. [Google Scholar] [CrossRef]
- Kanmani, P.; Lim, S.T. Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem. 2013, 141, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Jiang, Y.; Zhao, Y. Chitosan–whey protein isolate composite films for encapsulation and stabilization of fish oil containing ultra pure omega-3 fatty acids. J. Food Sci. 2011, 76, C133–C141. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Sunflower protein films incorporated with clove essential oil have potential application for the preservation of fish patties. Food Hydrocoll. 2013, 33, 74–84. [Google Scholar] [CrossRef]
- Simmonds, G.; Spence, C. Food imagery and transparency in product packaging. In Multisensory Packaging; Springer: Berlin/Heidelberg, Germany, 2019; pp. 49–77. [Google Scholar]
- Davidovich-Pinhas, M.; Danin-Poleg, Y.; Kashi, Y.; Bianco-Peled, H. Modified chitosan: A step toward improving the properties of antibacterial food packages. Food Packag. Shelf Life 2014, 1, 160–169. [Google Scholar] [CrossRef]
- Kandirmaz, E.A.; Ozcan, A. Antibacterial effect of Ag nanoparticles into the paper coatings. Nord. Pulp Pap. Res. J. 2019, 34, 507–515. [Google Scholar] [CrossRef]
- Nithya, V.; Murthy, P.; Halami, P. Development and application of active films for food packaging using antibacterial peptide of B acillus licheniformis M e1. J. Appl. Microbiol. 2013, 115, 475–483. [Google Scholar] [CrossRef]
- Fang, T.; Shen, X.; Hou, J.; Guo, M. Effects of polymerized whey protein prepared directly from cheese whey as fat replacer on physiochemical, texture, microstructure and sensory properties of low-fat set yogurt. LWT 2019, 115, 108268. [Google Scholar] [CrossRef]
- Braber, N.L.V.; Di Giorgio, L.; Aminahuel, C.A.; Vergara, L.I.D.; Costa, A.O.M.; Montenegro, M.A.; Mauri, A.N. Antifungal whey protein films activated with low quantities of water soluble chitosan. Food Hydrocoll. 2021, 110, 106156. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Picchio, M.L.; Linck, Y.G.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Igarzabal, C.I.A. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of active fish gelatin films with anthocyanins by compression molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodriguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Liu, A.; Wang, W. Improved thermal-stability and mechanical properties of type I collagen by crosslinking with casein, keratin and soy protein isolate using transglutaminase. Int. J. Biol. Macromol. 2017, 98, 292–301. [Google Scholar] [CrossRef]
- Ahmad, M.; Nirmal, N.P.; Danish, M.; Chuprom, J.; Jafarzedeh, S. Characterisation of composite films fabricated from collagen/chitosan and collagen/soy protein isolate for food packaging applications. RSC Adv. 2016, 6, 82191–82204. [Google Scholar] [CrossRef]
- Pranata, M.P.; González-Buesa, J.; Chopra, S.; Kim, K.; Pietri, Y.; Ng, P.K.W.; Matuana, L.M.; Almenar, E. Egg White Protein Film Production Through Extrusion and Calendering Processes and its Suitability for Food Packaging Applications. Food Bioproc. Technol. 2019, 12, 714–727. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.Q. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020. [Google Scholar] [CrossRef]
- Munir, S.; Hu, Y.; Liu, Y.; Xiong, S. Enhanced properties of silver carp surimi-based edible films incorporated with pomegranate peel and grape seed extracts under acidic condition. Food Packag. Shelf Life 2019, 19, 114–120. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.F.; Barbut, S.; Lim, L.-T. Fortification of dietary biopolymers-based packaging material with bioactive plant extracts. Food Res. Int. 2012, 49, 80–91. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.; Bean, S.R.; Sun, X.S.; Wang, D. Evaluation of adhesive performance of a mixture of soy, sorghum and canola proteins. Ind. Crops Prod. 2020, 157, 112898. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. Int. J. Biol. Macromol. 2017, 94, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Fărcaş, A.C.; Socaci, S.A.; Dulf, F.V.; Tofană, M.; Mudura, E.; Diaconeasa, Z. Volatile profile, fatty acids composition and total phenolics content of brewers’ spent grain by-product with potential use in the development of new functional foods. J. Cereal Sci. 2015, 64, 34–42. [Google Scholar] [CrossRef]
- Proaño, J.L.; Salgado, P.R.; Cian, R.E.; Mauri, A.N.; Drago, S.R. Physical, structural and antioxidant properties of brewer’s spent grain protein films. J. Sci. Food Agric. 2020, 100, 5458–5465. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, J.-H.; Yang, H.-J.; Song, K.B. Preparation and characterization of brewer’s spent grain protein-chitosan composite films. J. Food Sci. Technol. 2015, 52, 7549–7555. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and characterization of bioplastics from grass pea flour cast in the presence of microbial transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Takma, D.K.; Korel, F. Active packaging films as a carrier of black cumin essential oil: Development and effect on quality and shelf-life of chicken breast meat. Food Packag. Shelf Life 2019, 19, 210–217. [Google Scholar] [CrossRef]
- Badr, K.; Ahmed, Z.; El Gamal, M. Evaluation of the antimicrobial action of whey protein edible films incorporated with cinnamon, cumin and thyme against spoilage flora of fresh beef. Int. J. Agric. Res. 2014, 9, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Efficacy of activated alginate-based nanocomposite films to control Listeria monocytogenes and spoilage flora in rainbow trout slice. J. Food Sci. Technol. 2016, 53, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Caro, N.; Medina, E.; Díaz-Dosque, M.; López, L.; Abugoch, L.; Tapia, C. Novel active packaging based on films of chitosan and chitosan/quinoa protein printed with chitosan-tripolyphosphate-thymol nanoparticles via thermal ink-jet printing. Food Hydrocoll. 2016, 52, 520–532. [Google Scholar] [CrossRef]
- Escamilla-García, M.; Delgado-Sánchez, L.F.; Ríos-Romo, R.A.; García-Almendárez, B.E.; Calderón-Domínguez, G.; Méndez-Méndez, J.V.; Amaro-Reyes, A.; Di Pierro, P.; Regalado-González, C. Effect of transglutaminase cross-linking in protein isolates from a mixture of two quinoa varieties with chitosan on the physicochemical properties of edible films. Coatings 2019, 9, 736. [Google Scholar] [CrossRef] [Green Version]
- Porta, R.; Di Pierro, P.; Rossi-Marquez, G.; Mariniello, L.; Kadivar, M.; Arabestani, A. Microstructure and properties of bitter vetch (Vicia ervilia) protein films reinforced by microbial transglutaminase. Food Hydrocoll. 2015, 50, 102–107. [Google Scholar] [CrossRef]
- Porta, R.; Di Pierro, P.; Roviello, V.; Sabbah, M. Tuning the functional properties of bitter vetch (vicia ervilia) protein films grafted with spermidine. Int. J. Mol. Sci. 2017, 18, 2658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Moosavi, M.H.; Khani, M.R.; Shokri, B.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Modifications of protein-based films using cold plasma. Int. J. Biol. Macromol. 2020, 142, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, Q.; Gao, Q.; Chen, H.; Shi, S.Q.; Zhou, W.; Li, X.; Xia, C.; Li, J. Facile fabrication of self-healable and antibacterial soy protein-based films with high mechanical strength. ACS Appl. Mater. Interfaces 2019, 11, 16107–16116. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100, 105443. [Google Scholar] [CrossRef]
- Romani, V.P.; Olsen, B.; Collares, M.P.; Oliveira, J.R.M.; Prentice, C.; Martins, V.G. Cold plasma and carnauba wax as strategies to produce improved bi-layer films for sustainable food packaging. Food Hydrocoll. 2020, 108, 106087. [Google Scholar] [CrossRef]
- Acquah, C.; Zhang, Y.; Dubé, M.A.; Udenigwe, C.C. Formation and characterization of protein-based films from yellow pea (Pisum sativum) protein isolate and concentrate for edible applications. Curr. Res. Food Sci. 2020, 2, 61–69. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Dias, M.V.; de Siqueira Elias, H.H.; Fukushima, K.L.; Silva, E.K.; Carneiro, J.d.D.S.; Soares, N.d.F.F.; Borges, S.V. Effect of whey protein isolate films incorporated with montmorillonite and citric acid on the preservation of fresh-cut apples. Food Res. Int. 2018, 107, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Andrade, M.A.; Ribeiro-Santos, R.; Bonito, M.C.C.; Saraiva, M.; Sanches-Silva, A. Characterization of rosemary and thyme extracts for incorporation into a whey protein based film. LWT 2018, 92, 497–508. [Google Scholar] [CrossRef]
- Yuan, G.; Jia, Y.; Pan, Y.; Li, W.; Wang, C.; Xu, L.; Wang, C.; Chen, H. Preparation and characterization of shrimp shell waste protein-based films modified with oolong tea, corn silk and black soybean seed coat extracts. Polym. Test. 2020, 81, 106235. [Google Scholar] [CrossRef]
- Romani, V.P.; Olsen, B.; Collares, M.P.; Oliveira, J.R.M.; Prentice-Hernández, C.; Martins, V.G. Improvement of fish protein films properties for food packaging through glow discharge plasma application. Food Hydrocoll. 2019, 87, 970–976. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active edible furcellaran/whey protein films with yerba mate and white tea extracts: Preparation, characterization and its application to fresh soft rennet-curd cheese. Int. J. Biol. Macromol. 2020, 155, 1307–1316. [Google Scholar] [CrossRef]
- Perez-Gago, M.B.; Serra, M.; Alonso, M.; Mateos, M.; del Río, M.A. Effect of whey protein- and hydroxypropyl methylcellulose-based edible composite coatings on color change of fresh-cut apples. Postharvest Biol. Technol. 2005, 36, 77–85. [Google Scholar] [CrossRef]
- Conte, A.; Buonocore, G.G.; Sinigaglia, M.; Del Nobile, M.A. Development of immobilized lysozyme based active film. J. Food Eng. 2007, 78, 741–745. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Liang, S.; Wang, L. A natural antibacterial-antioxidant film from soy protein isolate incorporated with cortex phellodendron extract. Polymers 2018, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yu, M.; Wang, L. Preparation and characterization of antioxidant soy protein isolate films incorporating licorice residue extract. Food Hydrocoll. 2018, 75, 13–21. [Google Scholar] [CrossRef]
- Yang, H.-J.; Lee, J.-H.; Won, M.; Song, K.B. Antioxidant activities of distiller dried grains with solubles as protein films containing tea extracts and their application in the packaging of pork meat. Food Chem. 2016, 196, 174–179. [Google Scholar] [CrossRef]
- Wang, H.; Hu, D.; Ma, Q.; Wang, L. Physical and antioxidant properties of flexible soy protein isolate films by incorporating chestnut (Castanea mollissima) bur extracts. LWT Food Sci. Technol. 2016, 71, 33–39. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Rungraeng, N.; Rawdkuen, S. Characterization of fish myofibrillar protein film incorporated with catechin-Kradon extract. Int. J. Biol. Macromol. 2018, 107, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of antioxidant and antimicrobial coating based on whey protein nanofibrils with TiO2 nanotubes on the quality and shelf life of chilled meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef] [Green Version]
- Chollakup, R.; Pongburoos, S.; Boonsong, W.; Khanoonkon, N.; Kongsin, K.; Sothornvit, R.; Sukyai, P.; Sukatta, U.; Harnkarnsujarit, N. Antioxidant and antibacterial activities of cassava starch and whey protein blend films containing rambutan peel extract and cinnamon oil for active packaging. LWT 2020, 130, 109573. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B Biointerfaces 2019, 180, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Yazdi, F.T.; Shabani, A.; Mortazavi, S. Active gelatin-mannan film: Physicochemical, antifungal and aflatoxin binding properties. Int. Food Res. J. 2019, 26, 1803–1812. [Google Scholar]
- Bourtoom, T. Factors affecting the properties of edible film prepared from mung bean proteins. Int. Food Res. J. 2008, 15, 167–180. [Google Scholar]
- Bourtoom, T. Edible protein films: Properties enhancement. Int. Food Res. J. 2009, 16, 1–9. [Google Scholar]
- Liu, C.-C.; Tellez-Garay, A.M.; Castell-Perez, M.E. Physical and mechanical properties of peanut protein films. LWT Food Sci. Technol. 2004, 37, 731–738. [Google Scholar] [CrossRef]
- Coughlan, K.; Shaw, N.; Kerry, J.; Kerry, J. Combined effects of proteins and polysaccharides on physical properties of whey protein concentrate-based edible films. J. Food Sci. 2004, 69, E271–E275. [Google Scholar] [CrossRef]
- Garrido, T.; Leceta, I.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Tailoring soy protein film properties by selecting casting or compression as processing methods. Eur. Polym. J. 2016, 85, 499–507. [Google Scholar] [CrossRef]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Gennadios, A. Proteins as Raw Materials for Films and Coatings: Definitions, Current Status, and Opportunities. In Protein-based Films and Coatings; CRC Press: Boca Raton, FL, USA, 2002; pp. 21–62. [Google Scholar]
- Barreto, P.L.M.; Pires, A.T.N.; Soldi, V. Thermal degradation of edible films based on milk proteins and gelatin in inert atmosphere. Polym. Degrad. Stab. 2003, 79, 147–152. [Google Scholar] [CrossRef]
- Gialamas, H.; Zinoviadou, K.G.; Biliaderis, C.G.; Koutsoumanis, K.P. Development of a novel bioactive packaging based on the incorporation of Lactobacillus sakei into sodium-caseinate films for controlling Listeria monocytogenes in foods. Food Res. Int. 2010, 43, 2402–2408. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.R.; Yu, J. Properties of biodegradable thermoplastic pea starch/carboxymethyl cellulose and pea starch/microcrystalline cellulose composites. Carbohydr. Polym. 2008, 72, 369–375. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Rossi-Márquez, G.; Han, J.H.; García-Almendárez, B.; Castaño-Tostado, E.; Regalado-González, C. Effect of temperature, pH and film thickness on nisin release from antimicrobial whey protein isolate edible films. J. Sci. Food Agric. 2009, 89, 2492–2497. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG in prebiotic edible films. Food Chem. 2014, 159, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Yonekura, L.; Gan, H.-H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Dianina, I.B.; Jrb, A.G.O.; Pimentelc, T.C.; Hernandesa, N.F.; Costaa, G.N. Edible Biofilms Formulated with Whey Protein Isolate and L. casei Probiotic Culture: Characterization and Application in Tomatoes and Grapes. Chem. Eng. 2019, 75. [Google Scholar] [CrossRef]
- Pereira, J.O.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Mirzakhani, M.; Moini, S.; Emam-Djomeh, Z. Physical and mechanical features investigation of protein-based biodegradable films obtained from trout fish waste. J. Food Bioprocess. Eng. 2018, 2, 41–54. [Google Scholar]
- Coupland, J.N.; Shaw, N.B.; Monahan, F.J.; O’Riordan, E.D.; O’Sullivan, M. Modeling the effect of glycerol on the moisture sorption behavior of whey protein edible films. J. Food Eng. 2000, 43, 25–30. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.; Song, K.B. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015, 46, 208–215. [Google Scholar] [CrossRef]
- Kuswandi, B. Jumina, 12—Active and intelligent packaging, safety, and quality controls. In Fresh-Cut Fruits and Vegetables; Siddiqui, M.W., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 243–294. [Google Scholar]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Ly, M.H.; Aguedo, M.; Goudot, S.; Le, M.L.; Cayot, P.; Teixeira, J.A.; Le, T.M.; Belin, J.M.; Waché, Y. Interactions between bacterial surfaces and milk proteins, impact on food emulsions stability. Food Hydrocoll. 2008, 22, 742–751. [Google Scholar] [CrossRef] [Green Version]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: A brief overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Pavelková, A. Time temperature indicators as devices intelligent packaging. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Sabato, S.; Ouattara, B.; Yu, H.; D’aprano, G.; Le Tien, C.; Mateescu, M.; Lacroix, M. Mechanical and barrier properties of cross-linked soy and whey protein based films. J. Agric. Food Chem. 2001, 49, 1397–1403. [Google Scholar] [CrossRef]
- Schmid, M.; Müller, K. Whey Protein-Based Packaging Films and Coatings. In Whey Proteins; Deeth, H.C., Bansal, N., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 11; pp. 407–437. [Google Scholar]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Mesaros, A.; Vasile, B.S.; Toloman, D.; Pop, O.L.; Marinca, T.; Unguresan, M.; Perhaita, I.; Filip, M.; Iordache, F. Towards understanding the enhancement of antibacterial activity in manganese doped ZnO nanoparticles. Appl. Surf. Sci. 2019, 471, 960–972. [Google Scholar] [CrossRef]
- Cuibus, L.; Maggio, R.; Mureșan, V.*; Diaconeasa, Z.; Pop, O.L.; Socaciu, C. Preliminary discrimination of butter adulteration by ATR-FTIR spectroscopy. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2015, 72, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Pop, O.L.; Vodnar, D.C. Procyanidins and their effectiveness after incorporation in food systems. In Characterization, Antioxidant Roerties and Health Benefits; Chedea, V.S., Ed.; Nova Publisher: Hauppauge, NY, USA, 2016; p. 129. [Google Scholar]
- Mustapha, F.; Jai, J.; Hamidon, F.; Sharif, Z.M.; Yusof, N.M. Antimicrobial agents from Malaysian plants and their potential use in food packaging material. Chem. Eng. Res. Bull. 2017, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Blanke, M.M.; Shekarriz, R. Gold nanoparticles and sensor technology for sensitive ethylene detection. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 934, Lisbon, Portugal, 30 June 2012. [Google Scholar]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control. 2020, 112, 107086. [Google Scholar] [CrossRef]
- Baldea, I.; Florea, A.; Olteanu, D.; Clichici, S.; David, L.; Moldovan, B.; Cenariu, M.; Achim, M.; Suharoschi, R.; Danescu, S. Effects of silver and gold nanoparticles phytosynthesized with Cornus mas extract on oral dysplastic human cells. Nanomedicine 2020, 15, 55–75. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Barbu-Tudoran, L.; Coman, C.; Leopold, L.; Mesaros, A.; Pop, O.; Rugină, D.; Ştefan, R.; Tabaran, F.; Tripon, S. Cerium oxide nanoparticles and its cytotoxicity human lung cancer cells. Rom. Biotechnol. Lett. 2015, 20, 10679. [Google Scholar]
- Das, M.; Saxena, N.; Dwivedi, P.D. Emerging trends of nanoparticles application in food technology: Safety paradigms. Nanotoxicology 2009, 3, 10–18. [Google Scholar] [CrossRef]
- Brugè, F.; Damiani, E.; Marcheggiani, F.; Offerta, A.; Puglia, C.; Tiano, L. A comparative study on the possible cytotoxic effects of different nanostructured lipid carrier (NLC) compositions in human dermal fibroblasts. Int. J. Pharm. 2015, 495, 879–885. [Google Scholar] [CrossRef]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Cozma, A.; Sitar-Taut, A.; Urian, L.; Fodor, A.; Suharoschi, R.; Muresan, C.; Negrean, V.; Sampelean, D.; Zdrenghea, D.; Pop, D. Unhealthy lifestyle and the risk of metabolic syndrome-the Romanian experience. J. Mind Med. Sci. 2018, 5, 218–229. [Google Scholar] [CrossRef]
- Darmon, N.; Drewnowski, A. Contribution of food prices and diet cost to socioeconomic disparities in diet quality and health: A systematic review and analysis. Nutr. Rev. 2015, 73, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M. Global diets link environmental sustainability and human health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef]
- Heller, M.C.; Selke, S.E.; Keoleian, G.A. Mapping the influence of food waste in food packaging environmental performance assessments. J. Ind. Ecol. 2019, 23, 480–495. [Google Scholar] [CrossRef] [Green Version]
- Chis, M.S.; Paucean, A.; Stan, L.; Muste, S.; Suharoschi, R.; Man, S.M. Protein metabolic conversion of nutritional features during quinoa sourdough fermentation and its impact on baked goods. CyTA J. Food 2018, 280, 744–753. [Google Scholar] [CrossRef]
- West, P.C.; Gerber, J.S.; Engstrom, P.M.; Mueller, N.D.; Brauman, K.A.; Carlson, K.M.; Cassidy, E.S.; Johnston, M.; MacDonald, G.K.; Ray, D.K. Leverage points for improving global food security and the environment. Science 2014, 345, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Licciardello, F.; Piergiovanni, L. 6—Packaging and food sustainability. In The Interaction of Food Industry and Environment; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 191–222. [Google Scholar]
- Vanderroost, M.; Ragaert, P.; Verwaeren, J.; De Meulenaer, B.; De Baets, B.; Devlieghere, F. The digitization of a food package’s life cycle: Existing and emerging computer systems in the logistics and post-logistics phase. Comput. Ind. 2017, 87, 15–30. [Google Scholar] [CrossRef]
- Etxabide, A.; Garrido, T.; Uranga, J.; Guerrero, P.; de la Caba, K. Extraction and incorporation of bioactives into protein formulations for food and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Bandy, L.; Adhikari, V.; Jebb, S.; Rayner, M. The use of commercial food purchase data for public health nutrition research: A systematic review. PLoS ONE 2019, 14, e0210192. [Google Scholar] [CrossRef]
- World Health Organization. Global Database on the Implementation of Nutrition Action (GINA): Results of a User Survey; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Food and Architecture Organization of the United Nations. The State of Food Security and Nutrition in the World; United Nations World Food Programme: Rome, Italy, 2019. [Google Scholar]
- Vågsholm, I.; Arzoomand, N.S.; Boqvist, S. Food Security, Safety, and Sustainability—Getting the Trade-Offs Right. Front. Sustain. Food Syst. 2020, 4, 16. [Google Scholar] [CrossRef]
- Socaci, S.A.; Rugină, D.O.; Diaconeasa, Z.M.; Pop, O.L.; Fărcaș, A.C.; Păucean, A.; Tofană, M.; Pintea, A. Antioxidant compounds recovered from food wastes. In Functional Food—Improve Health through Adequate Food; IntechOpen: London, UK, 2017. [Google Scholar]
- Patil, S.R.; Morales, R.; Cates, S.; Anderson, D.; Kendall, D. An application of meta-analysis in food safety consumer research to evaluate consumer behaviors and practices. J. Food Prot. 2004, 67, 2587–2595. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Khaneghah, A.M. Role of Green Polymers in Food Packaging. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Oxford, UK, 2020; pp. 305–319. [Google Scholar]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and function of protein-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 25–56. [Google Scholar]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Edible films and coatings: Tomorrow’s packagings: A review. Crit. Rev. Food Sci. 1998, 38, 299–313. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid. Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid. Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef]
- Licciardello, F. Packaging, blessing in disguise. Review on its diverse contribution to food sustainability. Trends Food Sci. Technol. 2017, 65, 32–39. [Google Scholar] [CrossRef]
- Magrassi, F. Life Cycle Assessment of Ship Recycling: Metals Recovery; ECI Digital Archives, ECI Symposium Series: New York, NY, USA, 2016. [Google Scholar]



| Formulation | Attainment Method | Characteristics | References |
|---|---|---|---|
| Sodium caseinate, bee wax and fatty acids | Film-forming emulsions | ↑ rigidity ↓ whater permeability ↑ laminate-like structure | [34] |
| Gelatin, soluble starch and polyols | Drying casting aqueous solutions | ↓ elasticity and tensile strength in samples high in water, glycerol or sorbitol | |
| Bovine hide and pigskin gelatin | Casting technique | ↓ puncture force by sorbitol addition ↑ water permeability with the gelatin content | [35] |
| Gelatin vs gelatin and casein | Cross linked with transglutaminase | ↑ elongation no modification in tensile strength and water vapor barrier properties | [36] |
| Whey protein concentrate and sodium caseinate | Casting technique | ↑ mecanical and tensile strength ↑ resistance to puncture no modification in elongation at break, water vapor barrier properties and moisture content | [37] |
| Milk proteins | Transglutaminase-catalyzed polymerization | ↓ moisture transfer ↓ whater vaopr resistance | [38] |
| Chitosan–whey protein | Casting technique | good moisture content ↓ water activity | [39] |
| Sunflower protein | Casting technique | ↓ water solubility ↓ glass transmising temerature | [40] |
| Functionalization/Material | Outcome | Application | References |
|---|---|---|---|
| Whey protein and gluten protein films treated with cold air and argon plasma | ↑ tensile strength ↑ roughness of whey protein-films ↓ roughness of gluten protein-films ↓ gas permeability (oxygen) ↑ stability of whey protein-films against water ↓ stability of gluten-films against water ↑ hydrophilicity of whey protein-films | Biodegradable food packaging | [74] |
| Whey protein films (WPI) activated with low quantities of water soluble chitosan (WSCh) | ↓ film‘s solubility ↓ film‘s elongation mechanical resistance barrier to water vapor ↑ surface hydrophobicity ↑ antifungal properties | Food packaging | [46] |
| Soy protein isolate (SPI) material with integrated polyethyleneimine (PEI) an metal ions Cu(II) or Zn(II) | ↑ tensile strength mechanical properties can be tuned ↑ material stretchability, ↑ self-healing capability ↓ restoration time ↑ antibacterial activity | Tissue regeneration, gene delivery, packaging, adhesives, food packaging | [75] |
| Preparation of shrimp shell waste protein-based films modified with oolong tea, corn silk and black soybean seed coat extracts | ↑ thermal stability ↑ barrier against UV light ↑ antioxidant activity | Biodegradable films for active packaging | [82] |
| Incorporating cellulose nanocrystals (CNCs) and pine needle extract (PNE) into soy protein-based films. | ↓ moisture content ↓ elongation at break ↑ tensile strength ↓ water vapor permeability ↑ antioxidant activity | Active food packaging material | [80] |
| Incorporating montmorillonite and citric acid into whey protein isolate films to preserve fresh-cut apples | ↓ enzymatic browning ↓ loss of apple quality ↑ shelf-life | Active food packaging | [79] |
| Fish protein-films treated with cold plasma and carnauba wax coating | ↑ tensile strength ↑ barrier properties ↓ water vapor permeability ↑ adhesion properties | Food packaging | [77] |
| Fisch protein-films treated with glow discharge plasma | ↑ elongation at break (*) ↑ tensile strength (*) ↑ color properties (*) ↑ barrier properties (*) (*) dependent on plasma treatment time | Food packaging | [83] |
| Incorporating mango kernel extract into soy protein isolate films and into fish gelatin films | ↑ thickness ↑ tensile strength ↑ transparency ↑ antioxidant activity ↓ water vapor permeability (*) (*) in soy protein isolate films | Food packaging | [18] |
| Incorporating rosemary and thyme extracts into whey protein films | ↑ antimicrobial activity | Active food packaging | [81] |
| Adding tannins to caseinate films or gelatin films | ↑ antioxidant activity (*) ↑ antimicrobial activity (*) ↓ water solubility (*) ↓ water vapor permeability (*) ↓ stretchability (*) ↑ thickness (*) (*) only for caseinate films | Active food packaging | [76] |
| Incorporating yerba mate and white tea extracts into furcellaran/whey protein films | ↓ water solubility (*) ↓ water vapor permeability (*) ↓ water content (*) ↑ shelf-life ↑ antimicrobial activity ↑ thermal stability ↑ puncture strength (*) ↑ modulus elasticity (*) ↓ elongation at break (*) (*) only for yerba mate extract | Edible active food packaging | [84] |
| Film/Coating | Formulation | Antioxidant Capacity | Antimicrobial/Antifungal Activity Against | Reference |
|---|---|---|---|---|
| Edible coating | whey protein isolate whey protein concentrate hydroxypropyl methylcellulose beeswax or carnauba wax | decrease enzimatic browning (just for the whey protein-based coating) | [85] | |
| Film | Polyvinyalcohol with lysozyme | - | Micrococcus lysodeikticus | [86] |
| Film | mung bean protein pomegranate peel (0, 2.5, 12.5, and 25% w/w) | 13.88 mg GAE/g (gallic acid equivalents) (25% pomegranate peel) | Escherichia coli O157:H7 Listeria monocytogenes | [87] |
| Film | soy protein isolate with cortex phellodendron extract (0, 10, 12.5, 15, 17.5, 20, 22.5% w/w) | 14.87 mg GAE/g (22.5% phellodendron extract) | Staphylococcus aureus ↓ Escherichia coli | [88] |
| Film | soy protein isolate fish gelatin mango kernel extracts | 3.77 μg GAE/g film | - | [18] |
| Film | soy protein isolate licorice residue extract (10, 30, 50, 70 g/kg) | 20% higher than in the control | - | [89] |
| Film | distiller dried grains with soluble (protein) green, black and oolong tea extract (0.1, 0.3, 0.5%) | all 0.3% samples had over 50% higher antioxidant activity than control | - | [90] |
| Film | soy protein isolate chestnut (Castanea mollissima) bur extracts (20, 50, 80, and 100 g/kg) | at least 20% higher than the control | - | [91] |
| Film | fish myofibrillar protein catechin–Kradon extract | at least 40% higher than the control | - | [92] |
| Coating | Whey protein TiO2 nanotubes | over 50% higher than the control | Listeria monocytogenes Staphylococcus aureus Salmonella enteritidis Escherichia coli | [93] |
| Film | cassava starch and whey protein rambutan peel extract cinnamon oil | over 30% higher than the control | Bacillus cereus Staphylococcus aureus Escherichia coli | [94] |
| Aspergilus niger CGMCC | [95] | |||
| Film | soy protein isolate plant-sourced cinnamaldehyde zinc oxide nanosheets | - | ||
| Film | gelatin nano-chitin | - | Aspergillus niger | [96] |
| Film | gelatin mannoprotein (extracted from Saccharomyces cerevisiae cell wall) | - | Aspergillus flavus binding aflatoxin B1 | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihalca, V.; Kerezsi, A.D.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.C.; Dulf, F.V.; Socaci, S.A.; Fărcaș, A.; Mureșan, C.I.; et al. Protein-Based Films and Coatings for Food Industry Applications. Polymers 2021, 13, 769. https://doi.org/10.3390/polym13050769
Mihalca V, Kerezsi AD, Weber A, Gruber-Traub C, Schmucker J, Vodnar DC, Dulf FV, Socaci SA, Fărcaș A, Mureșan CI, et al. Protein-Based Films and Coatings for Food Industry Applications. Polymers. 2021; 13(5):769. https://doi.org/10.3390/polym13050769
Chicago/Turabian StyleMihalca, Vlad, Andreea Diana Kerezsi, Achim Weber, Carmen Gruber-Traub, Jürgen Schmucker, Dan Cristian Vodnar, Francisc Vasile Dulf, Sonia Ancuța Socaci, Anca Fărcaș, Carmen Ioana Mureșan, and et al. 2021. "Protein-Based Films and Coatings for Food Industry Applications" Polymers 13, no. 5: 769. https://doi.org/10.3390/polym13050769