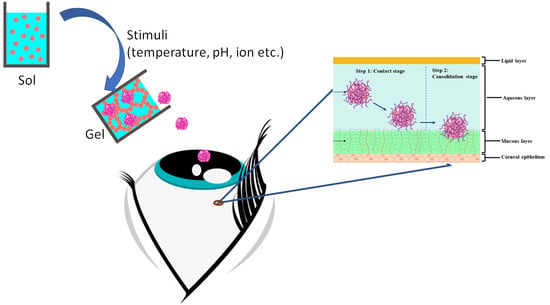
Potential of Stimuli-Responsive In Situ Gel System for Sustained Ocular Drug Delivery: Recent Progress and Contemporary Research
Abstract
:1. Introduction
2. Mucoadhesive Polymeric Approach
3. Smart Ocular Delivery Using Stimuli-Responsive In Situ Gel Platform
3.1. Thermo-Responsive In Situ Ocular Gelling Systems
3.1.1. Recent Research on the Applications of Thermo-Responsive In Situ Ocular Gelling Systems
Ocular Hypertension (Glaucoma)
Ocular Bacterial Infections
Ocular Fungal Infections
Ocular Viral Infections
Other Ocular Diseases
3.2. pH-Responsive In Situ Gelling Systems
3.2.1. Recent Researches on the Application of pH-Responsive In Situ Ocular Gelling Systems
Ocular Hypertension (Glaucoma)
Ocular Bacterial Infections
Ocular Fungal Infections
Ocular Viral Infections
Other Ocular Diseases
3.3. Ion-Responsive In Situ Ocular Gelling Systems
3.3.1. Recent Research on the Application of Ion-Responsive In Situ Ocular Gelling Systems
Ocular Hypertension (Glaucoma)
Ocular Bacterial Infections
Ocular Fungal Infections
Ocular Viral Infections
Other Ocular Diseases
3.4. Recent Research on the Application of Multi-Stimuli-Responsive In Situ Ocular Gelling Systems
3.4.1. Ocular Hypertension (Glaucoma)
3.4.2. Ocular Bacterial Infections
3.4.3. Ocular Fungal Infections
3.4.4. Ocular Viral Infections
3.4.5. Other Ocular Diseases
4. Expert Opinion
4.1. Expert Opinion on Thermo-Responsive In Situ Gelling Systems for Ocular Delivery
4.2. Expert Opinion on pH-Responsive In Situ Gelling Systems for Ocular Delivery
4.3. Expert Opinion on Ion-Responsive In Situ Gelling Systems for Ocular Delivery
4.4. Expert Opinion on Multi-Stimuli-Responsive In Situ Gelling Systems for Ocular Delivery
5. Safety Concern
5.1. Safety Concern Related to Thermo-Responsive In Situ Gel
5.2. Safety Concern Related to pH-Responsive In Situ Gel
5.3. Safety Concern Related to Ion-Responsive In Situ Gel
5.4. Safety Concern Related to Multi-Stimuli-Responsive In Situ Gel
6. Clinical and Safety Aspect
6.1. Clinical and Safety Aspect Related to Thermo-Responsive In Situ Gel for Ocular Delivery
6.2. Clinical and Safety Aspect Related to pH-Responsive In Situ Gel for Ocular Delivery
6.3. Clinical and Safety Aspect Related to Ion-Responsive In Situ Gel for Ocular Delivery
6.4. Clinical and Safety Aspect Related to Multi-Stimuli-Responsive In Situ Gel for Ocular Delivery
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [Green Version]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Chowhan, A.; Giri, T.K. Polysaccharide as renewable responsive biopolymer for in situ gel in the delivery of drug through ocular route. Int. J. Biol. Macromol. 2020, 150, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Su, J.S.T.; Tan, C.L.; Chin, W.Y.; Yip, K.Y. Advancement on sustained antiviral ocular drug delivery for herpes simplex virus keratitis: Recent update on potential investigation. Pharmaceutics 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.; Beg, S.; Kohli, K. Target Strategies for Drug Delivery Bypassing Ocular Barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Campos, P.M.; Petrilli, R.; Lopez, R.F.V. The prominence of the dosage form design to treat ocular diseases. Int. J. Pharm. 2020, 586, 119577. [Google Scholar] [CrossRef]
- Garg, A.; Garg, S.; Kumar, M.; Kumar, S.; Shukla, A.K.; Kaushik, S.P.C. Applications of natural polymers in mucoadhesive drug delivery: An overview. Adv. Pharm. J. 2018, 3, 38–42. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Yeo, E.; Yew Chieng, C.J.; Choudhury, H.; Pandey, M.; Gorain, B. Tocotrienols-rich naringenin nanoemulgel for the management of diabetic wound: Fabrication, characterization and comparative in vitro evaluations. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Sudheer, P. Mucoadhesive polymers: A review. J. Pharm. Res. 2018, 17, 47–55. [Google Scholar]
- Chatterjee, B.; Amalina, N.; Sengupta, P.; Mandal, U.K. Mucoadhesive polymers and their mode of action: A recent update. J. Appl. Pharm. Sci. 2017, 7, 195–203. [Google Scholar]
- Asati, S.; Jain, S.; Choubey, A. Bioadhesive or Mucoadhesive Drug Delivery System: A Potential Alternative to Conventional Therapy. J. Drug Deliv. Ther. 2019, 9, 858–867. [Google Scholar]
- Rhushikesh, S.; Suresh, S. A Review on Mucoadhesive Drug Delivery System. Int. J. Res. Anal. Rev. 2020, 7, 793–808. [Google Scholar]
- Tandel, H.T.; Parmar, H.K.; Pandya, K.K.; Pardasani, L.J.; Panchal, V.S. A Systematic Review on Mucoadhesive Drug Delivery System. World J. Pharm. Res. 2017, 6, 337–366. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Sharma, D.; Garg, R. Review on Mucoadhesive Drug Delivery System with Special Emphasis on Buccal Route: An Important Tool in Designing of Novel Controlled Drug Delivery System for the Effective Delivery of Pharmaceuticals. J. Dev. Drugs 2017, 06. [Google Scholar]
- Moustafa, M.A.; El-Refaie, W.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Gel in core carbosomes as novel ophthalmic vehicles with enhanced corneal permeation and residence. Int. J. Pharm. 2018, 546, 166–175. [Google Scholar] [CrossRef]
- El-Bary, A.A.; Ibrahim, H.K.; Haza’a, B.S.; Al Sharabi, I. Formulation of sustained release bioadhesive minitablets containing solid dispersion of levofloxacin for once daily ocular use. Pharm. Dev. Technol. 2019, 24, 824–838. [Google Scholar] [CrossRef]
- Morsi, N.; Ibrahim, M.; Refai, H.; El Sorogy, H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur. J. Pharm. Sci. 2017, 104, 302–314. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.E.Y.; Regdon, G.; Hamedelniel, E.I.; Sovány, T. Review of recently used techniques and materials to improve the efficiency of orally administered proteins/peptides. DARU J. Pharm. Sci. 2020, 28, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Park, C.G.; Kim, Y.K.; Kim, S.N.; Lee, S.H.; Huh, B.K.; Park, M.A.; Won, H.; Park, K.H.; Choy, Y. Bin Enhanced ocular efficacy of topically-delivered dorzolamide with nanostructured mucoadhesive microparticles. Int. J. Pharm. 2017, 522, 66–73. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Wolf, J.D.; Atyabi, F.; Bernkop-Schnürch, A. In situ gelling and mucoadhesive polymers: Why do they need each other? Expert Opin. Drug Deliv. 2018, 15, 1007–1019. [Google Scholar] [CrossRef]
- Khattab, A.; Marzok, S.; Ibrahim, M. Development of optimized mucoadhesive thermosensitive pluronic based in situ gel for controlled delivery of Latanoprost: Antiglaucoma efficacy and stability approaches. J. Drug Deliv. Sci. Technol. 2019, 53, 101134. [Google Scholar] [CrossRef]
- Sathyanarayana, S.D.; Rompicherla, N.C.; Vadakkepushpakath, A.N.; Nayak, P. Development of thermosensitive ophthalmic in situ gels of bimatoprost for glaucoma therapy. Indian J. Pharm. Educ. Res. 2020, 54, S154–S162. [Google Scholar] [CrossRef]
- Wei, Y.; Li, C.; Zhu, Q.; Zhang, X.; Guan, J.; Mao, S. Comparison of thermosensitive in situ gels and drug-resin complex for ocular drug delivery: In vitro drug release and in vivo tissue distribution. Int. J. Pharm. 2020, 578, 119184. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Chen, J.; Li, Y.; Huang, J.; Huang, Z.; Huang, Y.; Pan, X.; Wu, C. Thermo-sensitive gel in glaucoma therapy for enhanced bioavailability: In vitro characterization, in vivo pharmacokinetics and pharmacodynamics study. Life Sci. 2018, 212, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.; Koland, M. Development and evaluation of an in situ thermogelling system of ofloxacin for controlled ocular delivery. Asian J. Pharm. Clin. Res. 2019, 12, 567–570. [Google Scholar] [CrossRef]
- Saher, O.; Ghorab, D.M.; Mursi, N.M. Preparation and in vitro/in vivo evaluation of antimicrobial ocular in situ gels containing a disappearing preservative for topical treatment of bacterial conjunctivitis. Pharm. Dev. Technol. 2016, 21, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Sawant, D.; Dandagi, P.M.; Gadad, A.P. Formulation and evaluation of sparfloxacin emulsomes-loaded thermosensitive in situ gel for ophthalmic delivery. J. Sol-Gel Sci. Technol. 2016, 77, 654–665. [Google Scholar] [CrossRef]
- Yassir Al-Bazzaz, F.; Al-Kotaji, M. Ophthalmic in-situ sustained gel of ciprofloxacin, preparation and evaluation study. Int. J. Appl. Pharm. 2018, 10, 153–161. [Google Scholar] [CrossRef]
- Abdelkader, H.; Mansour, H.F. Comparative studies for ciprofloxacin hydrochloride pre-formed gels and thermally triggered (in situ) gels: In vitro and in vivo appraisal using a bacterial keratitis model in rabbits. Pharm. Dev. Technol. 2015, 20, 410–416. [Google Scholar] [CrossRef]
- Üstündağ Okur, N.; Yozgatlı, V.; Evren Okur, M.; Yoltaş, A.; Siafaka, P.I. Improving therapeutic efficacy of voriconazole against fungal keratitis: Thermo-sensitive in situ gels as ophthalmic drug carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 323–333. [Google Scholar] [CrossRef]
- Ustundag Okur, N.; Yozgatli, V.; Senyigit, Z. Formulation and detailed characterization of voriconazole loaded in situ gels for ocular application. Ankara Univ. Eczac. Fak. Derg. 2020, 44, 33–49. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Li, N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1282–1287. [Google Scholar] [CrossRef] [Green Version]
- Mahboobian, M.M.; Mohammadi, M.; Mansouri, Z. Development of thermosensitive in situ gel nanoemulsions for ocular delivery of acyclovir. J. Drug Deliv. Sci. Technol. 2020, 55, 101400. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, G.H.; Zhang, L.Y.; Zhang, Z.; Liao, Y.H.; Liu, X.T. Effect of nintedanib thermo-sensitive hydrogel on neovascularization in alkali burn rat model. Int. J. Ophthalmol. 2020, 13, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ Okur, N.; Yozgatli, V.; Evren Okur, M. In vitro–in vivo evaluation of tetrahydrozoline-loaded ocular in situ gels on rabbits for allergic conjunctivitis management. Drug Dev. Res. 2020, 81, 716–727. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, T.; Zhang, D.; He, W.; Wang, S.; Jiang, T. Disulfiram thermosensitive in-situ gel based on solid dispersion for cataract. Asian J. Pharm. Sci. 2018, 13, 527–535. [Google Scholar] [CrossRef]
- Sandeep, D.S.; Charyulu, R.N.; Narayanan, V.A. Smart in situ gels for Glaucoma-An Overview. Int. J. Pharm. Sci. Rev. Res. 2018, 50, 94–100. [Google Scholar]
- Xu, L.; Wang, X.; Wu, M. Topical medication instillation techniques for glaucoma. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.; Bhowmick, B.; Sarkar, G.; Rana, D.; Bain, M.K.; Bhowmik, M.; Chattopadhyay, D. Effect of methyl cellulose on gelation behavior and drug release from poloxamer based ophthalmic formulations. Int. J. Biol. Macromol. 2015, 72, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, K.N.G.; Prabagaran, S.R. Ocular bacterial infections: Pathogenesis and diagnosis. Microb. Pathog. 2020, 145, 104206. [Google Scholar] [CrossRef]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P. Common eye infections. Aust. Prescr. 2018, 41, 67–72. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Ma, Z.; Yin, Y.; Sun, L.; Yang, L.; Zheng, Y. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb. Pathog. 2020, 138, 103802. [Google Scholar] [CrossRef]
- Thakkar, R.; Patil, A.; Mehraj, T.; Dudhipala, N.; Majumdar, S. Updates in Ocular Antifungal Pharmacotherapy: Formulation and Clinical Perspectives. Curr. Fungal Infect. Rep. 2019, 13, 45–58. [Google Scholar] [CrossRef]
- Hatano, M.; Tokuda, K.; Kobayashi, Y.; Yamashiro, C.; Uchi, S.-H.; Kobayashi, M.; Kimura, K. Inhibitory effect of nintedanib on VEGF secretion in retinal pigment epithelial cells induced by exposure to a necrotic cell lysate. PLoS ONE 2019, 14, e0218632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güven, U.M.; Berkman, M.S.; Şenel, B.; Yazan, Y. Development and in vitro/in vivo evaluation of thermo-sensitive in situ gelling systems for ocular allergy. Braz. J. Pharm. Sci. 2019, 55, e17511. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef]
- Sharif, Z.; Sharif, W. Corneal neovascularization: Updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [PubMed]
- Fursova, A.Z.; Rumyantseva, Y.V.; Kolosova, N.G.; Kedik, S.A.; Panov, A.V.; Tyukova, V.S. Disulfiram inhibits cataract development in OXYS rats. Adv. Gerontol. 2016, 6, 212–216. [Google Scholar] [CrossRef]
- Lou, J.; Hu, W.; Tian, R.; Zhang, H.; Jia, Y.; Zhang, J.; Zhang, L. Optimization and evaluation of a thermoresponsive ophthalmic in situ gel containing curcumin-loaded albumin nanoparticles. Int. J. Nanomed. 2014, 9, 2517–2525. [Google Scholar]
- Deguchi, S.; Ogata, F.; Yamaguchi, M.; Minami, M.; Otake, H.; Kanai, K.; Kawasaki, N.; Nagai, N. In Situ Gel Incorporating Disulfiram Nanoparticles Rescues the Retinal Dysfunction via ATP Collapse in Otsuka Long-Evans Tokushima Fatty Rats. Cells 2020, 9, 2171. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Vyas, S.P. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.; Prabhu, P. Formulation and Evaluation of Stimuli-Sensitive Hydrogels of Timolol Maleate and Brimonidine Tartrate for the Treatment of Glaucoma. Int. J. Pharm. Investig. 2014, 4, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, X.; Li, J.; Pi, J.; Qi, D.; Guo, P.; Li, N.; Wu, Y.; Liu, Z. Increasing efficacy and reducing systemic absorption of brimonidine tartrate ophthalmic gels in rabbits. Pharm. Dev. Technol. 2018, 23, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Barse, R.K.; Tagalpallewar, A.A.; Kokare, C.R.; Sharma, J.P.; Sharma, P.K. Formulation and ex vivo–in vivo evaluation of pH-triggered brimonidine tartrate in situ gel for the glaucoma treatment using application of 32 factorial design. Drug Dev. Ind. Pharm. 2018, 44, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Bharath, S.; Hariharan, S.; Karuppaiah, A.; Siram, K.; Santhanam, R.; Veintramuthu, S. Development and evaluation of a ph triggered in situ ocular gel of brimonidine tartrate. J. Res. Pharm. 2020, 24, 416–424. [Google Scholar] [CrossRef]
- Kouchak, M.; Mahmoodzadeh, M.; Farrahi, F. Designing of a pH-Triggered Carbopol®/HPMC In Situ Gel for Ocular Delivery of Dorzolamide HCl: In Vitro, In Vivo, and Ex Vivo Evaluation. AAPS PharmSciTech 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sheshala, R.; Ming, N.J.; Kok, Y.Y.; Singh, T.R.R.; Dua, K. Formulation and characterization of pH induced in situ gels containing sulfacetamide sodium for ocular drug delivery: A combination of Carbopol®/HPMC polymer. Indian J. Pharm. Educ. Res. 2019, 53, 654–662. [Google Scholar] [CrossRef] [Green Version]
- Sethuraman, N.; Balu, A.; Selvaraj, R.; Johnson, T.; Seetharaman, S. Formulation and characterization of ph based stimuli sensitive based hydrogels for the treatment of ocular infection. J. Young Pharm. 2018, 10, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Anand, A.; Banarasi, B.; Mamman, K.M. Concomitant delivery of Ofloxacin and Diclofenac sodium via pH triggered in-situ ophthalmic gel: In-vitro and in-vivo consideration. Sch. Res. Libr. 2016, 8, 233–242. [Google Scholar]
- Prasanth, V.; Parambi, D.G.T.; Ranjan, S. Formulation and evaluation of in situ ocular gel of levofloxacin. J. Drug Deliv. Ther. 2017, 7, 68–73. [Google Scholar] [CrossRef]
- Noreen, S.; Ghumman, S.A.; Batool, F.; Ijaz, B.; Basharat, M.; Noureen, S.; Kausar, T.; Iqbal, S. Terminalia arjuna gum/alginate in situ gel system with prolonged retention time for ophthalmic drug delivery. Int. J. Biol. Macromol. 2020, 152, 1056–1067. [Google Scholar] [CrossRef]
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221. [Google Scholar] [CrossRef]
- Upadhayay, P.; Kumar, M.; Pathak, K. Norfloxacin loaded pH triggered nanoparticulate in-situ gel for extraocular bacterial infections: Optimization, ocular irritancy and corneal toxicity. Iran. J. Pharm. Res. 2016, 15, 3–22. [Google Scholar] [PubMed]
- Jaiswal, M.; Kumar, M.; Pathak, K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surfaces B Biointerfaces 2015, 130, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.V.; Chetty, C.M.; Reddy, Y.D.; Ugandar, R.E.; Gladiola, B.D. Formulation and in vitro Characterization of Ocular in situ Gels of Valcyclovir. J. Pharm. Sci. Res. 2019, 11, 2974–2979. [Google Scholar]
- Kapadia, R.; Khambete, H.; Katara, R.; Ramteke, S. A Novel Approach for Ocular Delivery of Acyclovir Via Niosomes Entrapped In Situ Hydrogel System. J. Pharm. Res. 2009, 2, 745–751. [Google Scholar]
- Shetty, G.N.; Charyulu, R.N. A study on stability and in vivo drug release of naphazoline and antazoline in situ gelling system for ocular delivery. Int. J. Pharma Bio Sci. 2013, 4, 161–171. [Google Scholar]
- Shaikh, A.; Farheen, T.; Shahi, S. pH trigger in situ gel: Formulation developmenr and evaluation of in situ ophthalmic gel of olopatadine hydrochloride. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 180–185. [Google Scholar]
- Dawood, B.Y.; Kassab, H.J. Preparation and in vitro evaluation of naproxen as a pH sensitive ocular in-situ gel. Int. J. Appl. Pharm. 2019, 11, 37–44. [Google Scholar]
- Ni, X.; Guo, Q.; Zou, Y.; Xuan, Y.; Mohammad, I.; Ding, Q.; Hu, H. Preparation and characterization of bear bile-loaded pH sensitive in-situ gel eye drops for ocular drug delivery. Iran. J. Basic Med. Sci. 2020, 23, 922–929. [Google Scholar]
- Wu, H.; Liu, Z.; Peng, J.; Li, L.; Li, N.; Li, J.; Pan, H. Design and evaluation of baicalin-containing in situ pH-triggered gelling system for sustained ophthalmic drug delivery. Int. J. Pharm. 2011, 410, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.Y.; Tan, J.Y.P.; Choudhury, H.; Pandey, M.; Sisinthy, S.P.; Gorain, B. Development and optimization of chitosan coated nanoemulgel of telmisartan for intranasal delivery: A comparative study. J. Drug Deliv. Sci. Technol. 2021, 62, 102341. [Google Scholar] [CrossRef]
- Hoffmann, U.; Kuno, S.; Franke, G.; Fusch, C.; Haas, J.P. Adrenoceptor agonist poisoning after accidental oral ingestion of brimonidine eye drops. Pediatr. Crit. Care Med. 2004, 5, 282–285. [Google Scholar] [CrossRef]
- Wilson, C.G.; Zhu, Y.P.; Frier, M.; Rao, L.S.; Gilchrist, P.; Perkins, A.C. Ocular contact time of a carbomer gel (GelTears) in humans. Br. J. Ophthalmol. 1998, 82, 1131–1134. [Google Scholar] [CrossRef]
- Vass, C.; Hirn, C.; Sycha, T.; Findl, O.; Sacu, S.; Bauer, P.; Schmetterer, L. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Kumar, S.; Himmelstein, K.J. Modification of in situ gelling behavior of carbopol solutions by hydroxypropyl methylcellulose. J. Pharm. Sci. 1995, 84, 344–348. [Google Scholar] [CrossRef]
- Noveon® Polycarbophil—Mucoadhesive / Mucoadhesion Excipient Polymer—Lubrizol. Available online: https://www.lubrizol.com/Health/Pharmaceuticals/Excipients/Noveon-Polycarbophil (accessed on 23 August 2020).
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is chitosan mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur. J. Pharm. Biopharm. 2004, 57, 251–261. [Google Scholar] [CrossRef]
- Manikandan, P.; Abdel-Hadi, A.; Randhir Babu Singh, Y.; Revathi, R.; Anita, R.; Banawas, S.; Bin Dukhyil, A.A.; Alshehri, B.; Shobana, C.S.; Panneer Selvam, K.; et al. Fungal keratitis: Epidemiology, rapid detection, and antifungal susceptibilities of fusarium and aspergillus isolates from corneal scrapings. Biomed Res. Int. 2019, 2019, 6395840. [Google Scholar] [CrossRef] [Green Version]
- Bettini, R.; Catellani, P.L.; Santi, P.; Massimo, G.; Peppas, N.A.; Colombo, P. Translocation of drug particles in HPMC matrix gel layer: Effect of drug solubility and influence on release rate. J. Control. Release 2001, 70, 383–391. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Devel. Ther. 2018, 12, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhalerao, H.; Koteshwara, K.B.; Chandran, S. Brinzolamide Dimethyl Sulfoxide In Situ Gelling Ophthalmic Solution: Formulation Optimisation and In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2020, 21, 1–15. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Q.M.; Wang, X.; Liu, D.; Zhang, W.; Ye, T.; Yang, X.; Pan, W. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int. J. Pharm. 2015, 480, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, K.; Kant, S.; Pandit, J.K. Therapeutic Effectiveness in the Treatment of Experimental Bacterial Keratitis with Ion-activated Mucoadhesive Hydrogel. Ocul. Immunol. Inflamm. 2016, 24, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, H.; Koteshwara, K.B.; Chandran, S. Levofloxacin Hemihydrate In Situ Gelling Ophthalmic Solution: Formulation Optimization and In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, S.; Chen, H.; Zhang, S.; Yu, S.; Li, Y.; Cui, M.; Pan, W.; Yang, X. A novel ion-activated in situ gelling ophthalmic delivery system based on κ-carrageenan for acyclovir. Drug Dev. Ind. Pharm. 2018, 44, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Shelley, H.; Rodriguez-Galarza, R.M.; Duran, S.H.; Abarca, E.M.; Babu, R.J. In Situ Gel Formulation for Enhanced Ocular Delivery of Nepafenac. J. Pharm. Sci. 2018, 107, 3089–3097. [Google Scholar] [CrossRef]
- Paula, P.C.; Talarico, L.B.; Noseda, M.D.; Silvia, S.M.; Damonte, E.B.; Duarte, M.E.R. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carbohydr. Polym. 2006, 63, 459–465. [Google Scholar]
- Gupta, H.; Velpandian, T.; Jain, S. Ion-and pH-activated novel in-situ gel system for sustained ocular drug delivery. J. Drug Target. 2010, 18, 499–505. [Google Scholar] [CrossRef]
- Gupta, H.; Malik, A.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Physiologically active hydrogel (in situ gel) of sparfloxacin and its evaluation for ocular retention using gamma scintigraphy. J. Pharm. Bioallied Sci. 2015, 7, 195–200. [Google Scholar]
- Ameeduzzafar; Imam, S.S.; Bukhari, S.N.A.; Ali, A. Preparation and evaluation of novel chitosan: Gelrite ocular system containing besifloxacin for topical treatment of bacterial conjunctivitis: Scintigraphy, ocular irritation and retention assessment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 959–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, H.; Aqil, M.; Khar, R.; Ali, A.; Bhatnagar, A.; Mittal, G. An alternative in situ gel-formulation of levofloxacin eye drops for prolong ocular retention. J. Pharm. Bioallied Sci. 2015, 7, 9–14. [Google Scholar] [PubMed]
- Ranch, K.; Patel, H.; Chavda, L.; Koli, A.; Maulvi, F.; Parikh, R.K. Development of in situ ophthalmic gel of dexamethasone sodium phosphate and chloramphenicol: A viable alternative to conventional eye drops. J. Appl. Pharm. Sci. 2017, 7, 101–108. [Google Scholar]
- Pawar, P.; Kashyap, H.; Malhotra, S.; Sindhu, R. Hp-β-CD-voriconazole in situ gelling system for ocular drug delivery: In vitro, stability, and antifungal activities assessment. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Ramyadevi, D.; Sandhya, P. Dual sustained release delivery system for multiple route therapy of an antiviral drug. Drug Deliv. 2014, 21, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef]
- Krishnatreyya, H.; Hazarika, H.; Saha, A.; Mandal, S.; Bora, N.S.; Kishor, S.; Bhutia, Y.D.; Goyary, D.; Karmakar, S.; Chattopadhyay, P. Amelioration from the ocular irritant Capsaicin: Development and assessment of a Capsazepine in situ gel system for ocular delivery. Expert Opin. Drug Deliv. 2020, 17, 863–880. [Google Scholar] [CrossRef]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and optimization of naringenin-loaded chitosan-coated nanoemulsion for topical therapy in wound healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef]
- Azari, A.A.; Barney, N.P. Conjunctivitis: A systematic review of diagnosis and treatment. J. Am. Med. Assoc. 2013, 310, 1721–1729. [Google Scholar] [CrossRef]
- Conjunctivitis (Pink Eye). 2019. Available online: https://www.mayoclinic.org/diseases-conditions/pink-eye/symptoms-causes/syc-20376355#:~:text=Pink%20eye%20(conjunctivitis)%20is%20an,%2C%20they’re%20more%20visible (accessed on 25 August 2020).
- Xu, X.; Weng, Y.; Xu, L.; Chen, H. Sustained release of avastin® from polysaccharides cross-linked hydrogels for ocular drug delivery. Int. J. Biol. Macromol. 2013, 60, 272–276. [Google Scholar] [CrossRef]
- Thomas, P.A.; Kaliamurthy, J. Mycotic keratitis: Epidemiology, diagnosis and management. Clin. Microbiol. Infect. 2013, 19, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Md Ali, S. Asthma and Allergic Conjunctivitis. Available online: http://pendidikanpesakit.myhealth.gov.my/en/asthma-and-allergic-conjunctivitis/ (accessed on 15 August 2020).
- Solomonidou, D.; Cremer, K.; Krumme, M.; Kreuter, J. Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films. J. Biomater. Sci. Polym. Ed. 2001, 12, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Prajna, N.V. Topical Fluoroquinolones: Current Perspectives. Delhi J. Ophthalmol. 2015, 25, 267–271. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Agarwal, K.L.; Mehta, N.; Namdev, A.; Gupta, A.K. In-Situ Gel Formation for Ocular Drug Delivery System an Overview. Asian J. Biomed. Pharm. Sci. 2011, 1, 1–7. [Google Scholar]
- Final report on the safety assessment of carbomers-934, -910, -934P, -940, -941, and -962. Sage Publ. 1982, 109–141.
- Krenzer, K.L.; Zhang, J.Z.; Coffey, M.J.; Richardson, M.E. Safety of repeated topical ocular administration of a polycarbophil-based formulation in several models of ocular surgery in rabbits. J. Cataract Refract. Surg. 2012, 38, 696–704. [Google Scholar] [CrossRef]
- Fernández-Ferreiro, A.; González Barcia, M.; Gil-Martínez, M.; Vieites-Prado, A.; Lema, I.; Argibay, B.; Blanco Méndez, J.; Lamas, M.J.; Otero-Espinar, F.J. In vitro and in vivo ocular safety and eye surface permanence determination by direct and Magnetic Resonance Imaging of ion-sensitive hydrogels based on gellan gum and kappa-carrageenan. Eur. J. Pharm. Biopharm. 2015, 94, 342–351. [Google Scholar] [CrossRef]
- Shah, H.R.; Reichel, E.; Busbee, B.G. A novel lidocaine hydrochloride ophthalmic gel for topical ocular anesthesia. Local Reg. Anesth. 2010, 3, 57–63. [Google Scholar]
- U.S. Food & Drug Administration Akten. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022221_akten_toc.cfm (accessed on 25 July 2020).
- U.S. Food & Drug Administration Zirgan. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022211_zirgan_toc.cfm (accessed on 25 July 2020).
- Kaufman, H.E.; Haw, W.H. Ganciclovir ophthalmic gel 0.15%: Safety and efficacy of a new treatment for herpes simplex keratitis. Curr. Eye Res. 2012, 37, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.Y.; Hong, B.Y. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: Background, effectiveness, tolerability, safety, and future applications. Ther. Clin. Risk Manag. 2014, 10, 665–681. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Gong, L.; Sun, X.-H.; Zhao, N.-Q.; Chen, W.; Yuan, H.-P.; Shao, Y.; Gao, M.-H.; Tang, H. Effectiveness and safety of 0.15% ganciclovir in situ ophthalmic gel for herpes simplex keratitis -a multicenter, randomized, investigator-masked, parallel group study in Chinese patients. Drug Des. Devel. Ther. 2013, 7, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demailly, P.; Allaire, C.; Trinquand, C. Ocular hypotensive efficacy and safety of once daily carteolol alginate. Br. J. Ophthalmol. 2001, 85, 921–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, K.; Walters, T.; DaVanzo, R.; Lindstrom, R.L. A randomized double-masked study to compare the ocular safety, tolerability, and efficacy of bromfenac 0.075% compared with vehicle in cataract surgery subjects. Clin. Ophthalmol. 2016, 10, 2311–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Food & Drug Administration BromSite. 1997. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/206911Orig1s000TOC.cfm (accessed on 10 September 2020).








| Objective | Disease/Drug | Types of Stimuli/Polymer Used | Membrane/ Cell Line/ Animal Model | Outcome | Source |
|---|---|---|---|---|---|
| To develop thermo-responsive in situ gel for ocular delivery of latanoprost. | Glaucoma/latanoprost | Temperature/Pluronic F-127, Pluronic F-68, HPMC E5, and HPMC E50 | New Zealand White (NZW) rabbits | Gelation temperature: 34.3 °C pH: 6.53 Mucoadhesive properties: Mucoadhesion: 0.06 mJ Maximum detachment force: 0.09 N Ex vivo permeation: 53.5 μg/cm2 In vivo antiglaucoma efficacy: the optimized formula showed a 2.9-fold higher AUC value than Ioprost eyedrop. In vivo ocular irritation test: no sign of corneal tissue damage. | [24] |
| To prepare and investigate sustained-release thermo-responsive in situ gel of bimatoprost. | Glaucoma/bimatoprost | Thermo-responsive/ poloxamer 188, poloxamer 407, and HPMC K4M | Chorioallantoic membrane (CAM) | Gelation temperature: 37.5 °C pH: 7.3 ± 0.31 Gelling capacity: +++ (rapid gelation and remained for 10 h) Viscosity: 7.65–82.24 cps (sol) 238.16–2335.52 cps (gel) Rheology: pseudoplastic In vitro release: 80.37–86.63% of drug released for 10 h. Ex vivo permeation: 67.45% of the drug was permeated up to 12 h. In vitro ocular irritation test (HET-CAM): irritation score of 0.04 indicating no irritation. | [25] |
| To evaluate and compare the effectiveness of betaxolol hydrochloride loaded thermo-responsive in situ gels with ophthalmic resin suspension. | Glaucoma/betaxolol hydrochloride | Thermo-responsive/poloxamer 407 + HPMC (poloxamer-based gel) MC+PEG 4000 (MC-based gel) | Rabbits | Poloxamer-based in situ gel Gelation temperature: 29.16 ± 0.21 °C Gel capacity: 9.48 ± 0.29 min In vitro drug release: Increasing P407 concentration from 18% to 22% decreased the release percentage from 85% to 53% in 6 h. Sustained release In vivo distribution: the AUC and MRT of were 2-fold higher than the commercial resin suspension (Betoptic S® eye drops). MC-based in situ gel Gelation temperature: 34 °C In vitro drug release: increasing PEG4000 concentration from 3% to 7% decreased the release percentage from 68% to 43% in 4 h. Sustained release In vivo distribution: the AUC and MRT of both in situ formulations were 2-fold higher than the commercial resin suspension (Betoptic S® eye drops). | [26] |
| To prepare thermo-responsive in situ gel to improve the bioavailability of timolol maleate. | Glaucoma/timolol maleate | Thermo-responsive/poloxamer 407 and poloxamer 188 | NZW Rabbits | Gelation temperature: 32 °C pH: 6.70 ± 0.10 Rheology: pseudoplastic In vivo pharmacokinetics: MRT of TM in situ gel was 1.6-fold higher than TM eye drops. AUC of TM in situ gel was higher than TM eye drops. In vivo pharmacodynamics: TM loaded in situ gel exhibited a more significant IOP lowering effect compared to TM eye drops. Histopathological study: the formulation was well biocompatible, and no irritation was observed. | [27] |
| To fabricate controlled drug release thermosensitive in situ gel of ofloxacin. | Bacterial conjunctivitis/ofloxacin | Thermo-responsive/ poloxamer 407 HPMC, and PVA (mucoadhesive polymers) | - | Gelation temperature: <32 °C pH: 6.6–7.2 In vitro drug release: initial burst effect was observed. The prepared formulations demonstrated sustained drug release for 9 h. In vitro antimicrobial studies: all in situ formulations are effective in antimicrobial but the ZOI was smaller compared to the marketed formulation (0.3% w/v ofloxacin eye drops). | [28] |
| To formulate levofloxacin hemihydrate loaded ocular in situ gel. | Bacterial conjunctivitis/levofloxacin | Thermo-responsive emulsomal/Pluronic F-127 and Pluronic F-68 | Rabbits | Gelation temperature: 32.75 ± 0.35 °C Rheology: non-linear plastic flow In vitro drug release: optimized formulation exhibited prolonged drug release up to 12 h. Optimized formulation exhibited prolonged drug release up to 12 h. No change in release pattern over 3 months. In vivo microbiological susceptibility: optimized formulation Retention time: 12 h Inhibition zone: decreased after 8 h Levoxin® eye drops Retention time: 4 h. No inhibition zone was observed after 4 h. In vivo ocular irritation test: non-irritant. | [29] |
| To synthesize thermo-responsive emulsomal in situ gel to enhance the therapeutic efficacy of sparfloxacin. | Bacterial conjunctivitis/sparfloxacin | Thermo-responsive/ Pluronic F-127, and Pluronic F-68 | Rabbits | Gelation temperature: ≈35 °C pH: 7.4 Gelling capacity: +++ (gelation occurred within 2–3 s and remained for >2 h.) Viscosity: Before gelation (25 ± 1 °C): 107.46 ± 6.74 cps After gelation: (37 ± 1 °C): 1669 ± 13.89 cps In vitro release: 75.274 ± 0.17% of drug released at 12 h. Ex vivo permeation: 66.203 ± 2.39% of the drug was permeated through goat cornea. In vivo antimicrobial efficacy: the symptoms were reduced within 4–5 days with the use of optimized formulation. In vivo ocular irritation test: non-irritant. | [30] |
| To fabricate and investigate the sustained release in situ gel of ciprofloxacin. | Bacterial keratitis/ciprofloxacin | Thermo-responsive/poloxamer 407 and HPMC | Rabbits | Gelation temperature: 30 ± 0.01 °C pH: 4.57 Gelling capacity: +++ (rapid gelation and remained for an extended period) Rheology: pseudoplastic In vitro drug release: 83% of drug release from the optimized formulation in 8 h while the nearly total amount of drug release from marketed eye drop solution in 10 min. In vivo elimination study: elimination of optimized in situ gel was within 1 h. In vivo ocular irritation (Draize test): non-irritant. | [31] |
| To compare the characterisation and evaluation of pre-formed gel and in situ gel for delivery of ciprofloxacin. | Bacterial keratitis/ciprofloxacin | Thermo-responsive/MC and CMC (preformed cellulose-based gel) Pluronic F-127 (in situ gel) | Rabbits | Pluronic-based in situ gel Gelation temperature: 35 °C pH: 5.0 Rheology: pseudoplastic In vitro drug release: 20%.h−0.5 In vivo study: faster corneal healing was observed (<5 days) compared to ciprofloxacin solution. Histological examination: significant stromal edema MC-based pre-formed gel pH: 5.1 Viscosity: 10,000 mPa.s In vitro drug release: 33%.h−0.5 In vivo study: faster corneal healing was observed (<5 days) compared to ciprofloxacin solution. Histological examination: minimal stromal edema CMC-based pre-formed gel pH: 6.5 Viscosity: 8300 mPa.s In vitro drug release: 31%.h−0.5 In vivo study: faster corneal healing was observed (<5 days) compared to ciprofloxacin solution. Histological examination: minimal stromal edema | [32] |
| To fabricate and evaluate voriconazole-loaded thermo-responsive in situ gel. | Fungal keratitis/voriconazole | Thermo-responsive/poloxamer 188, poloxamer 407 and/or CMC | NZW rabbits | Gelation temperature: 34.13 ± 0.32 °C pH: 6.80 ± 0.03 In vitro drug release: 83.5% of drug released from the formulation at 12 h indicating sustained release. Ex vivo permeation: 42.92 ± 6.81% of drug permeated through cornea after 24 h. Voriconazole concentration in tear (in vivo): the drug concentration in tear fluid was higher for optimized in situ gel compared to the solution. In vivo Draize Rabbit Eye test: irritation score <1, non-irritant. | [33] |
| To synthesize, characterize, and investigate the sustained release in situ formulations of voriconazole. | Fungal keratitis/voriconazole | Thermo-responsive/poloxamer 188, poloxamer 407, and poloxamer 388 | - | Gelation temperature: 32.200 ± 0.265 °C pH: 6.357 ± 0.006 Gelling capacity: 1.233 ± 0.058 s Adhesiveness: 0.558 ± 0.005 g⋅s at 25 °C 5.600 ± 1.024 g⋅s at 32 °C Rheology: pseudoplastic In vitro drug release: prolonged drug release up to 24 h. | [34] |
| To prepare PNIPAAM–hyaluronic acid-based in situ gel to deliver ketoconazole. | Fungal keratitis/ketoconazole (KCL) | Thermo-responsive/PNIPAAM-hyaluronic acid | NZW rabbits | Gelation temperature: 33 °C pH: 6.0–7.5 In vitro drug release: The drug release of in situ gel (30% drug released at 2 h) slower than in free drug (95% drug released at 2 h). In vivo antimicrobial study: 91.7% and 66.7% of cure rate were obtained in ketoconazole in situ gel and commercial ketoconazole eye drops, respectively. In vivo ocular irritation test: irritation score of zero was obtained for developed formulation. | [35] |
| To develop and evaluate thermo-responsive in situ gel nanoemulsions in delivering acyclovir. | Herpes simplex keratitis/acyclovir (ACV) | Thermo-responsive nanoemulsion/ Triacetin, and Transcutol® P (nanoemulsion) poloxamer 407 and poloxamer 188 (in situ) | NZW rabbits (in vivo ocular irritation test) and CAM (in vitro ocular irritation test) | Gelation temperature: 30.9 °C pH: 4.58 ± 0.068 Viscosity: 103.03 ± 4.68 mPa.s In vitro drug release efficiency: 80.78 ± 1.82% The optimized formulations displayed a sustained release manner. Ex vivo permeation: the permeation of ACV was 2.83-fold higher in optimized formulation compared to ACV solution. In vivo ocular irritation test: minimal conjunctival redness but disappeared after 2 h of administration. In vitro ocular irritation test (HET-CAM): cumulative score of 0.33 ± 0.58 indicating non-irritant | [36] |
| To evaluate the efficacy of nintedanib thermosensitive hydrogel. | Neovascularization/nintedanib | Thermo-responsive/poloxamer 407 | Rat | Gelation temperature: 37 °C In vivo studies: The CNV area of alkali burn rats was significantly reduced from day 3 to day 14. The CNV area was found to be the lowest in NTH group on day 14 compared to model group, dexamethasone group and normal control group. Immunofluorescence: the developed formulation reduced the expression of VEGFR-2 and CD31 | [37] |
| To fabricate and optimize sustained-release thermosensitive in situ formulations of tetrahydrozoline. | Ocular allergy/tetrahydrozoline (THZ) | Thermo-responsive/poloxamer 407 and poloxamer 188 | NZW rabbits | Gelation temperature: 31.08 ± 0.34 °C pH: 7.010 ± 0.017 Gelling capacity: 1.9 ± 0.1 s Viscosity: 504.2 ± 3.5 cps In vitro drug release: 88.745 ± 8.275% of THZ release from optimized formulation at the end of 24 h. The sustained release was achieved. Ex vivo permeation: 2.035 ± 0.062% of THZ was permeated. Ex vivo penetration: 0.582 ± 0.035% of THZ was detected in corneal tissue. In vivo studies: the concentration of THZ (1.029 μg/mL) in tear sample was higher in optimized formulation compared to THZ marketed product (no drug detected) at 6 h. In vivo ocular irritation: non-irritant | [38] |
| To develop disulfiram solid dispersion thermosensitive in situ gel to enhance corneal permeability and aqueous solubility. | Cataract/disulfiram | Thermo-responsive/poloxamer 407 and poloxamer 188 | NZW rabbits | In vitro corneal permeation: the formulation with the ratio of 1:5 (DSF to poloxamer 188) showed 2.31-fold higher apparent permeability coefficient than DSF eye drops. In vivo precorneal retention study: the formulation successfully prolonged the retention of fluorescence for 30 min. In vivo efficacy: thermosensitive in situ formulation had better anti-cataract efficacy among blank group, model group, DSF suspensions group, and DSFSD suspensions group at day 7 post-administration. In vivo ocular irritation: non-irritant. | [39] |
| Objective | Disease/Drug | Types of Stimuli/Polymer Used | Membrane/ Cell Line/ Animal Model | Outcome | Source |
|---|---|---|---|---|---|
| To develop in situ gel formulation of timolol maleate based on gelling properties of Carbopol and chitosan combination and evaluate reduction of IOP compared with the liposomal and marketed formulation. | Glaucoma/Timolol maleate | pH-responsive/Carbopol and Chitosan | - | pH: 6.00 Gelation pH: 7.4 (STF) Gelation time: +++ (gel immediately and remained extended period) Viscosity: 1040.00 cps (gel) Rheology: pseudoplastic flow In vitro release: cumulative release 60.9% up to 24 h. In vivo distribution: Developed in situ gel showed a 2.4-fold greater AUC compared to marketed eye drops. Developed in situ gel showed a 2.1-fold greater AUC compared to the liposomal formulation. | [56] |
| To investigate the potential of pH-responsive hydrogel containing a combination of timolol maleate and brimonidine tartrate as an ocular drug delivery system to treat glaucoma. | Glaucoma/Timolol Maleate and Brimonidine tartarate | pH-responsive/Carbopol 934P and HPMC | White rabbits | Gelation pH: 7.4 (phosphate buffer) Viscosity: 33.2 cps (pH 4) and 56.3 cps (pH 7.4) In vitro release: cumulative released ≈80% of combination drug up to 8 h. In vivo efficacy: demonstrate 7 h longer IOP reduction compared to marketed eye drops. In vivo ocular irritation study: no sign of corneal damage. | [57] |
| To develop in situ gelling of brimonidine tartrate with a lower concentration (0.05%, 0.1%, and 0.2% w/v) and evaluate its efficacy with eye drops (0.2% w/v) and reduction of systemic absorption. | Glaucoma/Brimonidine tartarate | pH-responsive/Carbopol 974P and HPMC E4M | New Zealand White (NZW) rabbits | pH: 5.93–6.07 Residence time: up to 3 h In vivo efficacy: The lowest concentration (0.05%) showed 1.2 times better IOP reduction compared to 0.2% eye drops. All in situ gel formulation showed low systemic absorption, suggesting fewer systemic side effects. In vivo ocular irritation study: No sign of corneal damage. Histological examination demonstrated that epithelial remained intact. | [58] |
| To evaluate ex vivo and in vivo performance of brimonidine tartrate in situ gel as compared to marketed eye drops. | Glaucoma/Brimonidine tartarate | pH-responsive/Carbopol 974P and HPMC K4M | - | Gelation pH: 7.4 (artificial tears fluid) Gelation time: +++ (gel immediately and remained extended period) Viscosity: 4062 ± 138 cps (gel) In vitro release: cumulative released 90.77% up to 8 h. Ex vivo permeation: Permeability coefficient (P): Permeation flux (J): 76.83% of brimonidine was permeated up to 5 h. In vivo efficacy: demonstrated 3.4 times better IOP reduction and 5 h longer than marketed eye drops. | [59] |
| To develop an in situ gel system that can reside for a long time and prolong the drug release by using Carbopol 940 and HPMC. | Glaucoma/Brimonidine tartarate | pH-responsive/Carbopol 940 and HPMC | NZW rabbits | pH: 4.2 Gelation pH: 6.7 (artificial tears fluids) Gelation time: +++ (gel immediately and remained extended period) Viscosity: 754 ± 0.01 cps (gel) In vitro release: cumulative released 87.46 ± 0.15% up to 8 h. In vivo ocular irritation study: no sign of corneal damage. | [60] |
| To develop pH-responsive in situ gel for ophthalmic delivery of dorzolamide hydrochloride by using Carbopol. | Glaucoma/Dorzolamide hydrochloride | pH-responsive/Carbopol 940 and HPMC F4M | - | pH: 5.16 ± 0.01 Gelation pH: 7.4 (STF) Viscosity: 5.531 p (sol) and 18.374 p (gel) Rheology: pseudoplastic flow Mucoadhesion force: 0.3346 ± 0.001 nm (positive absorbance indicates interaction between formulation with mucin fluid) In vitro release: sustained release up to 24 h. Ex vivo permeability: Permeability coefficient: 1.476 ± 0.138 cm/h Permeation flux: 0.148 ± 0.014 In vivo efficacy: demonstrated a 1.7-fold better IOP reduction compared to marketed eye drops. | [61] |
| To investigate the combination of different grades of Carbopol with HPMC in formulating a sustained release in situ gelling system containing sulfacetamide sodium. | Bacterial conjunctivitis/Sulfacetamide sodium | pH-responsive/Carbopol 940 and HPMC E4M | - | pH: 6.02 Gelation pH: 7.4 (STF) Gelation time: +++ (gel immediately and remained for more than 6–8 h) Viscosity: 1209.00 ± 28.28 cps (gel) In vitro release: cumulative released 90% up to 8 h. In vitro efficacy: demonstrate similar ZOI as marketed eye drops. | [62] |
| To formulate and characterize the pH-responsive-based hydrogels for effective treatment of the infected eye. | Bacterial conjunctivitis and corneal ulcers/Ofloxacin | pH-responsive/Carbopol and HPMC | - | pH: 6.31 Gelation pH: 7.4 (STF) Gelation time: +++ (gel immediately and remained for more than 8–10 h) In vitro release: cumulative released 97.08% up to 8 h. Ex vivo release: 95.57% of ofloxacin was permeated up to 7 h. In vitro efficacy: demonstrate similar ZOI as standard formulation. | [63] |
| To evaluate pH-responsive in situ ophthalmic gel system for concomitant delivery of ofloxacin and diclofenac sodium to facilitate sustained release. | Ocular infection and inflammation/Ofloxacin and Diclofenac | pH-responsive/Carbopol 934P and HPMC | Albino rabbits | pH: 6.5 ± 0.2 Gelation pH: 7.2 (STF) Gelation time: +++ (gel within 90 sec and remained for more than 7-8 h) Viscosity: 35 cps (sol) & 1500 cps (gel) Rheology: pseudoplastic flow In vitro release: cumulative released ≈96% of combination drug up to 8 h. Ex vivo permeability: ≈88% of combination drugs permeated for up to 8 h. In vitro efficacy: Demonstrate similar ZOI as standard formulation. Developed in situ gel showed ≥86% antimicrobial effectiveness than the standard solution. In vivo ocular irritation study: no sign of corneal damage. | [64] |
| To evaluate in situ ocular gel of levofloxacin hydrochloride with increase ocular contact time, enhance the corneal permeability and site-specificity for the better treatment of conjunctivitis and corneal ulceration. | Bacterial conjunctivitis and corneal ulcers/Levofloxacin hydrochloride | pH-responsive/Noveon® AA-1 polycarbophil and HPMC E50LV | Albino rabbits | pH: 6.98 Gelation pH: 7.4 (STF) Gelation time: +++ (gel immediately and remained extended period) Rheology: pseudoplastic flow In vitro release: cumulative released 96.19% up to 8 h. In vitro efficacy: demonstrate similar ZOI as standard formulation. In vivo ocular irritation study: no sign of corneal damage. | [65] |
| To develop a pH-responsive in situ gel system using moxifloxacin hydrochloride, sodium alginate, and Terminalia arjuna gel as gelling agents. | Bacterial conjunctivitis/Moxifloxacin hydrochloride | pH-responsive/Terminalia arjuna and sodium alginate | MCF 7 breast cancer cell line (in vitro cytotoxic) Chorioallantoic membrane (CAM) (ex vivo ocular irritation) Rabbit models (in vivo irritation) | pH: 5.76 Gelation pH: 7.4 (artificial tear solution) Gelation time: ++ (gel immediately and remains for h) Rheology: pseudoplastic flow In vitro release: cumulative released 73.29 ± 1.6% up to 12 h. Ex vivo permeability: demonstrated a 2.3-fold greater amount of permeated drug compared to marketed eye drops. In vitro efficacy: demonstrate similar ZOI as standard formulation. In vitro cytotoxic: displayed negligible cytotoxic effect towards MCF cell lines. Ex vivo ocular irritation study: no toxicity on fertilized eggs. In vivo ocular irritation study: no sign of corneal damage. | [66] |
| To minimize ocular irritation and prolong the pharmacological action of vancomycin via formulation into nanosized spherical niosome loaded into pH-responsive in situ forming gel. | Ocular infection in MRSA patient/Vancomycin | pH-responsive niosome/Carbopol 934P and HPMC (in situ) with Span 60, Tween 40 and cholesterol (niosome) | Albino rabbits | pH: 5.0 ± 0.2 Gelation pH: 7.4 ± 0.1 (STF) Gelation time: +++ (gel immediately and remained for more than 2 h). Viscosity: 60.8 ± 1.4 Pa·s (gel) Rheology: pseudoplastic flow Mucoadhesion force: 5.2 ± 0.5 Pa In vitro release: cumulative released 39.2 ± 3.2% up to 24 h. In vitro efficacy: Demonstrate similar ZOI as vancomycin solution. Displayed a 2-fold more effective MIC compared to vancomycin solution. In vivo efficacy: showed a 2.5-fold effective in lowered MRSA CFU compared to vancomycin solution. In vivo ocular irritation study: No sign of corneal damage. Histological examination demonstrated that epithelial remained intact. | [67] |
| To synergize nanoparticle and in situ gel to obtain a norfloxacin formulation with improved residence time and provide sustained release. | Bacterial infection/Norfloxacin | pH-responsive chitosan nanoparticle/Carbopol 934P | CAM (in vitro irritation) Goat cornea (in vivo irritation) | pH: 5.84 ± 0.63 Gelation time: +++ (gel immediately and remained extended period) Rheology: pseudoplastic flow Mucoadhesion force: In vitro release: cumulative released 88.01 ± 0.48% up to 12 h. In vitro efficacy: demonstrate similar ZOI as marketed eye drops. In vitro ocular irritation study: HET-CAM test showed an irritation score of 0.33 indicated mild irritation. In vivo ocular irritation study: histological examination demonstrated that epithelial remained intact. | [68] |
| To investigate the amphiphilic block copolymer-based polymeric micellar incorporated in situ ocular gel of itraconazole to manage fungal keratitis. | Fungal keratitis/Itraconazole | pH-responsive polymeric micelle/Carbopol 943P (in situ) with Pluronic-F127 (polymeric micelle) | CAM (in vitro irritation) Goat cornea (in vivo irritation) | pH: 3.20 ± 0.4 (sol) and 6.84 ± 0.34 (gel) Gelation pH: 7.4 (STF) Gelation time: 47.3 ± 4.5 s Mucoadhesion force: 6242.03 Ex vivo permeation: Developed micellar in situ gel showed a 5.6-fold higher cumulated drug permeability than itraconazole suspension for up to 8 h. Demonstrated a 3-fold higher permeability flux compared to marketed eye drops. In vitro efficacy: remarkable better ZOI compared to marketed eye drops. In vitro ocular irritation study: HET-CAM test showed an irritation score of 0.67 indicated mild irritation. In vivo ocular irritation study: histological examination demonstrated that epithelial remained intact. | [69] |
| To develop and evaluate valacyclovir ophthalmic in situ gels based on the concept of pH-responsive in situ gelling systems for the prolonged corneal residence time. | Herpes keratitis/Valacyclovir | pH-responsive/Carbopol 940 and HPMC K100M | - | Gelation pH: 7.4 (STF) Gelation time: +++ (gel immediately and remained extended period) Viscosity: 1090 3.54 cps (gel) Rheology: pseudoplastic flow In vitro release: cumulative released 81.12% up to 8 h. | [70] |
| To formulate a niosome entrapped in situ hydrogels for sustained release and prolonged the residence time of the formulation. | Herpes keratitis/Acyclovir | pH-responsive niosome/ Carbopol 934 and MC (in situ) with Span 60 and cholesterol (niosome) | Rabbits | Gelation pH: 7.4 (STF) Viscosity: 298 cps (gel) In vitro release: cumulative released 76.5% up to 16 h. In vivo ocular irritation study: no sign of corneal damage. | [71] |
| To formulate better stability and extensive drug release of naphazoline and antazoline in an in situ gelling systems for ocular allergies. | Allergy conjunctivitis/Naphazoline and Antazoline | pH-responsive/Carbopol 940 and HPMC K4M | Rabbits | pH: 5.75 ± 0.4 Gelation pH: 7.4 (STF) Gelation time: +++ (gel immediately and remained extended period) Viscosity: 10.59 ± 0.34 cps (sol) and 55.96 ± 0.92 cps (gel) Developed in situ gel showed transparent, clear, same gelling capacity and pH value in different storage conditions for 15, 30, and 60 days. In vitro release: cumulative released ≈90% up to 8 h. In vivo distribution: AUC of both drugs was greater compared with marketed eye drops. In vivo ocular irritation study: no sign of corneal damage. | [72] |
| To develop an optimized formulation of in situ ophthalmic gel of olopatadine hydrochloride by using pH-responsive polymer. | Allergy conjunctivitis/Olopatadine hydrochloride | pH-responsive/Carbopol 974 and HPMC E50LV | Rabbits | pH: 7.0-7.5 Gelation pH: 7.4 artificial tear fluid Gelation time: ++ (gel immediately and remained for 3–4 h) Viscosity: 150.45 cps (gel) In vitro and Ex vivo release: released drug 6 h longer than marketed eye drops. In vivo ocular irritation: no sign of corneal damage. | [73] |
| To prepare ocular in situ gel of naproxen using carbomer as a pH-responsive gelling agent with different concentrations of a hydrophilic mucoadhesive polymer. | Post-surgery ocular inflammation/Naproxen | pH-responsive/Carbopol 940 and HPMC K100 | Rabbits | pH: 5.6 ± 0.02 Gelation pH: 7.4 (STF) Gelation time: +++ (gel immediately and remained extended period) Rheology: pseudoplastic flow. HPMC K100 was favored over HPMC K40 in achieving excellent gelling capacity in combination with Carbopol 940 In vitro release: cumulative release properties for up to 3 h. In vivo ocular irritation: no sign of corneal damage. | [74] |
| To develop a bear bile-loaded pH-sensitive in situ gel formulation by using a mixture of Carbopol 974 and HPMC K4M. | Glaucoma, Retinitis pigmentosa, age-related macular degeneration and prevent cataract formation/Tauroursodeoxycholic acid (bear bile) | pH-responsive/Carbopol 974 and HPMC K4M | NZW rabbits | pH: 5.0 ± 0.1 Rheology: pseudoplastic flow Residence time: up to 28 min In vitro release: cumulative release properties up to 160 min. In vivo ocular irritation: No sign of corneal damage. Histological examination demonstrated that epithelial remained intact. | [75] |
| To investigate the correlation between the stability of baicalin and in situ pH-triggered gelling system. | Anti-inflammatory, anti-Chlamydia, anti-bacterial, anti-oxidative, and anti-cataract/Baicalin | pH-responsive/Carbopol 974P and HPMC E4M | NZW rabbits | pH: 5.8 Gelation pH: 6.8 (phosphate buffer) Gelation time: ++ (gel immediately and remained for few h) Rheology: pseudoplastic flow In vitro release: cumulative released ≈95% up to 8 h. In vivo distribution: demonstrated a 6.1-fold greater AUC compared to the control solution. In vivo ocular irritation: no sign of corneal damage. | [76] |
| Objective | Disease/Drug | Types of Stimuli/Polymer used | Membrane/ Cell Line/ Animal Model | Outcome | Source |
|---|---|---|---|---|---|
| To develop a sustained ocular delivery of brinzolamide | Glaucoma/brinzolamide | Ion-activated/deacetylated gellan gum | New Zealand White (NZW) rabbits | pH: 6.32 Gelling capacity: +++ Viscosity: 500 mPa s (solution) and 2200 mPa s (gel) In vitro release: 92% drug release after 12 h. In vivo efficacy: IOP remained lower than baseline after 6 h. In vivo irritation: slight conjunctival hyperaemia in the rabbits. | [87] |
| To evaluate a sustained release ophthalmic formulation of brinzolamide | Glaucoma/brinzolamide | Ion-activated/deacetylated gellan gum | NZW rabbits | pH: 7 Gelling capacity: +++ Mean residence time: 7.4–17.7 h In vitro release: prolonged-release up to 48 h. In vivo efficacy: Decreased IOP for a longer period than marketed product AUC (∆IOP vs. t) 2–9 times higher than marketed product In vivo irritation: no sign of irritation on NZW rabbits. | [88] |
| To prepare timolol maleate liposomes dispersed into ion-sensitive in situ ophthalmic gel to enhance drug bioavailability and histocompatibility | Glaucoma/timolol maleate | Ion-activated/deacetylated gellan gum | NZW rabbits | pH: 7.00 Viscosity: 5.07 ± 0.38 mPa s Apparent permeability: 1.93-fold more than eye drops Mean residence time: longer retention time on cornea observed by fluorescence imaging study. In vitro release: sustained release for ≈20 h. In vivo efficacy: Achieved minimum IOP 1 h after use Decreased IOP for 5 h Least IOP increment after induced by water loading. In vivo irritation: TM L-SG showed no significant difference with 0.9% sodium chloride. | [89] |
| To investigate the therapeutic effectiveness of gatifloxacin ion-activated hydrogel | Bacterial keratitis/gatifloxacin | Ion-activated/gellan gum, sodium alginate | NZW rabbits | High mucoadhesive force In vitro release: retardation of drug release rate In vivo efficacy: Less dosing frequency (twice daily) compared to the marketed formulation (4 times daily) More rapid recovery of bacterial eye infection. In vivo irritation: no sign of irritation on NZW rabbits. | [90] |
| To evaluate levofloxacin hemihydrate in situ gelation ophthalmic solution | Bacterial conjunctivitis/levofloxacin hemihydrate | Ion-activated gellan gum | NZW rabbits | pH: ≈7.00 Gelling time: <10 s In vitro release: extended-release (18–24 h) In vivo efficacy: 2.7-fold higher ocular bioavailability Maintained levofloxacin MIC for 8–12 h In vivo irritation: no irritation to rabbits’ eyes. | [91] |
| To fabricate ion-sensitive in situ hydrogels of natamycin (NT)-loaded bilosomes for improved ocular pharmacotherapy | Fungal keratitis/natamycin loaded bilosomes | Ion-activated/gellan gum | Human corneal limbal epithelial cells (HCLE) | pH: 6.4 ± 0.3 Viscosity: 37.5 ± 2.4 cP (solution) and 514.9 ± 3.8 cP (gel) Adhesiveness: 1.39 ± 0.006 g/s In vitro permeation: 6- to 9-fold enhancement in the transcorneal flux. In vivo efficacy: Higher mean dose in cornea Slow-release of bilosomes into ocular tissues. In vitro irritation: cell viability was the same as the negative control. | [92] |
| Preparation and evaluation of ion-activated in situ gel ophthalmic delivery system of acyclovir based on kappa-carrageenan | Ocular herpes infection/acyclovir- hydroxypropyl-β-cyclodextrin complex | Ion-activated/kappa-carrageenan | NZW rabbits | Rheology: pseudoplastic fluid Gelling capacity: gel rapidly after contacted with tear fluid, maintained for a long time. In vitro release: 80% drug released after 6 h In vitro permeability: 2.16-fold higher apparent permeability. In vivo irritation: no irritation to rabbits’ eyes. | [93] |
| To formulate an in situ gel for improved residence time and sustained release of nepafenac to the corneal surface | Postoperative corneal pain and inflammation/nepafenac-hydroxypropyl-β-cyclodextrin complex | Ion-activated/sodium alginate | - | pH: 5.63–5.73 In vitro release: sustained drug release over 24 h without burst effect. In vitro permeability: 10 times higher drug penetration (p < 0.001) Ex vivo ocular distribution: higher drug retention in cornea, sclera and retina compared to marketed product. | [94] |
| Objective | Disease/Drug | Types of Stimuli/Polymer Used | Membrane/ Cell Line/ Animal Model | Outcome | Source |
|---|---|---|---|---|---|
| To characterize and evaluate the transcorneal permeation profile, ocular irritation, and sterility of timolol maleate in pH- and ion-triggered in situ ocular drug delivery system. | Glaucoma/ Timolol maleate | pH-activated/ chitosan and ion-activated/ gellan gum | Goat cornea HET-CAM test New Zealand White (NZW) rabbits | Gelation pH: 6.5–7.0 Viscosity (sol): 40 ± 2.3 cps Viscosity (gel): 150 ± 9.5 cps Have bioadhesive property. Ex vivo permeability assay: improved transcorneal permeation and extended the timolol maleate retention at the corneal site. In vitro ocular irritation (HET-CAM): no irritation. Gamma scintigraphy study: less nasolacrimal drainage and no timolol was observed in systemic circulation. | [96] |
| To develop and evaluate a combination of chitosan and gellan gum in situ gel of sparfloxacin to lengthen the corneal retention time. | Ocular bacterial infections/ sparfloxacin | pH-activated/chitosan and ion-activated/ gellan gum | HET-CAM test NZW rabbits | Gelation pH: 7.06 ± 0.1 Viscosity (sol): 42.33 ± 1.75 cps Viscosity (gel): 247.33 ± 5.92 cps Have mucoadhesive property. In vitro release study: prolonged release of sparfloxacin. In vitro ocular irritation (HET-CAM): no irritation. Gamma scintigraphy study: better corneal retention. | [97] |
| To prepare, characterize, and evaluate a combination of chitosan and gellan gum in situ gel formulations for sustained ophthalmic delivery of besifloxacin. | Bacterial conjunctivitis/ besifloxacin | Thermo and pH-activated/ chitosan and ion-activated/ gellan gum | Rabbits’ eye Goat cornea HET-CAM test | Gelation pH: 7.4 Have mucoadhesive properties. In vivo besifloxacin release study: have superior sustain drug release properties. Ex vivo permeation study: enhanced the retention of BSF at corneal surface. In vitro ocular irritation (HET-CAM): no irritation. Histopathology study: no damage seen on corneal membrane. Gamma scintigraphy study: higher amount of BSF retained on the corneal surface. Antimicrobial Study: superior anti-bacterial activity. | [98] |
| To formulate a combination of chitosan and sodium alginate in situ gel formulation of levofloxacin to prolong contact duration with the ocular surface. | Bacterial keratitis/ levofloxacin | pH-activated/chitosan and ion-activated/ sodium alginate | Albino rabbits | Gelation pH: 7.08 ± 0.07 Viscosity (sol): 52.16 ± 3.48 cps Viscosity (gel): 299.16 ± 29.39 cps Has mucoadhesive properties. In vitro release study: have sustained and control release characteristics. Gamma scintigraphy study: even levofloxacin distribution and none were detected in the systemic circulation. | [99] |
| To develop combination of Carbopol 940 and gellan gum in situ ophthalmic gel to sustain release of chloramphenicol and dexamethasone sodium phosphate. | Bacterial endophthalmitis/chloramphenicol and dexamethasone sodium phosphate | pH-activated/Carbopol 940 and ion-activated/ gellan gum | - | Viscosity (sol): 50–160 cps Viscosity (gel): 471–6500 cps Mucoadhesive strength: 8.5 ± 0.71–23.6 ± 0.43 g In vitro release study: have sustained release characteristics. | [100] |
| To design a combination of thermosensitive and ionic sensitive in situ ophthalmic delivery systems to prolong the effect of HP-β-CD Voriconazole (VCZ). | Fungal keratitis/ voriconazole | Thermo-activated/Pluronic F-127 or with combination of Pluronic F-68 and ion-activated/ sodium alginate | Goat cornea | Gelation temperature: 24.36 ± 0.41 to 37.33 ± 0.73 °C Viscosity (gel): 235.7 ± 6.66 to 414.3 ± 10.0 cps Mucoadhesive strength: 17.24 to 28.28 dynes/cm2 In vitro drug release study and ex vivo permeation study: Slowed down with increasing concentration of each polymer. Anti-fungal efficiency: have prolonged effect and retained its properties against fungal infection. | [101] |
| To design polymeric nanoparticles of Acyclovir incorporated in in situ gelling system to provide a dual sustained release effect, whereby the duration of action and bioavailability through different routes of administration could be improved. | Ocular viral infection/acyclovir | Thermo-activated/Pluronic F-127 and pH-activated/ Carbopol | - | Gelation temperature: 25 ± 0.20 to 35 ± 0.46 °C Gelation time: 2 to 4 min In vitro drug release study: better sustained release characteristics, with non-Fickian diffusion mechanism of drug release. | [102] |
| To formulate, optimize, and evaluate the in situ gel for the ophthalmic drug delivery using the combination of gellan gum and Carbopol 934P. | Allergic conjunctivitis/ olopatadine HCl | Thermo-activated/ Carbopol 934P and ion-activated/ gellan gum | NZW rabbits Goat cornea | Viscosity (sol): 34.1 ± 1.2 to 300.7 ± 21.6 cps Viscosity (gel): 471.1 ± 23.5 to 6500.1 ± 234.3 33 cps Mucoadhesive strength: 9.3 ± 0.7 to 33.3 ± 5.2 dynes/cm2 In vivo drug release study: sustained olopatadine release Ex vivo permeation study: within desired value range Ocular irritation study and histopathology study: no irritation. | [103] |
| To formulate capsazepine ocular in situ formulation to treat capsaicin induced ocular tissue inflammation. | Eye irritation cause by capsaicin/ capsazepine | Thermo-activated/ Pluronic F-127 and pH-activated/ chitosan | Oryctolagus cuniculus rabbits Rattus norvegicus rats Bovine eyes Human Corneal Epithelial Cells and Human Retinal Microvascular Endothelial Cells | Gelation temperature: 28.5 ± 0.62 °C Gelation time: 18 ± 0.68 s In vivo efficacy study: irritation and inflammatory symptoms caused by capsaicin were successfully treated. Ex vivo permeation study: weaker drug permeation. Histopathological study: eased inflammatory ocular responses caused by capsaicin. Cytotoxic study: capsazepine was not cytotoxic in optimum dose. | [104] |
| Type of In-Situ Formulation | Advantages | Disadvantages | Polymer Used |
|---|---|---|---|
| Thermo-responsive |
| Not suitable to be having around in hotter climate countries, Leakage of gel may occur if there is no immediate phase transition from sol to gel. | Poloxamer, PNIPAAM, cellulose derivatives |
| pH-responsive | Stability of basic drugs may be challenging as pH-sensitive polymers used are acidic Sudden shift of pH may cause mild irritation of the eyes. Leakage of gel may occur if there is no immediate phase transition from sol to gel. | Carbomer-910, -934, -941, -940, PCP, Chitosan | |
| Ion-responsive | Leakage of gel may occur if there is no immediate phase transition from sol to gel. | Carrageenan, gellan gum, alginate | |
| Multi-stimuli-responsive | All of the above | All polymers as mentioned above |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, M.; Choudhury, H.; binti Abd Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Su, J.S.T.; Tan, C.L.; Chin, W.Y.; Yip, K.Y. Potential of Stimuli-Responsive In Situ Gel System for Sustained Ocular Drug Delivery: Recent Progress and Contemporary Research. Polymers 2021, 13, 1340. https://doi.org/10.3390/polym13081340
Pandey M, Choudhury H, binti Abd Aziz A, Bhattamisra SK, Gorain B, Su JST, Tan CL, Chin WY, Yip KY. Potential of Stimuli-Responsive In Situ Gel System for Sustained Ocular Drug Delivery: Recent Progress and Contemporary Research. Polymers. 2021; 13(8):1340. https://doi.org/10.3390/polym13081340
Chicago/Turabian StylePandey, Manisha, Hira Choudhury, Azila binti Abd Aziz, Subrat Kumar Bhattamisra, Bapi Gorain, Jocelyn Sziou Ting Su, Choo Leey Tan, Woon Yee Chin, and Khar Yee Yip. 2021. "Potential of Stimuli-Responsive In Situ Gel System for Sustained Ocular Drug Delivery: Recent Progress and Contemporary Research" Polymers 13, no. 8: 1340. https://doi.org/10.3390/polym13081340