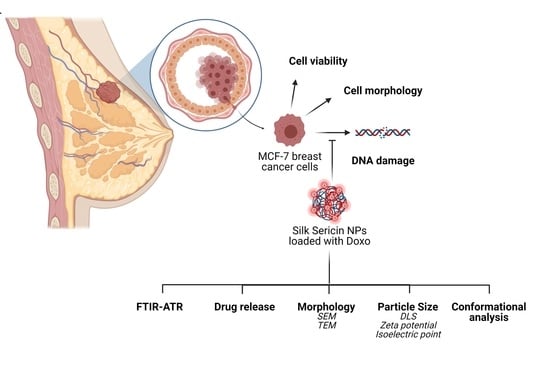
In Vitro Interaction of Doxorubicin-Loaded Silk Sericin Nanocarriers with MCF-7 Breast Cancer Cells Leads to DNA Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silk Sericin Nanoparticles
2.3. Drug Loading in Sericin Nanoparticles
2.4. Drug Release Behavior
2.5. Characterization Methods
2.5.1. FTIR–ATR Analysis
2.5.2. Morphological Characterization
2.5.3. Dynamic Light Scattering (DLS)
2.5.4. Conformational Analysis by Circular Dichroism (CD)
2.6. In Vitro Biological Evaluation of Free and Doxorubicin-Loaded Sericin Nanocarriers
2.6.1. Cell Culture Model
2.6.2. Cell Viability Assay
2.6.3. Cytoskeleton Investigation and DAPI staining
2.6.4. Measurement of DNA Damage by Comet Assay
2.6.5. Statistical Analysis
3. Results and Discussions
3.1. FTIR–ATR Analysis
3.2. Drug Release Behavior
3.2.1. Neutral Medium
3.2.2. Acidic Medium
3.2.3. Enzymatic Media
3.3. Morphological Characterization
3.3.1. SEM Analysis
3.3.2. TEM Analysis
3.4. Dynamic Light Scattering, Zeta Potential, and Isoelectric Point
Molecular Weight Evaluation by DLS
3.5. Conformational Analysis by Circular Dichroism (CD)
3.6. In Vitro Antitumor Activity Evaluation of DOX-Loaded Sericin Nanocarriers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagaraju, J.; Goldsmith, M.R. Silkworm Genomics–Progress and Prospects. Curr. Sci. 2002, 83, 415–425. [Google Scholar]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural Protective Glue Protein, Sericin Bioengineered by Silkworms: Potential for Biomedical and Biotechnological Applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk Fibroin Biomaterials for Tissue Regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Urry, D.W.; Luan, C.-H.; Harris, C.M.; Parker, T.M. Protein-based materials with a profound range of properties and applications: The elastin ΔT t hydrophobic paradigm. In Protein-Based Materials; Springer: Berlin/Heidelberg, Germany, 1997; pp. 133–177. [Google Scholar]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk Sericin: A Versatile Material for Tissue Engineering and Drug Delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef]
- Radu, I.-C.; Biru, I.-E.; Damian, C.-M.; Ion, A.-C.; Iovu, H.; Tanasa, E.; Zaharia, C.; Galateanu, B. Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges. Molecules 2019, 24, 4096. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef]
- McGrath, K.; Kaplan, D. Protein-Based Materials; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 3-7643-3848-2. [Google Scholar]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-Based Biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Carissimi, G.; Lozano-Pérez, A.A.; Montalbán, M.G.; Aznar-Cervantes, S.D.; Cenis, J.L.; Víllora, G. Revealing the Influence of the Degumming Process in the Properties of Silk Fibroin Nanoparticles. Polymers 2019, 11, 2045. [Google Scholar] [CrossRef] [Green Version]
- Chirila, T.V.; Suzuki, S.; Bray, L.J.; Barnett, N.L.; Harkin, D.G. Evaluation of Silk Sericin as a Biomaterial: In Vitro Growth of Human Corneal Limbal Epithelial Cells on Bombyx Mori Sericin Membranes. Prog. Biomater. 2013, 2, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, S.; Kundu, S.C. Silk Protein Sericin: Promising Biopolymer for Biological and Biomedical Applications. Biomater. Nat. Adv. Devices Ther. Wiley Soc. Biomater. 2016, 142–154. [Google Scholar]
- Aramwit, P.; Bang, N.; Ratanavaraporn, J.; Ekgasit, S. Green Synthesis of Silk Sericin-Capped Silver Nanoparticles and Their Potent Anti-Bacterial Activity. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar]
- Zhaorigetu, S.; Yanaka, N.; Sasaki, M.; Watanabe, H.; Kato, N. Silk Protein, Sericin, Suppresses DMBA-TPA-Induced Mouse Skin Tumorigenesis by Reducing Oxidative Stress, Inflammatory Responses and Endogenous Tumor Promoter TNF-α. Oncol. Rep. 2003, 10, 537–543. [Google Scholar] [PubMed]
- Zhaorigetu, S.; Sasaki, M.; Kato, N. Consumption of Sericin Suppresses Colon Oxidative Stress and Aberrant Crypt Foci in 1, 2-Dimethylhydrazine-Treated Rats by Colon Undigested Sericin. J. Nutr. Sci. Vitaminol. 2007, 53, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Perteghella, S.; Faragò, S.; Torre, M.L. Association of Silk Sericin and Platelet Lysate: Premises for the Formulation of Wound Healing Active Medications. Int. J. Biol. Macromol. 2018, 119, 37–47. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, B.B. Silk Biomaterials in Wound Healing and Skin Regeneration Therapeutics: From Bench to Bedside. Acta Biomater. 2020, 103, 24–51. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.C.; Montoya, Y.; Peresin, M.S.; Bustamante, J.; Álvarez-López, C. Silk Sericin as a Biomaterial for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–15. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The Effect of Sericin from Various Extraction Methods on Cell Viability and Collagen Production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk Sericin Ameliorates Wound Healing and Its Clinical Efficacy in Burn Wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef]
- Cui, Y.; Xing, Z.; Yan, J.; Lu, Y.; Xiong, X.; Zheng, L. Thermosensitive Behavior and Super-Antibacterial Properties of Cotton Fabrics Modified with a Sercin-NIPAAm-AgNPs Interpenetrating Polymer Network Hydrogel. Polymers 2018, 10, 818. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.Y.; Moon, J.Y.; Lee, Y.W.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Kim, K.H.; Cho, C.S. Preparation of Self-Assembled Silk Sericin Nanoparticles. Int. J. Biol. Macromol. 2003, 32, 36–42. [Google Scholar] [CrossRef]
- Akturk, O.; Gun Gok, Z.; Erdemli, O.; Yigitoglu, M. One-pot Facile Synthesis of Silk Sericin-capped Gold Nanoparticles by UVC Radiation: Investigation of Stability, Biocompatibility, and Antibacterial Activity. J. Biomed. Mater. Res. A 2019, 107, 2667–2679. [Google Scholar] [CrossRef]
- Das, S.K.; Dey, T.; Kundu, S. Fabrication of Sericin Nanoparticles for Controlled Gene Delivery. RSC Adv. 2014, 4, 2137–2142. [Google Scholar] [CrossRef]
- Hazeri, N.; Tavanai, H.; Moradi, A.R. Production and Properties of Electrosprayed Sericin Nanopowder. Sci. Technol. Adv. Mater. 2012, 13, 035010. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S. Self-Assembled Silk Sericin/Poloxamer Nanoparticles as Nanocarriers of Hydrophobic and Hydrophilic Drugs for Targeted Delivery. Nanotechnology 2009, 20, 355101. [Google Scholar] [CrossRef]
- He, H.; Cai, R.; Wang, Y.; Tao, G.; Guo, P.; Zuo, H.; Chen, L.; Liu, X.; Zhao, P.; Xia, Q. Preparation and Characterization of Silk Sericin/PVA Blend Film with Silver Nanoparticles for Potential Antimicrobial Application. Int. J. Biol. Macromol. 2017, 104, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Xu, Z.; Hu, Z.; Hu, B.; Yang, M.; Zhu, L. PH-Triggered Charge-Reversal Silk Sericin-Based Nanoparticles for Enhanced Cellular Uptake and Doxorubicin Delivery. ACS Sustain. Chem. Eng. 2017, 5, 1638–1647. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Wu, A.; Wang, J.; Ge, C.; Sun, Z. Self-Assembled Silk Fibroin Nanoparticles Loaded with Binary Drugs in the Treatment of Breast Carcinoma. Int. J. Nanomed. 2016, 11, 4373. [Google Scholar]
- Numata, K.; Kaplan, D.L. Silk-Based Delivery Systems of Bioactive Molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [Green Version]
- Radu, I.-C.; Hudita, A.; Zaharia, C.; Stanescu, P.O.; Vasile, E.; Iovu, H.; Stan, M.; Ginghina, O.; Galateanu, B.; Costache, M. Poly (Hydroxybutyrate-Co-Hydroxyvalerate)(PHBHV) Nanocarriers for Silymarin Release as Adjuvant Therapy in Colo-Rectal Cancer. Front. Pharmacol. 2017, 8, 508. [Google Scholar] [CrossRef] [Green Version]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Rodríguez, S.A.G.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Baishya, D.; Sarkar, T.; Edinur, H.A.; Pati, S. Synthesis and Characterization of Diosgenin Encapsulated Poly-ε-Caprolactone-Pluronic Nanoparticles and Its Effect on Brain Cancer Cells. Polymers 2021, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.O.; Catalan-Figueroa, J.; Landin, M.; Morales, J.O. Finding Key Nanoprecipitation Variables for Achieving Uniform Polymeric Nanoparticles Using Neurofuzzy Logic Technology. Drug Deliv. Transl. Res. 2018, 8, 1797–1806. [Google Scholar] [CrossRef]
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Kiafar, F.; Jelvehgari, M. Development of a Nanoprecipitation Method for the Entrapment of a Very Water Soluble Drug into Eudragit RL Nanoparticles. Res. Pharm. Sci. 2017, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xie, Z.; Ju, C.; Hu, X.; Yuan, D.; Zhao, W.; Shui, L.; Zhou, G. Dye-Doped Electrically Smart Windows Based on Polymer-Stabilized Liquid Crystal. Polymers 2019, 11, 694. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, F.; Isoardo, T.; Denis, S.; Tsapis, N.; Tselikas, L.; Nicolas, V.; Paci, A.; Fattal, E.; de Baere, T.; Huang, N. Biodegradable Pickering Emulsions of Lipiodol for Liver Trans-Arterial Chemo-Embolization. Acta Biomater. 2019, 87, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xie, L.; Xu, S.; Kuchel, R.P.; Dai, Y.; Jung, K.; Boyer, C. Dual Role of Doxorubicin for Photopolymerization and Therapy. Biomacromolecules 2020, 21, 3887–3897. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Yuan, Z.; Qian, H.; Xu, L.; Sidransky, E.; Chen, S. Development of Zwitterionic Polypeptide Nanoformulation with High Doxorubicin Loading Content for Targeted Drug Delivery. Langmuir 2018, 35, 1273–1283. [Google Scholar] [CrossRef]
- Jørgensen, J.R.; Thamdrup, L.H.; Kamguyan, K.; Nielsen, L.H.; Nielsen, H.M.; Boisen, A.; Rades, T.; Müllertz, A. Design of a Self-Unfolding Delivery Concept for Oral Administration of Macromolecules. J. Control. Release 2021, 329, 948–954. [Google Scholar] [CrossRef]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Naoum, G.E.; Tawadros, F.; Farooqi, A.A.; Qureshi, M.Z.; Tabassum, S.; Buchsbaum, D.J.; Arafat, W. Role of Nanotechnology and Gene Delivery Systems in TRAIL-Based Therapies. Ecancermedicalscience 2016, 10, 660. [Google Scholar] [CrossRef] [Green Version]
- Orlandi, G.; Bari, E.; Catenacci, L.; Sorrenti, M.; Segale, L.; Faragò, S.; Sorlini, M.; Arciola, C.R.; Torre, M.L.; Perteghella, S. Polyphenols-Loaded Sericin Self-Assembling Nanoparticles: A Slow-Release for Regeneration by Tissue-Resident Mesenchymal Stem/Stromal Cells. Pharmaceutics 2020, 12, 381. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Tao, K.; Liu, J.; Qi, C.; Xu, L.; Chang, P.; Gao, J.; Shuai, X.; Wang, G.; Wang, Z. Design and Fabrication of Multifunctional Sericin Nanoparticles for Tumor Targeting and PH-Responsive Subcellular Delivery of Cancer Chemotherapy Drugs. ACS Appl. Mater. Interfaces 2016, 8, 6577–6585. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Zhang, J.; Huang, L.; Qi, C.; Xu, L.; Liu, X.; Wang, G.; Wang, L.; Wang, Z. Safe and Effective Reversal of Cancer Multidrug Resistance Using Sericin-coated Mesoporous Silica Nanoparticles for Lysosome-targeting Delivery in Mice. Small 2017, 13, 1602567. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.-S.; Campos, E.V.R.; Fraceto, L.F.; del Pilar Rodriguez-Torres, M.; Mariano, K.C.F.; de Araujo, D.R.; Fernández-Luqueño, F.; Grillo, R.; Patra, J.K. Sericin Based Nanoformulations: A Comprehensive Review on Molecular Mechanisms of Interaction with Organisms to Biological Applications. J. Nanobiotechnol. 2021, 19, 1–22. [Google Scholar] [CrossRef]
- Radu, I.C.; Hudita, A.; Zaharia, C.; Galateanu, B.; Iovu, H.; Tanasa, E.; Georgiana Nitu, S.; Ginghina, O.; Negrei, C.; Tsatsakis, A. Poly (3-Hydroxybutyrate-CO-3-Hydroxyvalerate) PHBHV Biocompatible Nanocarriers for 5-FU Delivery Targeting Colorectal Cancer. Drug Deliv. 2019, 26, 318–327. [Google Scholar] [CrossRef]
- Galindo-Rodriguez, S.; Allemann, E.; Fessi, H.; Doelker, E. Physicochemical Parameters Associated with Nanoparticle Formation in the Salting-out, Emulsification-Diffusion, and Nanoprecipitation Methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Blouza, I.L.; Charcosset, C.; Sfar, S.; Fessi, H. Preparation and Characterization of Spironolactone-Loaded Nanocapsules for Paediatric Use. Int. J. Pharm. 2006, 325, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Maaz, A.; Abdelwahed, W.; Tekko, I.A.; Trefi, S. Influence of Nanoprecipitation Method Parameters on Nanoparticles Loaded with Gatifloxacin for Ocular Drug Delivery. Int. J. Acad. Sci. Res. 2015, 3, 12. [Google Scholar]
- Gewirtz, D. A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, I.-C.; Zaharia, C.; Hudiță, A.; Tanasă, E.; Ginghină, O.; Marin, M.; Gălățeanu, B.; Costache, M. In Vitro Interaction of Doxorubicin-Loaded Silk Sericin Nanocarriers with MCF-7 Breast Cancer Cells Leads to DNA Damage. Polymers 2021, 13, 2047. https://doi.org/10.3390/polym13132047
Radu I-C, Zaharia C, Hudiță A, Tanasă E, Ginghină O, Marin M, Gălățeanu B, Costache M. In Vitro Interaction of Doxorubicin-Loaded Silk Sericin Nanocarriers with MCF-7 Breast Cancer Cells Leads to DNA Damage. Polymers. 2021; 13(13):2047. https://doi.org/10.3390/polym13132047
Chicago/Turabian StyleRadu, Ionuț-Cristian, Cătălin Zaharia, Ariana Hudiță, Eugenia Tanasă, Octav Ginghină, Minodora Marin, Bianca Gălățeanu, and Marieta Costache. 2021. "In Vitro Interaction of Doxorubicin-Loaded Silk Sericin Nanocarriers with MCF-7 Breast Cancer Cells Leads to DNA Damage" Polymers 13, no. 13: 2047. https://doi.org/10.3390/polym13132047